Molecular Conformations and Biological Activity of N-Hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides
Abstract
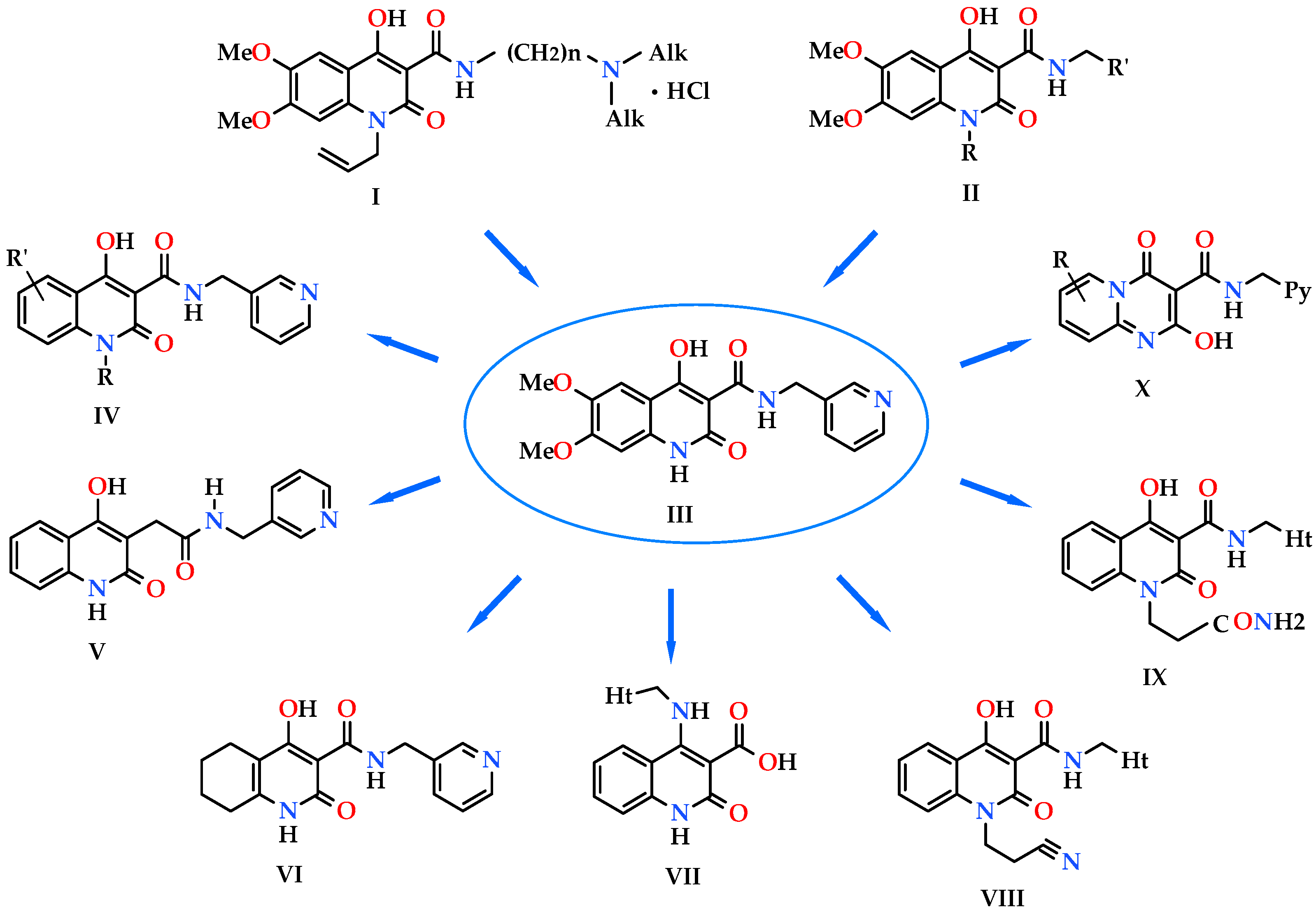
1. Introduction
2. Materials and Methods
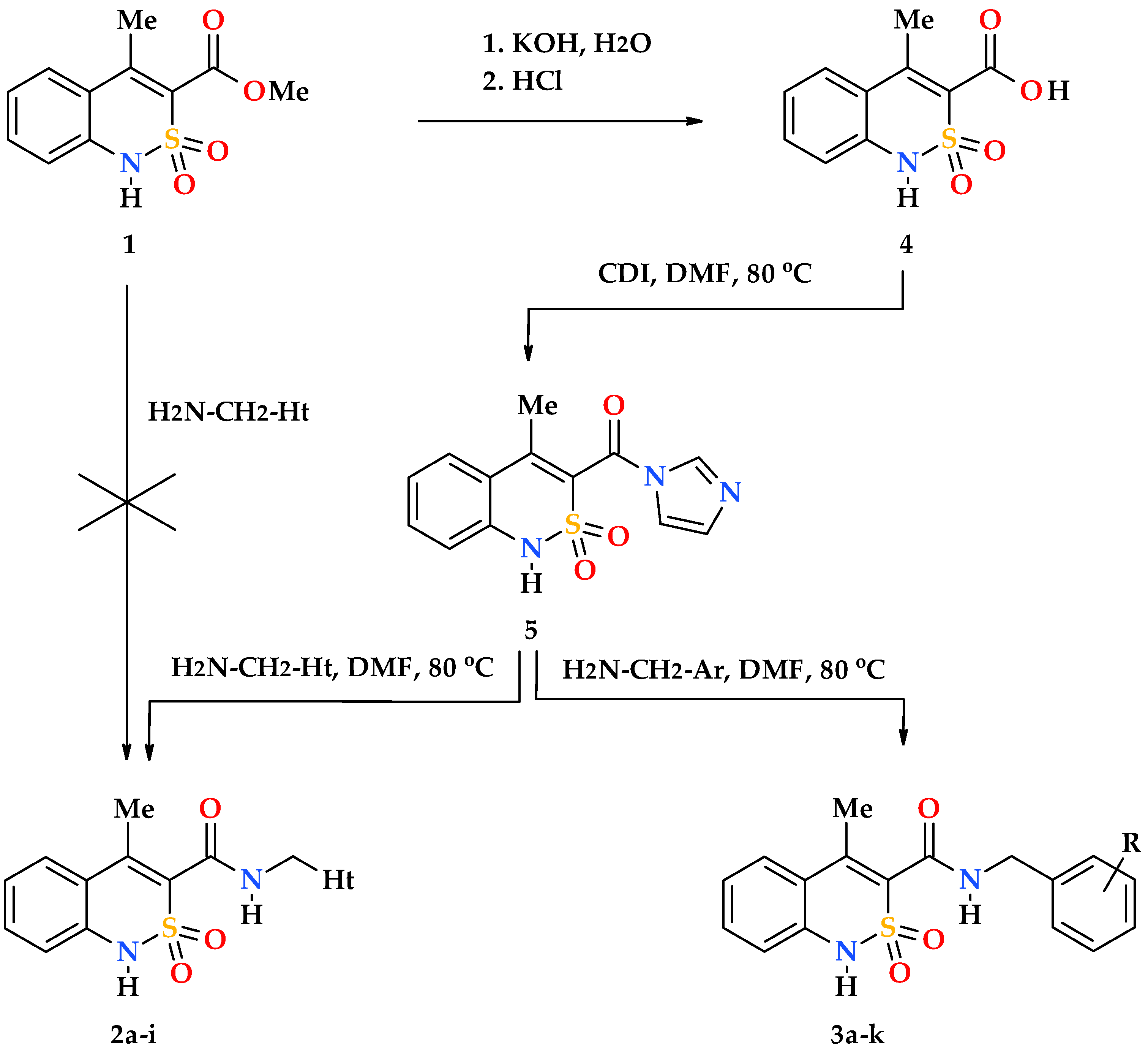
2.1. Chemistry
2.2. General Procedure for the Synthesis of N-Hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides 2a–i and 3a–k
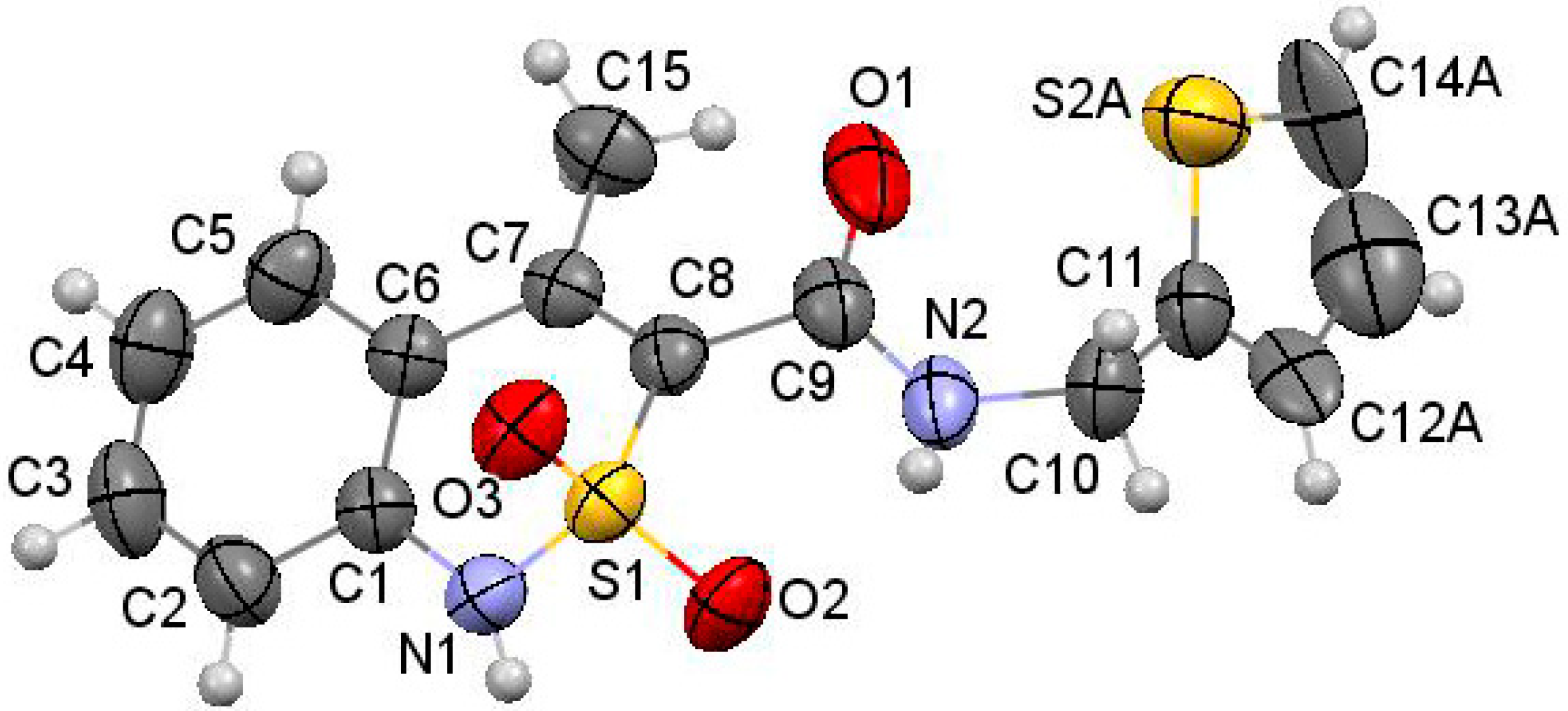
2.3. X-ray Structural Analysis of N-(Pyridin-4-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide monohydrate (2c)
2.4. X-ray Structural Analysis of N-(Furan-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2d)
2.5. X-ray Structural Analysis of N-(5-Methylfuran-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2e)
2.6. X-ray Structural Analysis of N-(Thiophen-2-ylmethyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (2g)
2.7. X-ray Structural Analysis of N-Benzyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3a)
2.8. X-ray Structural Analysis of N-(2-Chlorobenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3c)
2.9. X-ray Structural Analysis of N-(4-Chlorobenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3d)
2.10. X-ray Structural Analysis of N-(2-Methylbenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3e)
2.11. X-ray Structural Analysis of N-(3-Methylbenzyl)-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide (3f)
2.12. Pharmacology: Analgesic and Anti-Inflammatory Tests
3. Results and Discussion
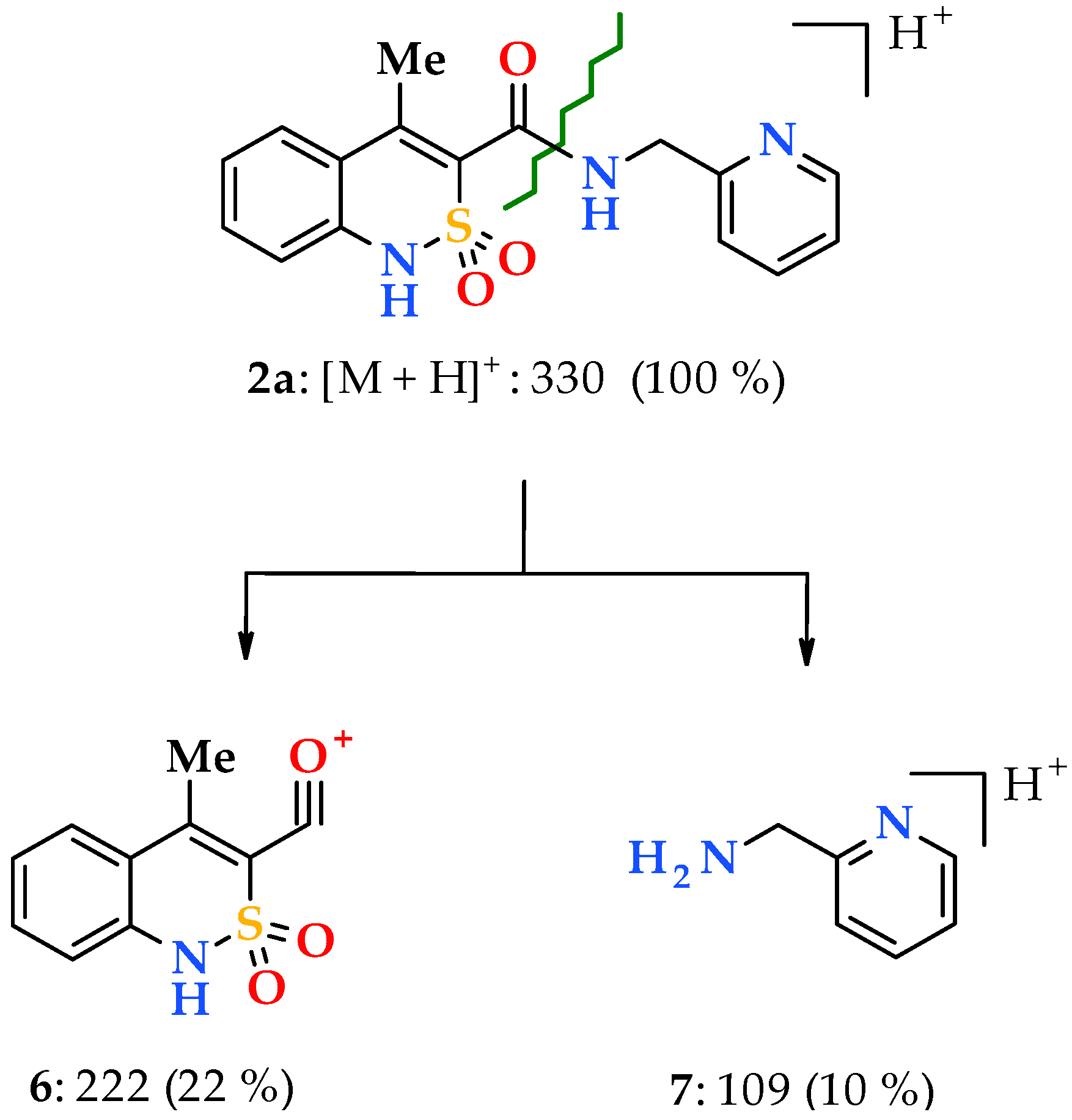
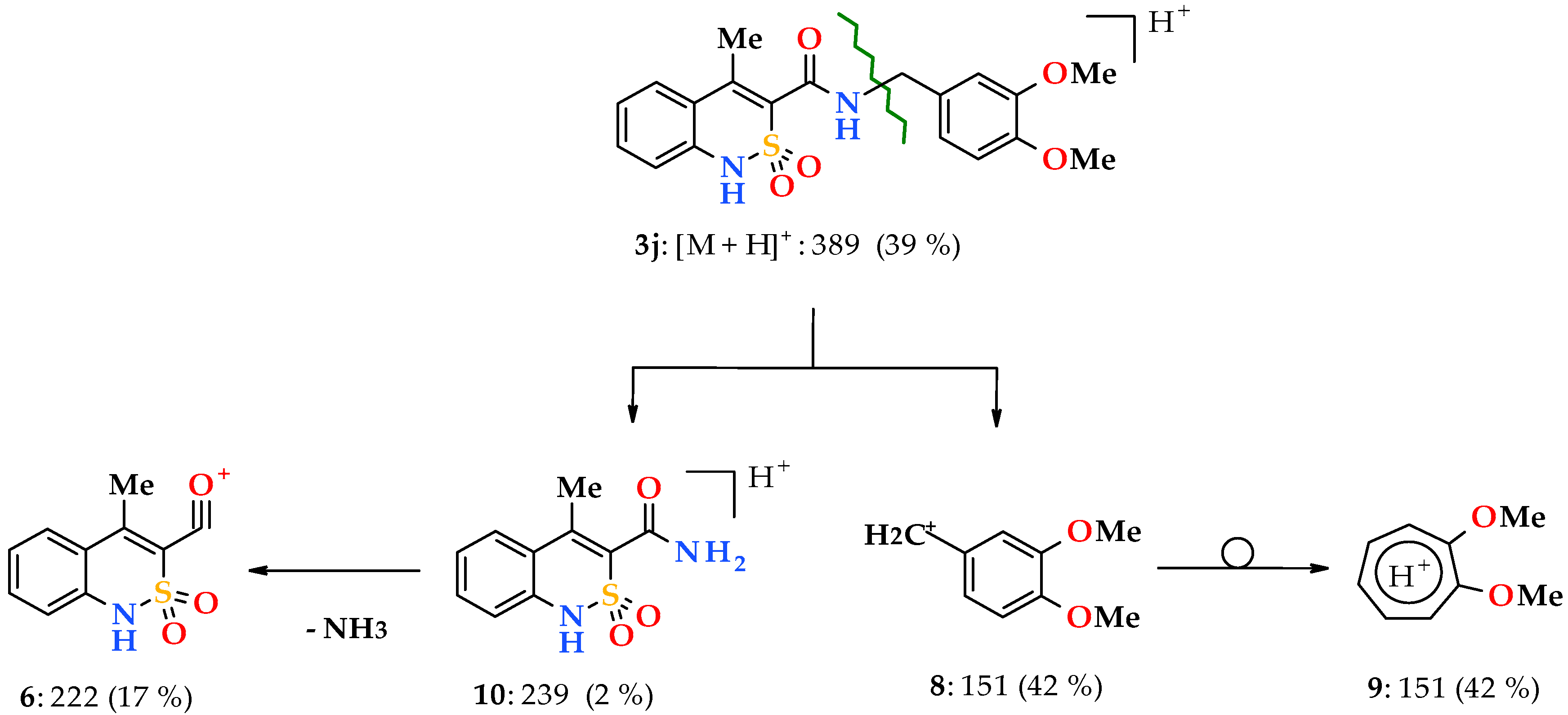
3.1. Chemistry
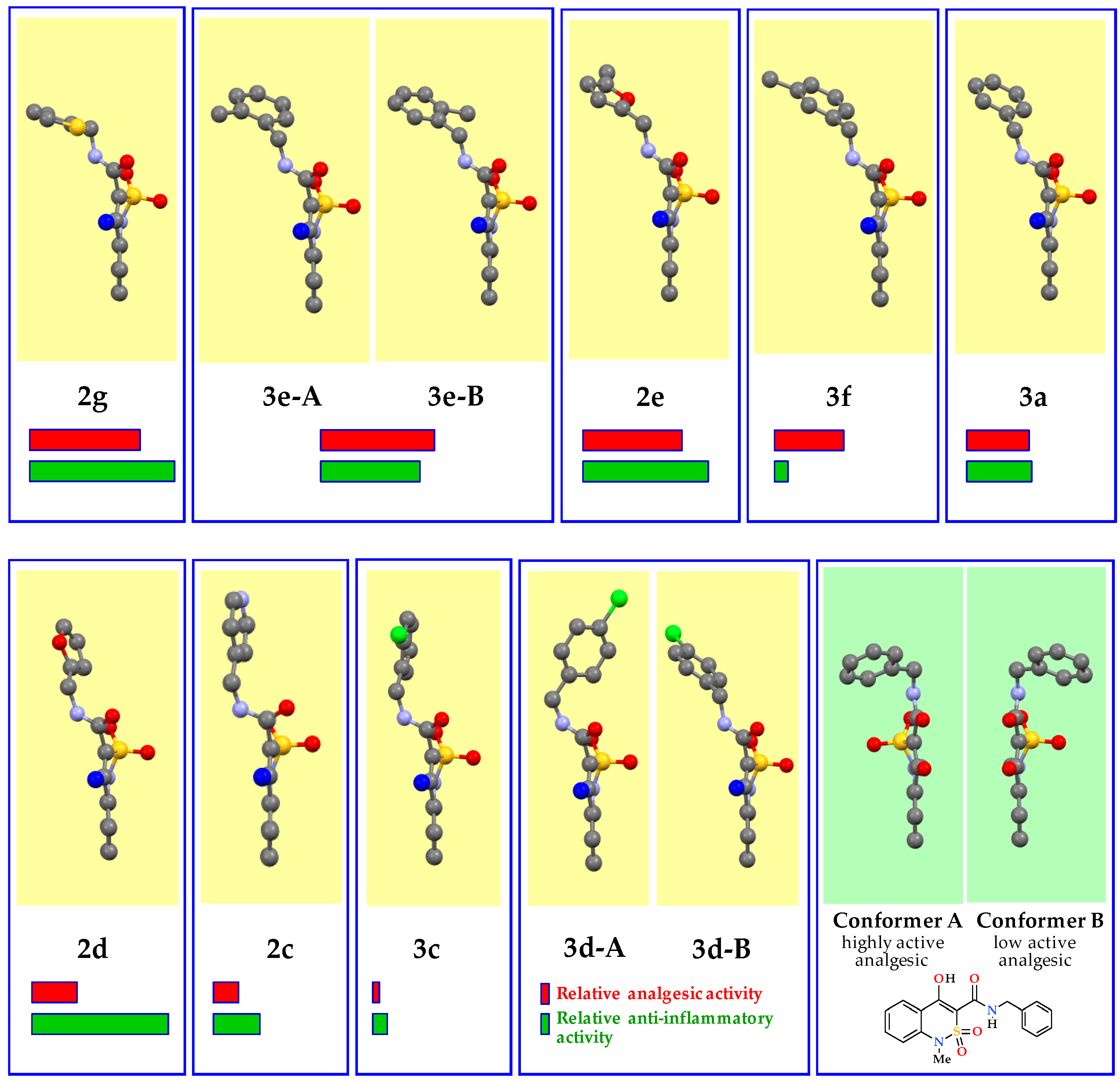
3.2. Evaluation of the Analgesic and Anti-Inflammatory Activity
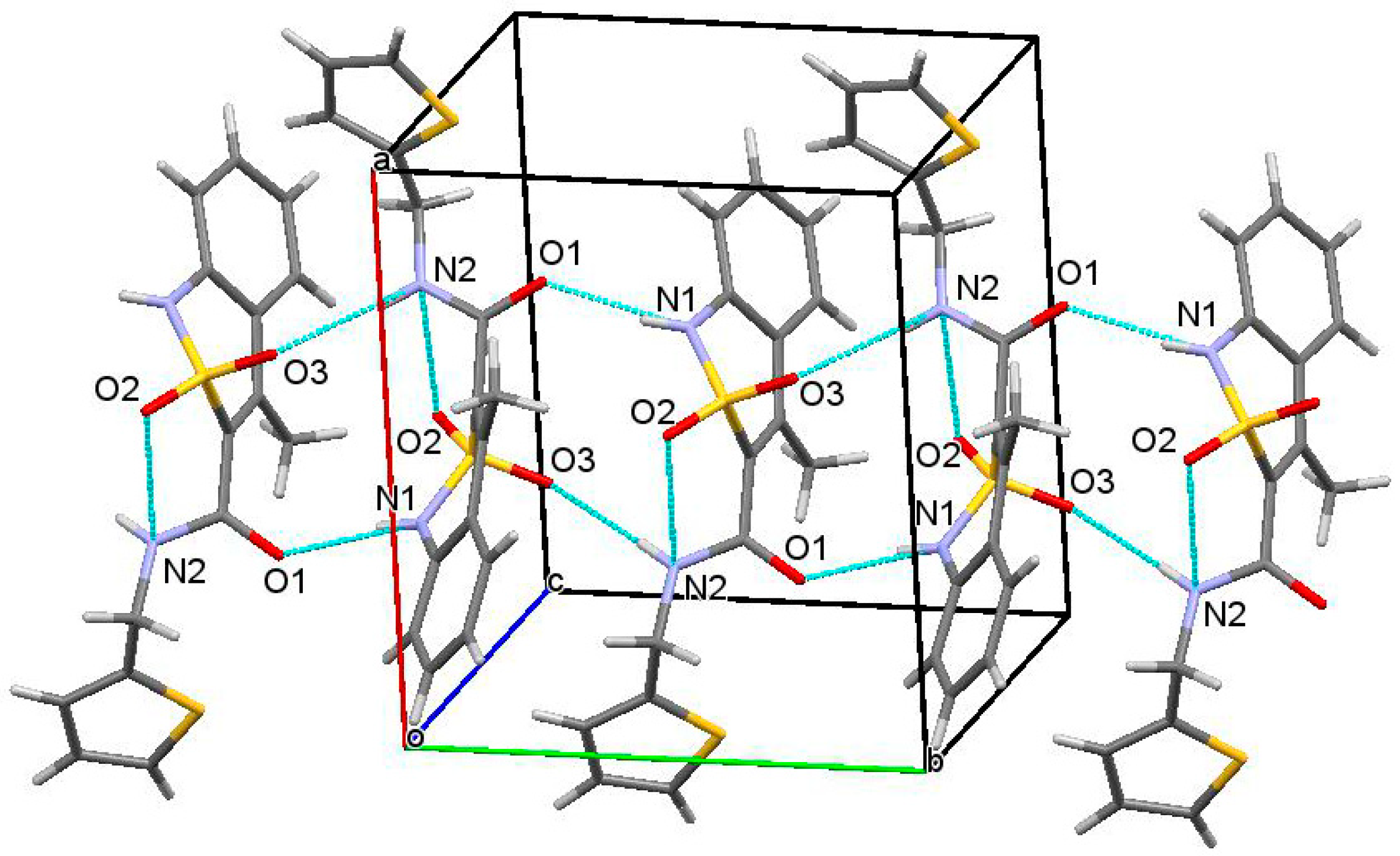
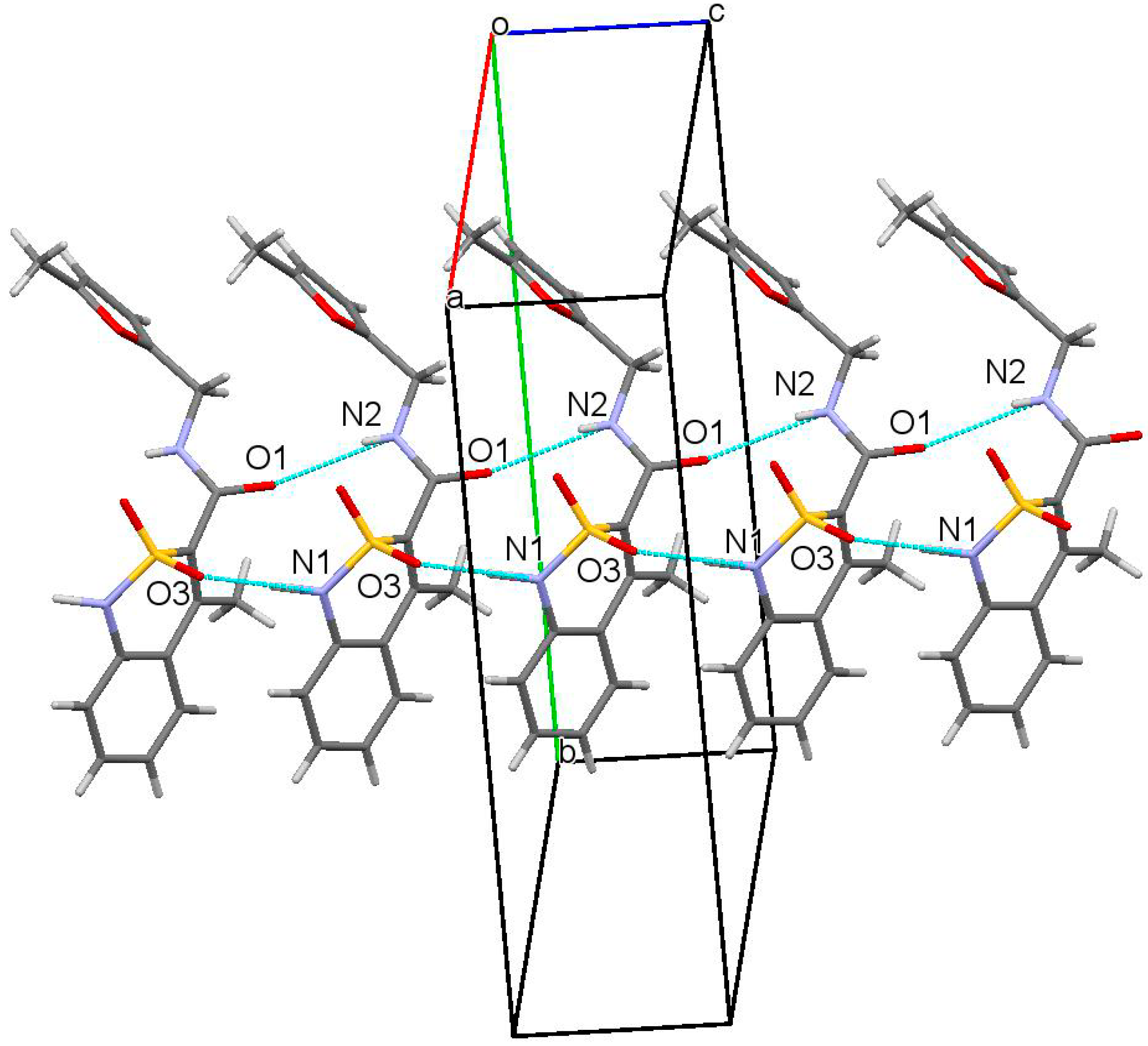
3.3. The Molecular and Crystal Structure Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ukrainets, I.V.; Sidorenko, L.V.; Davidenko, A.A.; Yarosh, A.K. 4-Hydroxy-2-quinolones. 174. Hydro-chlorides of [(alkylamino)alkyl]amides of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid—A new class of the opioid receptors antagonists. Chem. Heterocycl. Compd. 2010, 46, 445–451. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Davidenko, A.A.; Shishkina, S.V. 4-Hydroxy-2-quinolones. 180. Synthesis, chemical reactions, and analgesic activity of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid alkylamides. Chem. Heterocycl. Compd. 2010, 46, 1084–1095. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Jaradat, N.A.; Bevz, O.V.; Turov, A.V. 4-Hydroxy-2-quinolones. 204. Synthesis, bromination, and analgetic properties of 1-allyl-4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid arylalkylamides. Chem. Heterocycl. Compd. 2012, 48, 1347–1356. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Gorokhova, O.V.; Jaradat, N.A.; Petrushova, L.A.; Mospanova, E.V.; Savchenkova, L.V.; Kuz’min, V.E.; Lyahovsky, A.V. 4-Hydroxyquinolin-2-ones and their close structural analogues as a new source of highly effective pain-killers. In Pain and Treatment; Racz, G.B., Noe, C.E., Eds.; InTech: Rijeka, Croatia, 2014; pp. 21–73. [Google Scholar]
- Ukrainets, I.V.; Bevz, O.V.; Mospanova, E.V.; Savchenkova, L.V.; Yankovich, S.I. 4-Hydroxy-2-quinolones. 202. Synthesis, chemical and biological properties of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxylic acid alkylamides. Chem. Heterocycl. Compd. 2012, 48, 320–326. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Davidenko, A.A. Using bioisosteric replacements to enhance the analgesic properties of 4-hydroxy-6,7-dimethoxy-2-oxo-1,2-dihydroquinoline-3-carboxamides. Pharm. Chem. J. 2016, 50, 365–368. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, O.V.; Bereznyakova, N.L.; Davidenko, O.O. Polymorphism and the analgesic activity of N-(3-pyridylmethyl)-4-hydroxy-2-oxo-1,2,5,6,7,8-hexahydroquinoline-3-carboxamide. J. Org. Pharm. Chem. 2015, 13, 41–46. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Mospanova, E.V.; Savchenkova, L.V.; Yankovich, S.I. 4-Hydroxy-2-quinolones. 195. Synthesis of novel, potential analgesics based on 4-(hetarylmethyl)amino-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Chem. Heterocycl. Compd. 2011, 47, 67–73. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Davidenko, A.A.; Mospanova, E.V.; Sidorenko, L.V.; Svechnikova, E.N. 4-Hydroxy-2-quinolones. 176. 4-R-2-oxo-1,2-dihydroquinoline-3-carboxylic acids. Synthesis, physicochemical and biological properties. Chem. Heterocycl. Compd. 2010, 46, 559–568. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Gorokhova, O.V.; Andreeva, K.V. Transformation of 3-(3-arylalkylcarbamoyl-4-hydroxy-2-oxo-1,2-dihydroquinolin-1-yl)propanenitriles into amides and acids. Russ. J. Org. Chem. 2013, 49, 867–871. [Google Scholar] [CrossRef]
- Ukrainets, I.V.; Bereznyakova, N.L.; Sim, G.; Davidenko, A.A. Synthesis, structure, and analgesic activity of picolylamides of 2-hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-3-carboxylic acids. Pharm. Chem. J. 2018, 52, 601–605. [Google Scholar] [CrossRef]
- Meanwell, N.A. Synopsis of some recent tactical application of bioisosteres in drug design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, F.J.; Tovar, F.; Austin, N.; Tresadern, G.; Trabanco, A.A. Benzazaborinines as novel bioisosteric replacements of naphthalene: Propranolol as an example. J. Med. Chem. 2015, 58, 9287–9295. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Barreiro, E.J. Bioisosterism: A useful strategy for molecular modification and drug design. Curr. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Brogi, S.; Brindisi, M.; Butini, S.; Kshirsagar, G.U.; Maramai, S.; Chemi, G.; Gemma, S.; Campiani, G.; Novellino, E.; Fiorenzani, P.; et al. (S)-2-Amino-3-(5-methyl-3-hydroxyisoxazol-4-yl)propanoic acid (AMPA) and kainate receptor ligands: Further exploration of bioisosteric replacements and structural and biological investigation. J. Med. Chem. 2018, 61, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Patina, G.A.; LaVoie, E.J. Bioisosterism: A rational approach in drug design. Chem. Rev. 1996, 96, 3147–3176. [Google Scholar] [CrossRef]
- Azotla-Cruz, L.; Lijanova, I.V.; Ukrainets, I.V.; Likhanova, N.V.; Olivares-Xometl, O.; Bereznyakova, N.L. New synthesis, structure and analgesic properties of methyl 1-R-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylates. Sci. Pharm. 2017, 85, 2. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Shishkina, S.V.; Voloshchuk, N.I.; Malchenko, O.V. 4-Methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxylic acid. Peculiarities of preparation, structure, and biological properties. Sci. Pharm. 2018, 86, 9. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877570. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877571. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877572. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1865723. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 4 September 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877573. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877574. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877575. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877576. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Cambridge Crystallographic Data Center. Request Quoting via: CCDC 1877577. Available online: www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 November 2018).
- Ukrainian Law No. 3447-IV. On Protection of Animals from Severe Treatment. Available online: http://zakon2.rada.gov.ua/laws/show/3447-15 (accessed on 4 August 2017).
- Vogel, H.G. (Ed.) Drug Discovery and Evaluation: Pharmacological Assays, 2nd ed.; Springer: Berlin, Germany, 2008; pp. 1103–1106. [Google Scholar]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An overview of animal models of pain: Disease models and outcome measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.G.O.; Berger, W.; Domschke, G.; Fanghänel, E.; Faust, J.; Fisher, M.; Gentz, F.; Gewald, K.; Gluch, R.; Mayer, R.; et al. Organikum: Organisch-Chemisches Grundpraktikum; 24 Auflage; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 751–774. [Google Scholar]
- Terent’ev, P.B.; Stankiavichyus, A.P. Mass Spectrometry Bioactive Nitrogenous Bases; Mokslas: Vilnius, Lithuania, 1987; pp. 60–64. [Google Scholar]
- Shet, A.R.; Grant, D.J.W. Relationship between the structure and properties of pharmaceutical crystals. KONA Powder Part. J. 2005, 23, 36–48. [Google Scholar] [CrossRef]
- Galek, P.T.A.; Pidcock, E.; Wood, P.A.; Bruno, I.J.; Groom, C.R. One in half a million: A solid form informatics study of a pharmaceutical crystal structure. CrystEngComm 2012, 14, 2391–2403. [Google Scholar] [CrossRef]
- Larsen, E.M.; Wilson, M.R.; Taylor, R.E. Conformation–activity relationships of polyketide natural products. Nat. Prod. Rep. 2015, 32, 1183–1206. [Google Scholar] [CrossRef] [PubMed]
- Do, T.D.; Checco, J.W.; Tro, M.; Shea, J.-E.; Bowers, M.T.; Sweedler, J.V. Conformational investigation of the structure–activity relationship of GdFFD and its analogues on an achatin-like neuropeptide receptor of Aplysia californica involved in the feeding circuit. Phys. Chem. Chem. Phys. 2018, 20, 22047–22057. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Chen, Y.; Galvin, G.M.; Pabba, P.K. Conformation–activity relationships inpolyketide natural products. Towards the biologically active conformation of epothilone. Org. Biomol. Chem. 2004, 2, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Ukrainets, I.V.; Petrushova, L.A.; Shishkina, S.V.; Grinevich, L.A.; Sim, G. Synthesis, spatial structure and analgesic activity of sodium 3-benzylaminocarbonyl-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazin-4-olate solvates. Sci. Pharm. 2016, 84, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Zefirov, N.S.; Palyulin, V.A.; Dashevskaya, E.E. Stereochemical studies. XXXIV. Quantitative description of ring puckering via torsional angles. The case of six-membered rings. J. Phys. Org. Chem. 1990, 3, 147–158. [Google Scholar] [CrossRef]
- Zefirov, Y.V. Reduced intermolecular contacts and specific interactions in molecular crystals. Crystallogr. Rep. 1997, 42, 865–886. [Google Scholar]
- Orpen, A.G.; Brammer, L.; Allen, F.H.; Kennard, O.; Watson, D.G.; Taylor, R. Typical interatomic distances in organic compounds and organometallic compounds and coordination complexes of the d- and f-block metals. In Structure Correlation; Burgi, H.-B., Dunitz, J.D., Eds.; Wiley-VCH: Weinheim, Germany, 1994; Volume 2, pp. 741–926. [Google Scholar]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Stoica, C.; Verwer, P.; Meekes, H.; van Hoof, P.J.C.M.; Kaspersen, F.M.; Vlieg, E. Understanding the effect of a solvent on the crystal habit. Cryst. Growth Des. 2004, 4, 765–768. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J.; Bernstein, J. Conformational Polymorphism. Chem. Rev. 2014, 114, 2170–2191. [Google Scholar] [CrossRef] [PubMed]
- Leonidov, N.B. History of the development of the concept of polymorphism of chemical substances (short essay). Russ. Chem. J. (J. Russ. Chem. Soc. DI Mendeleyev’s) 1997, XLI, 10–21. [Google Scholar]
- Ukrainets, I.V.; Shishkina, S.V.; Baumer, V.N.; Gorokhova, O.V.; Petrushova, L.A.; Sim, G. The structure of two pseudo-enantiomeric forms of N-benzyl-4-hydroxy-1-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamide and their analgesic properties. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72 Pt 5, 411–415. [Google Scholar] [CrossRef]
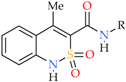
| Entry | Product | R | Pain Threshold on Damaged Extremity (g/mm2) | Pain Threshold on Non-Damaged Extremity (g/mm2) | Δ Pain Threshold | Analgesic Activity Compared to Control (%) |
|---|---|---|---|---|---|---|
| 1 | 2a |  | 432.0 ± 12.5 | 192.0 ± 18.5 | 240.0 ± 17.3 1,2,3 | +24.5 |
| 2 | 2b |  | 470.0 ± 50.9 | 345.0 ± 41.7 | 125.0 ± 36.1 1,2,3 | +60.7 |
| 3 | 2c |  | 522.0 ± 53.4 | 249.0 ± 36.9 | 273.0 ± 65.4 1,2,3 | +14.2 |
| 4 | 2d |  | 436.0 ± 36.6 | 202.0 ± 12.5 | 243.0 ± 55.1 1,2,3 | +26.4 |
| 5 | 2e | 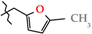 | 440.0 ± 35.8 | 306.0 ± 27.9 | 134.0 ± 30.5 1,2 | +57.9 |
| 6 | 2f | 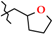 | 460.0 ± 27.4 | 219.0 ± 16.6 | 241.0 ± 43.3 1,2,3 | +24.2 |
| 7 | 2g | 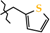 | 404.0 ± 37.1 | 288.0 ± 43.1 | 116.0 ± 20.0 1,2 | +63.5 |
| 8 | 2h | 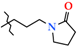 | 446.0 ± 49.9 | 306.0 ± 42.3 | 140.0 ± 45.4 1,2 | +56.0 |
| 9 | 2i | 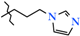 | 451.0 ± 21.1 | 344.0 ± 23.7 | 107.0 ± 32.2 1,2 | +66.4 |
| 10 | 3a | 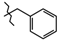 | 541.0 ± 11.9 | 339.0 ± 26.8 | 202.0 ± 48.2 1,2,3 | +36.5 |
| 11 | 3b | 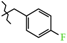 | 560.0 ± 23.5 | 274.0 ± 26.6 | 286.0 ± 40.4 1,2,3 | + 10.1 |
| 12 | 3c | 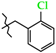 | 530.0 ± 40.2 | 224.0 ± 32.6 | 306.0 ± 36.1 1,2,3 | +3.8 |
| 13 | 3d | 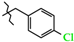 | 499.0 ± 11.3 | 195.0 ± 7.36 | 304.0 ± 13.2 | +4.4 |
| 14 | 3e | 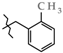 | 468.0 ± 29.6 | 380.0 ± 20.0 | 106.0 ± 28.8 | +66.7 |
| 15 | 3f | 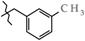 | 431.0 ± 19.4 | 183.0 ± 21.9 | 248.0 ± 11.0 1,2,3 | + 40.8 |
| 16 | 3g | 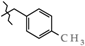 | 446.0 ± 24.0 | 352.0 ± 25.7 | 94.0 ± 18.9 1 | + 70.4 |
| 17 | 3h | 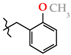 | 493.0 ± 8.56 | 366.0 ± 12.4 | 127.0 ± 7.64 1,2,3 | +60.1 |
| 18 | 3i | 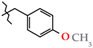 | 621.0 ± 13.9 | 590.0 ± 29.7 | 31.0 ± 15.3 1,3 | +90.3 |
| 19 | 3j | 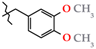 | 494.0 ± 15.6 | 259.0 ± 14.5 | 235.0 ± 49.2 1,2,3 | +26.1 |
| 20 | 3k | 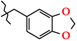 | 415.0 ± 21.4 | 212.0 ± 9.49 | 203.0 ± 40.4 1,2,3 | +36.2 |
| 21 | Lornoxicam | - | 441.0 ± 33.1 | 346.0 ± 30.2 | 95.0 ± 12.7 1 | +70.1 |
| 22 | Diclofenac | - | 538.0 ± 23.6 | 479.0 ± 32.8 | 59.0 ± 15.3 1 | +81.4 |
| 23 | Control | - | 593.0 ± 62.7 | 257.0 ± 41.4 | 318.0 ± 58.2 1 | 0 |
| Entry | Product | R | Volume of Damaged Extremity (mm3) | Volume of Non-Damaged Extremity (mm3) | Δ Volume (Volume Increase) | Anti-Inflammatory Activity Compared to Control (%) |
|---|---|---|---|---|---|---|
| 1 | 2a |  | 641.7 ± 72.9 | 321.5 ± 87.2 | 320.1 ± 69.3 1,2,3 | +22.6 |
| 2 | 2b | 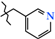 | 589.4 ± 17.3 | 339.2 ± 16.5 | 250.1 ± 13.3 1,2,3 | +39.6 |
| 3 | 2c |  | 594.9 ± 24.6 | 291.8 ± 49.6 | 303.1 ± 30.7 1,2,3 | +26.7 |
| 4 | 2d | 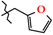 | 416.0 ± 50.5 | 330.3 ± 59.8 | 85.7 ± 6.0 1 | +79.3 |
| 5 | 2e | 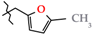 | 506.4 ± 62.4 | 397.6 ± 64.6 | 108.8 ± 6.3 1 | +73.7 |
| 6 | 2f |  | 473.7 ± 50.2 | 225.6 ± 54.2 | 248.0 ± 49.9 1,2,3 | +40.1 |
| 7 | 2g | 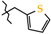 | 431.3 ± 36.7 | 379.4 ± 44.9 | 51.9 ± 10.5 1,2,3 | +87.5 |
| 8 | 2h | 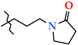 | 603.0 ± 68.6 | 425.3 ± 41.1 | 177.7 ± 37.5 1,2,3 | +57.0 |
| 9 | 2i |  | 561.7 ± 11.6 | 274.1 ± 21.0 | 287.7 ± 28.3 1,2,3 | +30.5 |
| 10 | 3a | 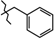 | 537.1 ± 32.5 | 281.7 ± 16.3 | 255.4 ± 43.41 1,2,3 | +38.3 |
| 11 | 3b | 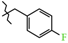 | 580.8 ± 62.7 | 297.6 ± 10.4 | 283.3 ± 53.2 1,2,3 | +31.5 |
| 12 | 3c | 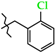 | 669.8 ± 30.2 | 292.3 ± 27.7 | 377.6 ± 28.1 | +8.7 |
| 13 | 3d | 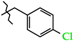 | 794.2 ± 37.1 | 393.8 ± 35.7 | 400.7 ± 41.9 | +4.2 |
| 14 | 3e | 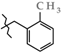 | 453.4 ± 51.3 | 279.1 ± 7.04 | 174.3 ± 50.7 1,2,3 | +57.9 |
| 15 | 3f | 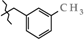 | 696.8 ± 28.6 | 314.4 ± 8.31 | 382.4 ± 31.6 | +7.6 |
| 16 | 3g | 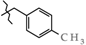 | 600.9 ± 13.6 | 326.2 ± 24.8 | 274.8 ± 27.0 1,2,3 | +33.6 |
| 17 | 3h | 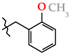 | 421.3 ± 18.5 | 271.1 ± 22.4 | 150.3 ± 26.7 1,2,3 | +63.7 |
| 18 | 3i | 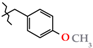 | 369.5 ± 65.4 | 296.6 ± 42.8 | 72.8 ± 24.7 1,2,3 | +82.4 |
| 19 | 3j | 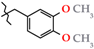 | 779.5 ± 50.2 | 369.7 ± 13.7 | 409.8 ± 54.5 | +0.9 |
| 20 | 3k | 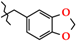 | 377.5 ± 39.2 | 306.6 ± 50.5 | 70.99 ± 16.3 | +82.8 |
| 21 | Lornoxicam | - | 360.5 ± 82.5 | 263.9 ± 60.9 | 96.6 ± 22.7 1 | +76.7 |
| 22 | Diclofenac | - | 397.6 ± 22.4 | 306.6 ± 17.6 | 91.0 ± 16.0 1 | +78.0 |
| 23 | Control | - | 768.7 ± 61.0 | 354.9 ± 22.9 | 413.7 ± 72.1 1 | 0 |
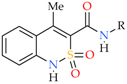
| Entry | Product | R | Conformer | Torsion Angle C(9)–N(2))–C(10))–C(11) | Torsion Angle N(2))–C(10))–C(11))–X | Torsion Angle N(2))–C(10))–C(11))–C(12) |
|---|---|---|---|---|---|---|
| 1 | 2g | 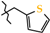 | 2g-A | −94.2(2) | +77.8(2) 1 | −107.0(2) |
| 2g-B | −94.2(2) | −94.2(2) 1 | +79.6(3) | |||
| 2 | 3e | 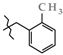 | 3e-A | +146.3(4) | +115.5(3) 2 | −64.4(3) |
| 3e-B | +171.0(3) | −73.2(3) 2 | +106.9(3) | |||
| 3 | 2e | 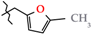 | 2e | +177.9(7) | −70.4(9) 3 | +114(1) |
| 4 | 3f |  | 3f | +152.5(2) | +122.2(3) 2 | −79.3(4) |
| 5 | 3a | 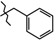 | 3a | +135.7(2) | +124.1(2) 2 | −55.8(3) |
| 6 | 2d |  | 2d-A | +111.0(3) | +171.6(2) 3 | −14.3(3) |
| 2d-B | +111.0(3) | +83.2(4) 3 | −96.0(5) | |||
| 7 | 2c | 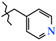 | 2c | +87.8(2) | +8.9(3) 4 | −173.1(2) |
| 8 | 3c | 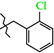 | 3c | +98.2(3) | +21.7(4) 2 | −159.8(3) |
| 9 | 3d | 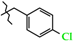 | 3d-A | −65(1) | −8(2) 2 | +172(1) |
| 3d-B | +150(1) | +128(1) 2 | −60(2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukrainets, I.V.; Hamza, G.M.; Burian, A.A.; Voloshchuk, N.I.; Malchenko, O.V.; Shishkina, S.V.; Grinevich, L.A.; Grynenko, V.V.; Sim, G. Molecular Conformations and Biological Activity of N-Hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Sci. Pharm. 2018, 86, 50. https://doi.org/10.3390/scipharm86040050
Ukrainets IV, Hamza GM, Burian AA, Voloshchuk NI, Malchenko OV, Shishkina SV, Grinevich LA, Grynenko VV, Sim G. Molecular Conformations and Biological Activity of N-Hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Scientia Pharmaceutica. 2018; 86(4):50. https://doi.org/10.3390/scipharm86040050
Chicago/Turabian StyleUkrainets, Igor V., Ganna M. Hamza, Anna A. Burian, Natali I. Voloshchuk, Oxana V. Malchenko, Svitlana V. Shishkina, Lina A. Grinevich, Vasyl V. Grynenko, and Galina Sim. 2018. "Molecular Conformations and Biological Activity of N-Hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides" Scientia Pharmaceutica 86, no. 4: 50. https://doi.org/10.3390/scipharm86040050
APA StyleUkrainets, I. V., Hamza, G. M., Burian, A. A., Voloshchuk, N. I., Malchenko, O. V., Shishkina, S. V., Grinevich, L. A., Grynenko, V. V., & Sim, G. (2018). Molecular Conformations and Biological Activity of N-Hetaryl(aryl)alkyl-4-methyl-2,2-dioxo-1H-2λ6,1-benzothiazine-3-carboxamides. Scientia Pharmaceutica, 86(4), 50. https://doi.org/10.3390/scipharm86040050





