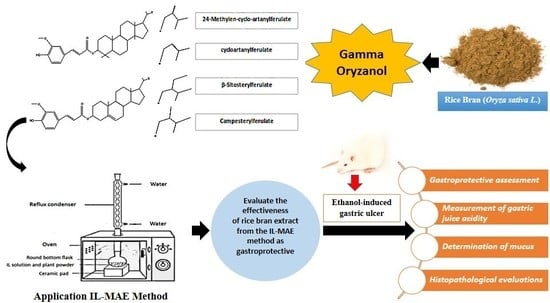
Potential Gastroprotective Activity of Rice Bran (Oryza sativa L.) Extracted by Ionic Liquid-Microwave-Assisted Extraction against Ethanol-Induced Acute Gastric Ulcers in Rat Model
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Extraction with the Ionic Liquid-Microwaved Assisted Extraction (IL-MAE) Method
2.3. High-Performance Liquid Chromatography (HPLC) Analysis
2.4. Pharmacological Study
2.4.1. Gastroprotective Assessment
2.4.2. Measurement of Gastric Juice Acidity
2.4.3. Determination of Mucus
2.5. Histopathological Evaluations
- (1)
- Mild (+): a layer of thick keratin coating the surface of the mucosa of the stomach.
- (2)
- Moderate (++): the accumulation of edema fluid under the keratin layer, hemorrhage, edema of the submucosa accompanied by infiltration of inflammatory cells.
- (3)
- Heavy (+++): discontinuity layer of keratin, atrophy of the coats of epithelium, congestion, hemorrhage, edema of the submucosa and infiltration of inflammatory cells, dominated by neutrophils [18].
- (1)
- Mild (+): 25% of the blood vessels experiencing congestion.
- (2)
- Moderate (++): 50% of blood vessels experiencing congestion.
- (3)
- Heavy (+++): 75% or more of blood vessels experiencing congestion [18].
2.6. Statistical Analysis
3. Results and Discussion
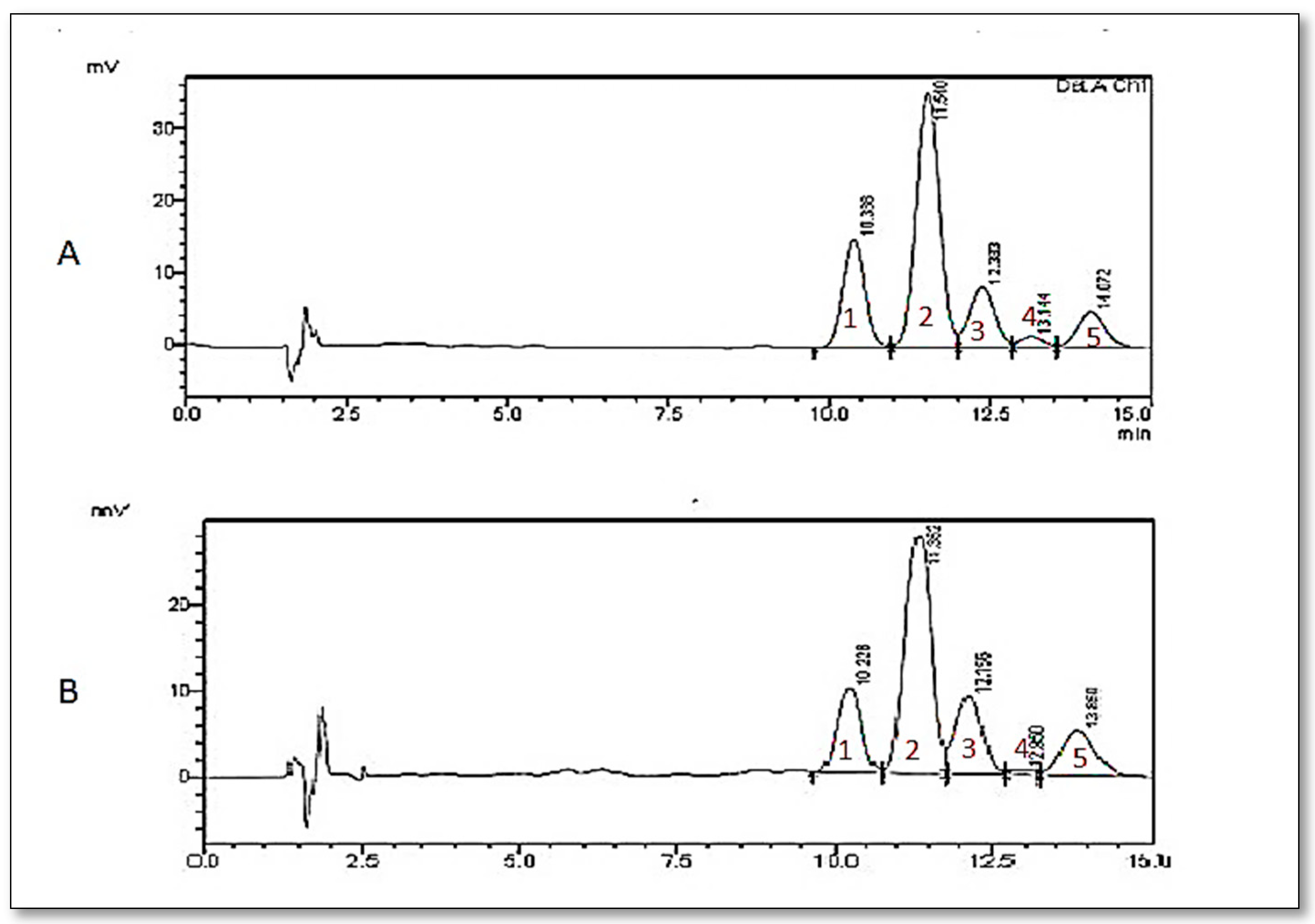
3.1. Analysis of Gamma-Oryzanol in the Extract
3.2. In Vivo Studies
3.2.1. Effect of Rice Bran Extract on Gastric Lesions Induced by Ethanol
3.2.2. Effect of Rice Bran Extract on Gastric Acidity
3.2.3. Effect of Rice Bran Extract on Mucus in Gastric Content
3.2.4. Histopathological Evaluations of Gastric Lesions
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zullaikah, S.; Melwita, E.; Jui, Y.H. Isolation of oryzanol from crude rice bran oil. Bioresour. Technol. 2009, 100, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Naik, S.N. Gamma Oryzanol from Rice Bran Oil-A Review. J. Sci. Ind. Res. 2004, 63, 569–578. [Google Scholar]
- Makynen, K.; Chitchumroonchokchai, C.; Adisakwattana, S.; Faillla, M.L.; Tipayanate, A. Effect of gamma-oryzanol on the bioaccessibility and synthesis of cholesterol. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 49–56. [Google Scholar] [PubMed]
- Bergman, C.J.; Xu, Z. Genotype and Environment Effects on Tocopherol, Tocotrienol, and gamma oryzanol Contents of Southern U.S. Cereal Chem. 2003, 80, 446–449. [Google Scholar] [CrossRef]
- Espino, M.; Fernandez, M.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. Trends Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Trinovita, E.; Sigalingging, E.; Saputri, F.C.; Mun’im, A. Optimization of ionic liquid 1-Butyl-3-Methylimidazolium Hexafluorophosphate ([Bmim]PF6)-based microwave assisted extraction method for gamma oryzanol from rice bran (Oryza sativa L.). J. Appl. Pharm. Sci. 2017, 7. [Google Scholar] [CrossRef]
- Islam, S.; Nagasaka, R.; Ohara, K.; Hosoya, T.; Ozaki, H.; Ushio, H.; Hori, M. Biological Abilities of Rice Bran-Derived Antioxidant Phytochemicals for Medical Therapy. Curr. Top. Med. Chem. 2011, 11, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Cossu, M.; Alamanni, M.C.; Piu, L. Antioxidant Activity of Gamma-Oryzanol: Mechanism of action dan its effect on oxidative stability of pharmaceutical oils. Int. J. Pharm. 2005, 299, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Kanter, M.; Demir, H.; Karakaya, C.; Ozbek, H. Gastroprotective activity of Nigella sativa L. oil and its constituent, thymoquinone against acute alcohol-induced gastric mucosal injury in rats. World J. Gastroenterol. 2005, 11, 6662–6666. [Google Scholar] [CrossRef] [PubMed]
- Minatel, I.O.; Francisqueti, F.V.; Correa, C.R.; Pace, G.; Lima, P. Antioxidant Activity of γ-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef] [PubMed]
- Price, S.A.; Wilson, L.M. Patofisiologi: Konsep Klinis Proses-Proses Penyakit; Terj dari Pathophysiology: Clinical Consepts of Disease Processes, oleh Pendit Bu, Hartanto, Ed 6. 1.; EGC: Jakarta, Indonesia, 2005; pp. 377–383. [Google Scholar]
- Sowndhararajan, K.; Paul, S.; Kwon, G.S.; Hwang, C.W.; Kang, S.C. Protective effect of polyamine extract of salt-stressed and sprouted soybean seeds against ethanol-induced gastric ulcer in rats. Biotechnology 2014, 23, 711–716. [Google Scholar] [CrossRef]
- Song, D.U.; Jang, M.S.; Kim, H.W.; Yoon, H.J.; Chay, K.O.; Joo, Y.E. Gastroprotective Effects of Glutinous Rice Extract against Ethanol, Indomethacin and Stress-induced Ulcers in Rats. Chonnam Med. J. 2014, 50, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Saputri, F.C.; Mun’im, A.; Sari, S.P. Gastroprotective Activity of Combination of Turmeric (Curcuma domestica Linn.) Rhizome Extracts and Neem (Azadirachta indica A. Juss) Bark. Jurnal Bahan Alam Indonesia 2009, 7, 44–46. [Google Scholar]
- Suzuki, Y.; Hayashi, M.; Ito, M.; Yamagami, I. Antiulcer effects of 40-(2-carboxyethyl) phenyl trans-4-aminomethyl cyclohexane carboxylate hydrochloride (Cetraxate) on various experimental gastric ulcers in rats. Jpn. J. Pharmacol. 1976, 26, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Raji, Y.; Oguwande, I.A.; Osadebe, C.A.; John, G. Effect of Azadirachta indica Extract on Gastric Ulceration and Acid Secretion in Rats. J. Ethnopharmacol. 2004, 90, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Klein-Junior, L.C.; Mota da Silva, L.; Boeing, T.; Bordignon, S.L.; Beber, A.P.; Rocha, A.R.J.; Henriques, T.A.; Andrade, F.S.; Filho, C.V. The Protective Potential of Phyllanthus niruri and Corilagin on Gastric Lesions Induced in Rodents by Different Harmful Agent. Planta Med. 2017, 83, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J. Peptic Ulcer Disease and Its Complication. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. In Pathophysiology/Diagnosis/Management, 7th ed.; Feldman, M., Friedman, L.S., Sleisenger, M.H., Eds.; Saunders: Philadelphia, PA, USA, 2002; p. 715. [Google Scholar]
- Xu, J.; Wang, W.; Liang, H.; Zhang, Q.; Li, Q. Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind. Crops Prod. 2015, 276, 487–493. [Google Scholar] [CrossRef]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction dan analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.J.L.; Martínez, J.M.H.; Alfonso, E.F.S.; Mendonca, C.R.B.; Ramos, G.R. Composition, Industrial processing, and Applications of Rice Bran γ-Oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Katary, M.A.; Salahuddin, A. Gastroprotective Effect of Punicalagin against Ethanol-Induced Gastric Ulcer:The Possible Underlying Mechanisms. Biomarkers 2017, 3, 1–8. [Google Scholar]
- Abdulla, M.A.; Ahmed, K.A.; Al-bayaty, F.H.; Masood, Y. Gastroprotective effect of Phyllanthus niruri leaf extract against ethanol-induced gastric mucosal injury in rats. Afr. J. Pharm. Pharmacol. 2010, 4, 226–230. [Google Scholar]
- Xu, Z.; Hua, N.; Godber, J.S. Antioxidant Activity of Tocopherols, Tocotrienols, and Gamma Oryzanol Components from Rice Bran Against Cholesterol Oxidation Accelerated by 2,2’-Azobis (2-methylpropionamidine) dihydrochloride. J. Agric. Food Chem. 2001, 49, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.G.; Portela, T.Y.; Di Stasi, L.C. Antiulcerogenic and analgesic effects of Maytenus aquifolium, Sorocea bomplandii and Zolernia ilicifolia. J. Ethnopharmacol. 2001, 77, 41–47. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, B.; Lv, L.; Wang, Z.; He, J.; Chen, Z.; Wen, X.; Zhang, Y.; Sun, W.; Li, Y.; et al. Gastroprotective effect of aucubin against ethanol-induced gastric mucosal injury in mice. Life Sci. 2017, 189, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Boligon, A.A.; Freitas, R.B.D.; Bruma, T.F.D.; Waczukc, E.F.; Klimaczewskic, C.V.; Avilac, D.S.D.; Athaydea, M.L.; Bauermann, L.D.F. Antiulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm. Sin. B 2014, 4, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Roschek, B.; Fink, R.C.; Li, D.; McMichael, M.; Tower, C.M.; Smith, R.D.; Alberte, R.S. Pro-Inflammatory Enzymes, Cyclooxygenase 1, Cyclooxygenase 2, and 5-Lipooxygenase, Inhibited by Stabilized Rice Bran Extracts. J. Med. Food 2009, 12, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.A.; Balan, T.; Azemi, A.K.; Omar, M.H.; Mohtarrudin, N.; Ahmad, Z.; Abdullah, M.N.H.; Nasir, M.; Desa, M.; Teh, L.K.; et al. Mechanism(s) of action underlying the gastroprotective effect of ethyl acetate fraction obtained from the crude methanolic leaves extract of Muntingia calabura. BMC Complement. Altern. Med. 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Tsutsumi, S.; Tomisato, W.; Hwang, H.J.; Tsuchiya, T.; Mizushima, T. Prostaglandin E2 Protects Gastric Mucosal Cells from Apoptosis via EP2 and EP4 Receptor Activation. J. Biol. Chem. 2003, 278, 12752–12758. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Chin, N.L. Antioxidant and Anti-ulcer Effects of Ethyl Acetate Fraction of Merremia Tridentata (L.) Hallier F. Root. Agric. Agric. Sci. Procedia 2014, 2, 406–414. [Google Scholar] [CrossRef]
- Vakil, N.; Fennerty, M.B. Systematic review: Direct comparative trials of the efficacy of proton pump inhibitors in the management of gastro-oesophageal reflux disease and peptic ulcer disease. Aliment. Pharmacol. Ther. 2003, 18, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Srikanta, B.M.; Siddaraju, M.N.; Dharmesh, S.M. A novel phenol-bound pectic polysaccharide from Decalepis hamiltonii with multi-step ulcer preventive activity. World J. Gastroenterol. 2007, 13, 5196–5207. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Murata, T.; Fujisawa, M.; Nagasaka, R.; Ushio, H.; Bari, A.M.; Hori, M.; Ozaki, H. Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol. 2008, 154, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Miura, S.; Uehara, K.; Mizumori, M.; Kishikawa, H.; Hokari, R.; Higuchi, H.; Adachi, M.; Nakamizo, H.; Ishii, H. Nuclear Factor-κB and TNF-α Mediate Gastric Ulceration Induced by Phorbol Myristate Acetate. Dig. Dis. Sci. 2002, 47, 2070–2078. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, R.; Chotimarkorn, C.; Shafiqul, I.; Hori, M.; Ozaki, H.; Ushio, H. Anti-inflammatory effects of hydroxycinnamic acid derivatives. Biochem. Biophys. Res. Commun. 2007, 358, 615–619. [Google Scholar] [CrossRef] [PubMed]

 = Epithelial erosion of mucosal layers.
= Epithelial erosion of mucosal layers.
 = Epithelial erosion of mucosal layers.
= Epithelial erosion of mucosal layers.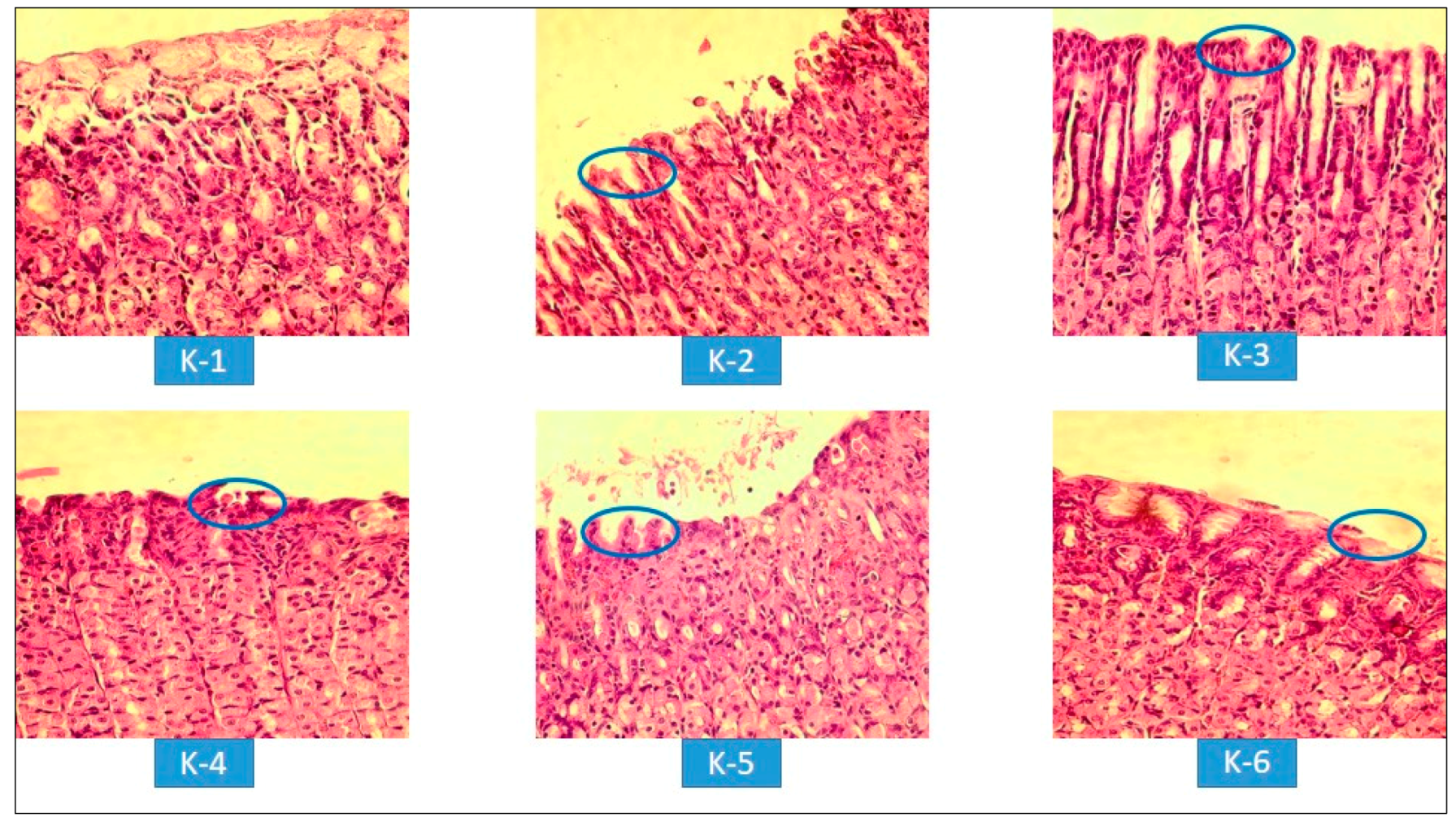
 Infiltration of inflammatory cells (neutrophils);
Infiltration of inflammatory cells (neutrophils);  = Congestion.
= Congestion.
 Infiltration of inflammatory cells (neutrophils);
Infiltration of inflammatory cells (neutrophils);  = Congestion.
= Congestion.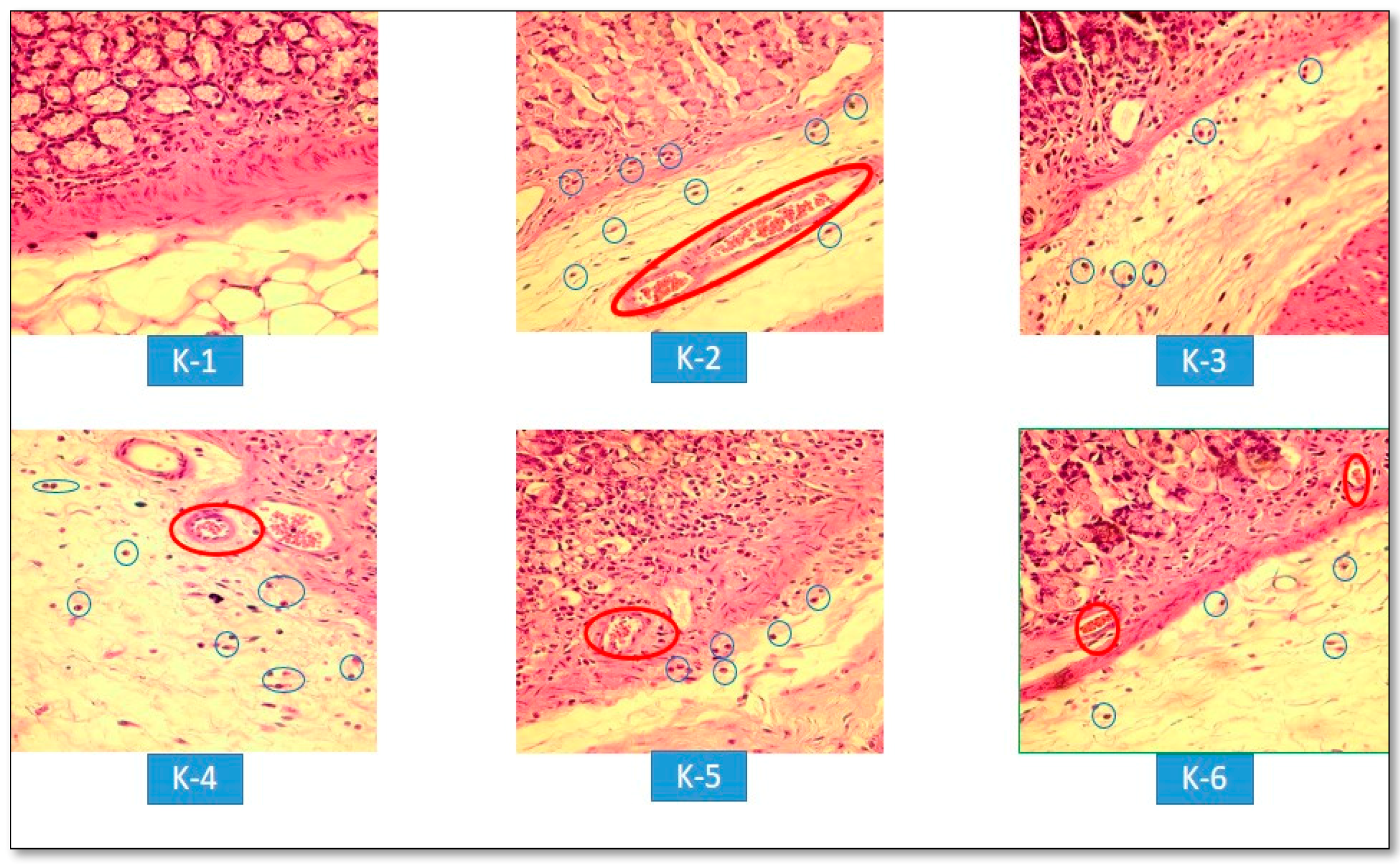
| Treatment Group | Dosage | Diameter of Ulcer (mm) | Ulcer Index | Inhibition of Ulceration (%) |
|---|---|---|---|---|
| Normal (pre-treated) | - | - | 0 | 100 |
| Negative control (CMC) | 5 mL/kg BW | 3.45 ± 0.70 | 4 | - |
| Positive control (OMZ) | 36 mg/kg BW | 0.47 ± 0.45 * | 0.67 | 83.25 |
| Rice Bran Extract | 100 mg/kg BW | 1.81 ± 0.55 * | 2.67 | 33.25 |
| 200 mg/kg BW | 1.47 ± 0.39 * | 2 | 50 | |
| 400 mg/kg BW | 0.83 ± 0.17 * | 1.33 | 66.75 |
| Treatment Group | Dosage | Gastric Acidity (µek/200 g) |
|---|---|---|
| Normal (pre-treated) | - | 1.19 ± 0.57 * |
| Negative control (CMC) | 5 ml/kg BW | 3.94 ± 0.10 # |
| Positive control (OMZ) | 36 mg/kg BW | 1.21 ± 0.19 * |
| Rice Bran Extract | 100 mg/kg BW | 1.58 ± 0.38 * |
| 200 mg/kg BW | 1.45 ± 0.33 * | |
| 400 mg/kg BW | 1.34 ± 0.04 * |
| Treatment Group | Dosage | Determination of Mucus (Absorbance/g) |
|---|---|---|
| Normal (pre-treated) | - | 0.44 ± 0.04 * |
| Negative control (CMC) | 5 mL/kg BW | 0.20 ± 0.01 # |
| Positive control (OMZ) | 36 mg/kg BW | 0.40 ± 0.07 * |
| Rice Bran Extract | 100 mg/kg BW | 0.22 ± 0.03 # |
| 200 mg/kg BW | 0.28 ± 0.02 # | |
| 400 mg/kg BW | 0.36 ± 0.02 * |
| Group | Dosage | Degree of Epithelial Cell Erosion Severity on the Mucosal Surface of the Stomach |
|---|---|---|
| Normal (pre-treated) | - | - |
| Negative control (CMC) | 5 mL/kg BW | +++ |
| Positive control (OMZ) | 36 mg/kg BW | ++ |
| Rice Bran Extract | 100 mg/kg BW | ++ |
| 200 mg/kg BW | + | |
| 400 mg/kg BW | + |
| Group | Dosage | Severity of Congestion |
|---|---|---|
| Normal (pre-treated) | - | - |
| Negative control (CMC) | 5 mL/kg BW | +++ |
| Positive control (OMZ) | 36 mg/kg BW | + |
| Rice Bran Extract | 100 mg/kg BW | + |
| 200 mg/kg BW | + | |
| 400 mg/kg BW | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinovita, E.; Chany Saputri, F.; Mun’im, A. Potential Gastroprotective Activity of Rice Bran (Oryza sativa L.) Extracted by Ionic Liquid-Microwave-Assisted Extraction against Ethanol-Induced Acute Gastric Ulcers in Rat Model. Sci. Pharm. 2018, 86, 35. https://doi.org/10.3390/scipharm86030035
Trinovita E, Chany Saputri F, Mun’im A. Potential Gastroprotective Activity of Rice Bran (Oryza sativa L.) Extracted by Ionic Liquid-Microwave-Assisted Extraction against Ethanol-Induced Acute Gastric Ulcers in Rat Model. Scientia Pharmaceutica. 2018; 86(3):35. https://doi.org/10.3390/scipharm86030035
Chicago/Turabian StyleTrinovita, Elsa, Fadlina Chany Saputri, and Abdul Mun’im. 2018. "Potential Gastroprotective Activity of Rice Bran (Oryza sativa L.) Extracted by Ionic Liquid-Microwave-Assisted Extraction against Ethanol-Induced Acute Gastric Ulcers in Rat Model" Scientia Pharmaceutica 86, no. 3: 35. https://doi.org/10.3390/scipharm86030035
APA StyleTrinovita, E., Chany Saputri, F., & Mun’im, A. (2018). Potential Gastroprotective Activity of Rice Bran (Oryza sativa L.) Extracted by Ionic Liquid-Microwave-Assisted Extraction against Ethanol-Induced Acute Gastric Ulcers in Rat Model. Scientia Pharmaceutica, 86(3), 35. https://doi.org/10.3390/scipharm86030035