Abstract
Eudragit® L100 is a commonly used polymer in a coating layer of modified-release drug formulation to prevent drug release in the stomach. The amount of Eudragit® L100 in the formula determines the dissolution profile of drug at its release medium. Hence, its quantification in reference product will facilitate the formulation of a bioequivalent drug product. Some analytical methods including size-exclusion chromatography (SEC) have been reported for characterization of Eudragit® L100 either as single component or its conjugate with the enzyme, but none for its quantification in drug formulation. In this work, an SEC method with charged-aerosol detection (CAD) was developed for determination of Eudragit® L100 in an enteric-coated tablet formulation using Waters Ultrahydrogel 1000 and Waters Ultrahydrogel 120 columns in series. The mobile phase was a mixture of 90:10 (v/v) 44.75 mM aqueous ammonium acetate buffer, pH 6.6 and acetonitrile pumped at a constant flow rate of 0.8 mL/min in isocratic mode. The method was validated for specificity, working range, limit of detection (LOD), limit of quantification (LOQ), accuracy and precision. The method was shown to be specific for Eudragit® L100 against the diluent (mobile phase) and placebo of a coating layer for the tablet. A good correlation coefficient (r = 0.9997) of CAD response against Eudragit® L100 concentration from 0.1–1.0 mg/mL was obtained using polynomial regression. LOD and LOQ concentrations were 0.0015 and 0.0040 mg/mL, respectively. The mean recovery of Eudragit® L100 was in the range of 88.0–91.1% at three levels of working concentration: 50%, 100% and 150%. Six replicated preparations of samples showed good precision of the peak area with % relative standard deviation (RSD) 2.7. In conclusion, the method was suitable for quantification of Eudragit® L100 in an enteric-coated tablet formulation.
1. Introduction
Modified-release formulation technology is popular nowadays due to the capability of delivering drugs at a rate dictated by the needs of the body and targeting specific sites in the body. An example of this is delayed-release dosage form, generally achieved by enteric-coating to prevent the release of drugs in the stomach [1]. For this application, several types of polymers are used in the formulation, one of which is Eudragit® L100 (Figure 1). Eudragit® L100 is an anionic copolymer of methacrylic acid and methyl methacrylate with ratio of the free carboxyl groups to the ester groups of approximately 1:1. The polymer is insoluble in acid medium, hence when used in a coating layer of drug formulation it will protect the acid-unstable drug from degradation once it reaches the stomach [2,3,4]. Moreover, the quantity of Eudragit® L100 in the coating layer determines the dissolution profile of the drug at its release medium (intestinal fluid). Therefore, determination of Eudragit® L100 in enteric-coated tablet formulation is very helpful in formulating a bioequivalent drug product.
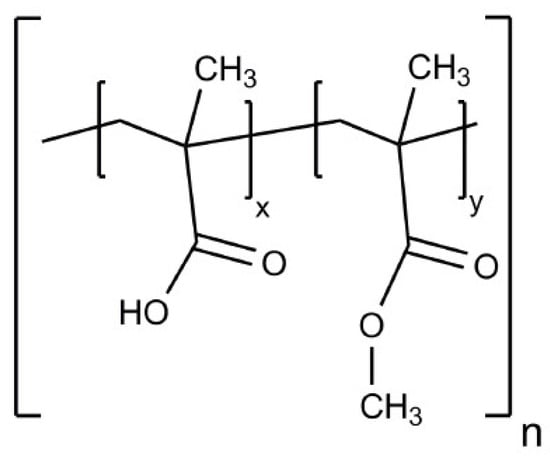
Figure 1.
Chemical structure of Eudragit® L100; x ≈ y ≈ 1 (ratio of methacrylic acid and methyl methacrylate units); n = degree of polymerization [3].
Some analytical methods for analysis of Eudragit® L100 raw material are available in common pharmacopoeias (European Pharmacopoeia, British Pharmacopoeia, United States Pharmacopoeia-National Formulary). The methods specified in the pharmacopoeias employ acid-base titration to determine the assay of methacrylic acid unit in Eudragit® L100 as well as a reversed-phase High Performance Liquid Chromatography (HPLC) method to determine limit of methacrylic acid and methyl methacrylate in Eudragit® L100 using UV detection at 202 nm [5,6,7]. However, the above-mentioned methods are only applicable for analysis of residual monomer units of Eudragit® L100 instead of the whole polymer. The analytical technique employing size-exclusion chromatography (SEC) has been reported for determination of the molar mass of Eudragit® L100 [8,9] as well as to study the properties of its conjugate with cellulase enzyme [10]. To the best of our knowledge, quantification of Eudragit® L100 in drug formulation has not yet been reported in any literature. Therefore, in this work, we develop and validate an SEC method employing charged-aerosol detection (CAD) to determine the content of Eudragit® L100 in an enteric-coated tablet formulation.
2. Materials and Methods
2.1. Chemicals, Reagents and Samples
The raw material of Eudragit® L100 was commercially obtained from Evonik Industries, Essen, Germany and used as a working standard. A local market sample of an enteric-coated tablet formulation containing Eudragit® L100 was purchased and analyzed as a representative sample. The synthetic mixture consisted of Eudragit® E100 (Evonik Industries, Essen, Germany), propylene glycol (Dow Chemical Pacific Pvt. Ltd., Singapore), and talc (Takehara Kagaku Kogyo Co. Ltd., Hiroshima, Japan). It was prepared as a placebo according to the formula of the coating layer of the tablet. Ammonium acetate GR grade, methanol and acetonitrile HPLC grade were procured from Merck, Darmstadt, Germany. Ammonium hydroxide 10% solution was prepared by diluting ammonia solution 25% GR grade obtained from Merck, Darmstadt, Germany. Water used for chromatography was prepared using Milli-Q water purification system from Merck Millipore (Molsheim, France).
2.2. Chromatographic Conditions
HPLC analysis was performed on a Waters Alliance e2695 separation module combined with Corona® CAD® Plus detector from Thermo Scientific (Germering, Germany). The chromatographic columns were Waters Ultrahydrogel 1000 (7.8 × 300 mm, 12 µm) tandem with Waters Ultrahydrogel 120 (7.8 × 300 mm, 6 µm) as a stationary phase that were kept at 40 °C. The mobile phase was a mixture of 90:10 (v/v) 44.75 mM aqueous ammonium acetate buffer, pH 6.6 and acetonitrile which had been pre-mixed before being flowed into the HPLC system. The flow rate was kept at 0.8 mL/min during the run time and the injection volume was 100 µL. The Corona® CAD® Plus detector was set at medium filter mode. Data collection and processing were performed using Empower 2 chromatographic data acquisition system (Waters Corporation, Milford, MA, USA).
2.3. Preparation Procedures
2.3.1. Preparation of Mobile Phase
An amount of 3.45 g of ammonium acetate was weighed and dissolved in 1000 mL of water and adjusted to pH 6.6 using ammonium hydroxide 10% solution. The solution was filtered through 0.45 µm cellulose nitrate membrane. The mobile phase was prepared by mixing 900 mL of the aqueous solution with 100 mL of acetonitrile. The mixture was degassed for 15 min prior to use.
2.3.2. Preparation of Placebo Solution
An amount of 9.5 mg of synthetic mixture containing Eudragit® E100, propylene glycol and talc (3:2.25:4.28, w/w/w) was diluted in 50 mL of mobile phase and sonicated for about 15 min, then centrifuged at 5000 rpm for 15 min to separate the insoluble material.
2.3.3. Preparation of Series Standard Solution for Working Range
Accurately weighed 250 mg of Eudragit® L100 was dissolved in 50 mL of methanol. Then 0.2–2.0 mL of solution was pipetted and transferred into 10 mL volumetric flask and diluted with mobile phase to make 0.1–1.0 mg/mL solution.
2.3.4. Preparation for Limit of Detection (LOD) and Limit of Quantification (LOQ)
A series of standard solution was prepared by diluting 0.1 mg/mL standard solution with mobile phase to make a 0.0010–0.0100 mg/mL solution. The limit of detection (LOD) and limit of quantification (LOQ) were established by identifying the concentration which gave signal-to-noise ratio of about 3 and 10, respectively. The precision at LOD and LOQ level was conducted by preparing six replicated standard preparations at each level.
2.3.5. Preparation of Placebo Spiked Solution for Accuracy
To each 9.5 mg of synthetic mixture was added 15 mg, 30 mg, and 45 mg of Eudragit® L100 in a 50 mL volumetric flask. The mixture was diluted with mobile phase and sonicated for 15 min, then centrifuged for 15 min at 5000 rpm. Each of the concentration levels was prepared in triplicate.
2.3.6. Preparation of Sample Solution for Precision
Six replicated preparations of the sample were performed for precision study. For each preparation, the coating layer of the enteric-coated tablet (1 tablet) was peeled off and ground with a mortar and pestle, then transferred into a 50 mL volumetric flask. The sample was diluted with methanol and centrifuged for 15 min at 5000 rpm.
3. Results and Discussion
3.1. Method Development
Eudragit® L100 was analyzed using SEC in which its retention in the column depended on the size of the molecule with respect to column pore size. Molecules with a larger size than the pore size of column are eluted rapidly, while the smaller molecules which enter the pore require a longer time to be eluted [11]. Since Eudragit® L100 is a hydrophilic polymer, Waters Ultrahydrogel columns were chosen since they are packed with hydrophilic polymethacrylate spherical beads which are suitable for analysis of water soluble polymers [12]. Eudragit® L100 has an average molecular weight of approximately 125,000 g/mol [3] which is within the exclusion limit of Waters Ultrahydrogel 1000 column (exclusion limit 1,000,000 pullulan; maximum pore size 1000 Å). The addition of an Ultrahydrogel 120 column was to obtain better peak shape of Eudragit® L100.
Since Eudragit® L100 showed poor UV response at 202 nm and there was interference from the placebo at the peak of interest (Figure 2a), other detectors were explored for the SEC analysis, namely refractive index detector (RID) and CAD. However, the peak of Eudragit® L100 was not detected in RID at the stated working concentration, hence CAD was chosen as the suitable detector for this analysis. The mobile phase was selected to accommodate both the solubility of Eudragit® L100 and CAD detector requirements. Eudragit® L100 is more soluble under basic conditions [3] due to ionization of the methacrylic acid units, but the same conditions will decrease the performance of CAD detector [13]. Therefore, ammonium acetate buffer pH 6.6 was chosen with respect to its compatibility with CAD detector as well. Acetonitrile was added in the mobile phase to increase the evaporation rate of the analyte. Methanol was chosen as the solvent in preparation of the standard stock solution and sample due to solubility of Eudragit® L100.
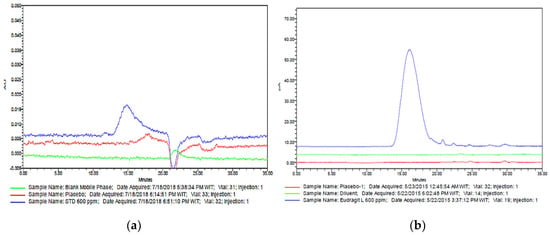
Figure 2.
Overlay chromatograms of diluent (green), synthetic mixture (red), and Eudragit® L100 0.6 mg/mL solution (blue) using (a) UV detector at 202 nm; (b) charged-aerosol detection (CAD) detector.
3.2. Method Validation
The method was validated for specificity, working range, limit of detection (LOD), limit of quantification (LOQ), accuracy and precision.
3.2.1. Specificity
Specificity is the parameter to measure the capability of a method to analyze a particular analyte of interest in the presence of other components with no interferences from the sample matrix [14]. Specificity study was demonstrated by injecting diluent (mobile phase), synthetic mixture solution, and Eudragit® L100 solution. The chromatograms in Figure 2b show that there were no interferences from diluent and synthetic mixture solution at the retention time of Eudragit® L100 which proved that the method was specific for Eudragit® L100 analysis. Eudragit® E100, propylene glycol and talc in a synthetic mixture solution did not give any responses in this method.
3.2.2. Working Range
The working range of an analytical procedure is the interval between the upper and lower concentrations of analyte in the sample for which the analytical procedure demonstrates a suitable level of precision, accuracy and direct proportional correlation between the test results and concentration (amount) of analyte in the sample [14]. The study was performed by analyzing a series concentration of Eudragit® L100 solution ranging from 0.1–1.0 mg/mL. The overlay chromatograms and calibration curve obtained by plotting area response against the concentration are presented in Figure 3a,b. The calibration curve shows a parabolic response and exhibits a quadratic function due to the CAD natural response [15]. The regression coefficient was calculated using the plot and gave a good correlation coefficient (r) of 0.9997 (r2 = 0.9995).
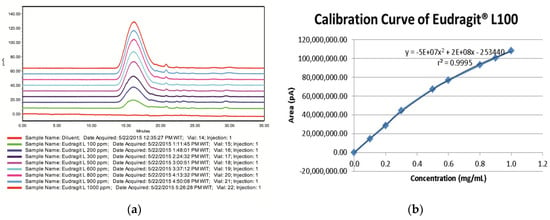
Figure 3.
(a) Overlay chromatograms and (b) calibration curve of Eudragit® L100 series concentration solutions (0.1–1.0 mg/mL).
3.2.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
The LOD of an analytical procedure is the lowest amount of analyte in sample which can be detected, but not necessarily quantitated as an exact value, while LOQ is the lowest amount which can be quantitatively determined with suitable precision and accuracy [14]. The LOD and LOQ of Eudragit® L100 were estimated by further diluting the standard to a range of low concentrations (0.0010–0.0100 mg/mL) and identifying the concentration which gave signal-to-noise ratio of about three and ten, respectively. From the signal-to-noise ratio, the LOD concentration was observed to be 0.0015 mg/mL and the LOQ concentration was 0.0040 mg/mL indicating good sensitivity of the method. Precision of six replicated preparations at individual LOD and LOQ level was performed and the relative standard deviation (%RSD) for each level was calculated (Table 1). The %RSD of peak area of Eudragit® L100 at LOD and LOQ levels were below 33 and 10, respectively.

Table 1.
Precision at LOD and LOQ levels of Eudragit® L100.
3.2.4. Accuracy
The accuracy of an analytical method is the closeness of the test results obtained by the method to the theoretical value. Accuracy may often be expressed as percent recovery by calculating the assay of known, added amounts of analyte [14]. The accuracy of Eudragit® L100 determination in the coating layer of enteric-coated tablet was determined by spiking Eudragit® L100 raw material into the synthetic mixture at three different levels of working concentration: 50% (0.3 mg/mL), 100% (0.6 mg/mL) and 150% (0.9 mg/mL), each in triplicate preparations and single injection. Percent recovery was calculated by comparing the amount of Eudragit® L100 recovered against the added amount (Table 2). The average of percent recovery at each concentration level was in the range of 88.0–91.1% which showed a fair accuracy of the polymer analysis in the sample matrix.

Table 2.
Recovery of Eudragit® L100 in placebo of coating layer of tablet.
3.2.5. Precision
The precision of an analytical procedure expresses the closeness between results obtained from a series of measurements from multiple sampling of the same homogenous sample under the prescribed condition [14]. The precision of Eudragit® L100 was evaluated by analyzing six test samples of enteric-coated tablet formulation and calculating the %RSD of the peak area of Eudragit® L100 (Table 3). The method showed good precision for peak area of the samples with %RSD 2.7.

Table 3.
Precision of Eudragit® L100 in analysis of local market samples of enteric-coated tablet.
3.3. Application of Method
As presented in Table 3, the method was applied to determine Eudragit® L100 content in local market samples of an enteric-coated tablet formulation. The result showed that Eudragit® L100 was used in the coating layer of the tablets (n = 6) with an average amount of 7.2% w/w (%RSD = 5.0) with respect to the coated-tablet weight.
4. Conclusions
A new SEC-CAD method was proposed for determination of Eudragit® L100 in an enteric-coated tablet formulation. The method was found to be relatively simple, specific, sensitive, accurate and precise. The developed method is applicable for the quantification of Eudragit® L100 in an enteric-coated tablet formulation which is useful for formulating a bioequivalent product of the same.
Author Contributions
The experiments were conceived and designed by M.W., J.G. and J.S.R.T. The experiments were performed by M.W. and J.G. The experimental data were analyzed and interpreted by M.W., J.G. and J.S.R.T. All the materials, reagents and analysis tools were provided by R.R.T. The manuscript was written by M.W. and J.G.
Acknowledgments
The authors acknowledge the management of Dexa Medica for the support in materials, reagents and instruments used for analysis. The authors would also like to thank the colleagues of the Analytical Development team of Dexa Development Center for their support and co-operation during this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagaraju, R.; Swapna, Y.; Babu, R.H.; Kaza, R. Design and evaluation of delayed and extended release tablets of mesalamine. J. Pharm. Sci. Technol. 2010, 2, 103–110. [Google Scholar]
- Skalsky, B. (Ed.) Enteric & GI targeting formulations. In Eudragit® Application Guidelines, 12th ed.; Evonik Industries AG: Darmstadt, Germany, 2012; Chapter 5; pp. 1–3. [Google Scholar]
- Technical Information of Eudragit® L100 and Eudragit® S100. Available online: www.pharosproject.net/uploads/files/cml/1389279051.pdf (accessed on 17 March 2015).
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009; pp. 525–533. ISBN 978 0 85369 792 3. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 9th ed.; EDQM: Strasbourg CEDEX, France, 2016; Volume 3, p. 3018. ISBN 978-92-871-8127-5. [Google Scholar]
- British Pharmacopoeia Commission. British Pharmacopoeia 2017; The Stationery Office: London, UK, 2016; Volume 2, pp. 240–241. ISBN 978 011 3230 204. [Google Scholar]
- United States Pharmacopeial Convention Inc. USP 41–NF 36 The United States Pharmacopoeia and National Formulary 2018; United Book Press Inc.: Baltimore, MD, USA, 2017; pp. 5444–5445. ISBN 978-3-7692-7022-8. [Google Scholar]
- Adler, M.; Pasch, H.; Meier, C.; Senger, R.; Koban, H.-G.; Augenstein, M.; Reinhold, G. Molar mass characterization of hydrophilic copolymers, 2 Size exclusion chromatography of cationic (meth)acrylate copolymers. e-Polymers 2005, 57, 1–11. [Google Scholar] [CrossRef]
- Porsch, B.; Hillang, I.; Karlsson, A.; Sundelöf, L.-O. Ion-exclusion controlled size-exclusion chromatography of methacrylic acid-methyl methacrylate copolymers. J. Chromatogr. A 2000, 872, 91–99. [Google Scholar] [CrossRef]
- Dourado, F.; Bastos, M.; Mota, M.; Gama, F.M. Studies on the properties of Celluclast/Eudragit L-100 conjugate. J. Biotechnol. 2002, 99, 121–131. [Google Scholar] [CrossRef]
- Mori, S.; Barth, H.G. Size Exclusion Chromatography, 1st ed.; Springer: Heidelberg, Germany, 1999; pp. 3–28. ISBN 3-540-65635-9. [Google Scholar]
- Ultrahydrogel Column Care and Use Manual. Available online: www.waters.com/webassets/cms/support/docs/wat011556.pdf (accessed on 16 January 2017).
- Operating and Maintenance Manual of Corona® CAD® Detector and Corona®Plus CAD® Detector. Available online: www.vtpup.cz/common/manual/Extern_RADANAL_ESA_CoronaCAD_manual_EN.pdf (accessed on 8 May 2017).
- ICH Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Available online: www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002662.pdf (accessed on 20 June 2017).
- Charged Aerosol Detection and Evaporative Light Scattering Detection—Fundamental Differences Affecting Analytical Performance. Available online: http://apps.thermoscientific.com/media/cmd/ hypersite-events/HPLC-2014/posters/PN70990_HPLC_2014-CAD-vs-ELSD.pdf (accessed on 25 May 2015).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).