Abstract
This study aimed to encapsulate Celastrus paniculatus seed oil (CPSO) in 2-hydroxypropyl-β-cyclodextrin (HPβCD) cavities and investigate their biological activity, physicochemical stability, and skin penetration by vertical Franz diffusion cells of the CPSO-HPβCD inclusion complex formulations. For biological activity studies—including 2,2-diphenyl-1-picryhydrazyl radical (DPPH) scavenging, metal ion chelating, and inhibition of lipid and tyrosinase inhibition activities—the CPSO-HPβCD inclusion complex exhibited lower inhibition activity than free CPSO. CPSO-HPβCD dispersion, serum, and gel formulations were prepared. All formulations containing the CPSO-HPβCD inclusion complex showed no significant changes in physical characteristics after three months’ storage. The percentages of oleic acid remaining in all formulations were over 80% of the initial amount during a three-month stability study. For the skin-penetration study, compared to other formulations, the CPSO-HPβCD serum formulation exhibited the highest cumulative amount of oleic acid in the whole skin and flux through receptor fluid, after six hours, of 32.75 ± 1.25 µg/cm2 and 1.02 ± 0.15 µg/cm2/h, respectively. The CPSO-HPβCD serum formulation also showed the proper viscosity. Hence, the CPSO-HPβCD inclusion complex will be beneficial for the further development of cosmeceutical products.
1. Introduction
Celastrus paniculatus is a member of the Celastraceae family, which is a large woody climber (called a climbing shrub) with a yellow corky bark [1]. It is a native of the Indian continent but is known to grow wildly in Indonesia, Laos, Malaysia, Myanmar, and Thailand, as well as numerous Pacific islands [2]. The plants exhibit varying degrees of therapeutic values, some of which include its use in the treatment of cognitive dysfunction, epilepsy, insomnia, rheumatism, gout, and dyspepsia [3]. The oil from its seeds, which are the most commonly used plant part, is rich in oleic acid (54.42%), which is the main fatty acid in the oil, together with palmitic acid (20.0%), linoleic acid (15.51%), and stearic acid (4.18%) [4]. In Northern Thailand, Celastrus paniculatus seed oil (CPSO) is used for massages with great benefits, for like muscle pain, paralysis, and joint stiffness from arthritis. Moreover, fatty acids, and especially oleic acid, have been reported for their antioxidant and anti-inflammatory activities [5,6], which may benefit topical skin applications. Our previous study reported that CPSO exhibits potent tyrosinase inhibition activity over the standard kojic acid, ascorbic acid, and arbutin [7]. However, the oil is oxidized during storage and processing, which affects its quality, stability, and safety [8]. Moreover, CPSO leaves a yellow-orange residue on the skin after application that cannot be removed by soap and water. CPSO, as the oily formulation, may not also be suitable for the delivery of CPSO active components into the skin.
Cyclodextrins are ring molecules with a remarkable ability to increase solubility and stabilize substances by including guest molecules within internal cavities [9,10]. Some derivatives, such as 2-hydroxypropyl-β-cyclodextrin (HPβCD), possess improved toxicological profiles compared to the parent substance [11]. Since the temporary encapsulation of certain active substances allows HPβCD to have controlled release properties [12,13], the aqueous solubility of poor water-soluble compounds can be increased by cyclodextrin inclusion complexes with the functional groups. Thus, the objective of this work was to improve the stability and skin penetration of oleic acid the bioactive compound in CPSO via an inclusion complex using HPβCD.
2. Materials and Methods
2.1. Materials
2-hydroxypropyl-β-cyclodextrin (HPβCD), ethylenediaminetetraacetic acid (EDTA), 2,2-diphenyl-1-picryhydrazyl radical (DPPH), vitamin C, kojic acid, ferric chloride, and ferrozine were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Oleic acid was procured from Wako Pure Chemical Industrial Ltd. (Osaka, Japan). l-tyrosine and tyrosinase (4187 U/mg) were obtained from Fluka (Buchs, Switzerland). All other chemical substances were of analytical grade.
2.2. Preparation of the C. paniculatus Seed Oil
The C. paniculatus seeds were gathered from the Samoeng district, Chiang Mai, Thailand, from January to June 2013. Voucher specimens No. HRDI 58 C0023-22 were deposited at Highland Research and Development Institute, Thailand. The dried seeds were cold-pressed using a Thai traditional cold-press machine. The yellowish-orange oil was obtained with a percentage yield of 26.09% w/w.
2.3. Determination of Oleic Acid Contents by HPLC
Oleic acid was selected since it contains the highest CPSO fatty acid profile. The oleic acid content in CPSO was determined by high performance liquid chromatography (HPLC) (Luna® C18 10 m 250 mm × 4.60 mm Column (Phenomenex, Torrance, CA, USA), LC1200UV/VIS detector and LC1100HPLC pump) using the mobile phase of 95% (v/v) acetonitrile mixed with 5% (v/v) of 0.1% (v/v) glacial acetic acid, an injection volume at 20 µL, a flow rate of 1 mL/min, and the ultraviolet (UV) detector at 205 nm [7]. The oleic acid content was determined from the HPLC chromatogram in comparing the standard oleic acid with the oleic standard curve equation: y = 4 × 10−5x − 0.1649, R2 = 0.99996.
2.4. Encapsulation of C. paniculatus Seed Oil in HPβCD
The co-evaporation method was used, with some modifications [14]. Briefly, HPβCD was dissolved with ethanol (95%) and mixed with CPSO at the weight ratio (CPSO:HPβCD) of 1:1, 1:2, 1:3, 1:4, and 1:5. Ethanol was eliminated by a rotary evaporator. The percentage yields of each formulation were calculated on a dry-weight basis.
2.5. Physicochemical Characteristics of Encapsulated HPβCD
2.5.1. Determination of Maximum Encapsulation
The concentrations of encapsulation were increased from 16.67 to 50% w/w at the weight ratio (CPSO:HPβCD) of 1:5, 1:4, 1:3, 1:2, and 1:1, respectively. The highest CPSO encapsulation was the target to involve the highest bioactive compounds in the inclusion complex. The maximum encapsulation was indicated from the maximum concentration, which produced no oil leakage on paper for 12 hours at 25 ± 2 °C. The inclusion ratio was calculated using Equation (1), whereas the total recovery was calculated using Equation (2) [15].
The CPSO content in Equation (1) was calculated from the HPLC analysis of biomarker oleic acid in CPSO, which could calculate the amount of CPSO in the inclusion complex.
2.5.2. Morphology and Particle Size of Inclusion Complex
The morphology of the HPβCD powder and the inclusion complex in the solid state were determined using a scanning electron microscope (SEM; JSM5410-LV, JEOL, Tokyo, Japan) at 200× and 100× magnification, with JEOL SemAfore software (Tokyo, Japan). The diameters of the empty and CPSO-loaded inclusion complexes in the form of solutions were determined using dynamic light scattering (DLS), Zetasizer 300HSA (Malvern Instruments, Malvern, UK), based on photon correlation spectroscopy. The analysis (n = 5) was carried out for 100 s at room temperature (27 ± 2 °C). All samples were diluted 30 times with freshly filtrated Millipore water (Millipore Corp., Bedfore, MA, USA) for particle measurement.
2.6. Biological Studies of the CPSO-HPβCD Inclusion Complex
The biological activities of the five serial concentrations of inclusion complex were investigated by comparing with non-encapsulated CPSO and standard reagents using the following methods: DPPH radical scavenging assay [16], lipid peroxidation inhibition activity [17], metal ion chelating [18], and tyrosinase inhibition assay [19]. Further, the sample concentrations providing 50% of each activity (IC50) were calculated.
2.7. Preparation of Formulations
The dispersion of the inclusion complex (CPSO-HPβCD dispersion) was prepared by incorporating 3% w/w of the CPSO-HPβCD inclusion complex into propylene glycol. Briefly, 1% w/w of Carbopol® 941 (Lubrizol, Wickliffe, OH, USA) was dispersed in water with gentle stirring to prepare the stock solution of Carbopol gel. The serum base was prepared using 65% w/w of propylene glycol and 35% w/w of the stock solution of Carbopol® gel. The CPSO-HPβCD inclusion complex (3% w/w) was incorporated into the serum base (CPSO-HPβCD serum) and gel (CPSO-HPβCD gel) base.
2.8. Stability of CPSO and CPSO-HPβCD Inclusion Complex Formulations
All inclusion-complex formulations, CPSO, and oleic acid dispersions in propylene glycol were kept in glass vials with aluminum caps and stored at 4 ± 2, 25 ± 2, and 45 ± 2 °C (65 ± 5% relative humidity) for three months. At the baseline and one, two, and three months, the samples were withdrawn and assayed for physical (separation layer, color change, and pH) and chemical stability by HPLC using oleic acid as a marker.
2.9. In Vitro Skin Permeation Study
2.9.1. Skin Sample
The abdominal skin of male rats (Sprague-Dawley strain, 150–200 g) was shaved with an electric clipper. The rats were sacrificed, and the abdominal skin was excised. The excess subcutaneous fat was carefully removed. The protocol was approved by the ethical committee of the Faculty of Medicine, Chiang Mai University in Thailand (protocol number: 20/2553).
2.9.2. CPSO Sample Preparation
All formulations (CPSO-HPβCD dispersion, serum, and gel) contained 3% w/w of the inclusion complex with a CPSO:HPβCD ratio of 1:3. Thus, the final concentration of CPSO was 0.75% w/w. Free CPSO in propylene glycol (CPSO oily solution) at 0.75% w/w was used as a control of the permeation study.
2.9.3. Skin-Permeation Study
Skin-permeation studies of oleic acid from various formulations were introduced using vertical Franz diffusion cells [20] having an area between the donor and the receiver chamber of 2.46 cm2 and a volume of the receptor fluid of 13 mL. The receptor fluid contained phosphate-buffered saline (PBS, pH 7.4), which was constantly stirred at 100 rpm with a small magnetic bar and temperature was controlled at 32 ± 2 °C throughout the experiment. The skin was mounted with the stratum corneum side facing upward to the donor compartment, and the subcutaneous side was in contact with the medium. The sample (1 µL) was placed directly onto the skin and covered the donor compartment with paraffin film (n = 6). The cells were stopped at one, two, four, and six hours. Samples in the receptor fluid were analyzed by HPLC as described before. The skin was cut into small pieces; the oleic acid in the skin was extracted by absolute ethanol under sonication for 10 minutes in an ice bath and filtered. The filtrate was assayed for oleic content by HPLC. Cumulative amounts of oleic acid in the whole skin (WS) and receptor fluid (RF) were calculated.
2.10. Statistical Analysis
The results are expressed as mean ± standard deviation (SD). A Kruskal-Wallis test was used to evaluate the significance of differences at a p-value > 0.05.
3. Results and Discussion
3.1. Physical Characteristics of C. paniculatus Seed Oil (CPSO)
CPSO has a woody, earthy smell, and is yellowish orange in appearance. The HPLC analysis showed that the CPSO in this study was mainly composed of cis-9-oleic acid (43.85% w/w). Other compounds were also found in the oil, such as palmitic acid (24.12%), stearic acid (3.01%), and myristic acid (0.48%). The active constituents of CPSO have also been reported in several studies, and it is composed of several fatty acids [21,22,23].
3.2. Physical Characteristics of the CPSO-HPβCD Inclusion Complex
The inclusion ratio and total recovery of the CPSO-HPβCD inclusion complex at various weight ratios are shown in Table 1. When the encapsulated concentration of CPSO was more than 25% w/w of CPSO, oil spots on the surface of the paper were observed after standing for 12 hours. The suitable encapsulation of CPSO in HPβCD cavities occurred at the weight ratio (CPSO: HPβCD) of 1:3 or 25% w/w of CPSO. The total recovery was 87.5 ± 3.4%. Driving forces between HPβCD and the bioactive compounds in CPSO, such as fatty acids, have been proposed to explain the complex formation energy, including hydrogen bonds, van der Waals forces, hydrophobic interactions, and the release of high-energy water molecules from the cavity [24]. A previous study reported that hydrogen bonds and hydrophobic interactions were detected between a thyme essential oil constituent and β-cyclodextrin using IR and proton nuclear magnetic resonance (1H-NMR) spectroscopy [25,26]. Moreover, the main driving force of complex formation is the release of enthalpy-rich water molecules from the hydrophobic cavity of HPβCD [27]. In the encapsulation process, water molecules are displaced by more lipophilic guest molecules present in the solution, resulting in a nonpolar–nonpolar association and a decrease of the cyclodextrin ring strain; this results in a more stable, lower-energy state [28].

Table 1.
The inclusion ratio and total recovery of CPSO-HPβCD inclusion complex at various weight ratios.
Representative SEM images of HPβCD powder, blank HPβCD, which processed the inclusion procedure and CPSO-HPβCD inclusion complex, are illustrated in Figure 1. The HPβCD powder before the inclusion procedure had a round porous shape with an average size of about 70 ± 24 µm. The blank HPβCD had a matte surface, whereas the CPSO-HPβCD inclusion complex in solid state showed irregular-shaped crystals with a glossy smooth surface without CPSO excess on their surface at a size of about 554 ± 78 µm. The CPSO-HPβCD inclusion complex appeared as the mean particle sizes in the solution form of HPβCD powder, blank HPβCD, and the CPSO-HPβCD inclusion-complex solutions measured by DLS were 103.30 ± 11.74, 112.71 ± 6.97, and 240.95 ± 13.38 nm with the pdI of 0.32, 0.28, and 0.39, respectively.
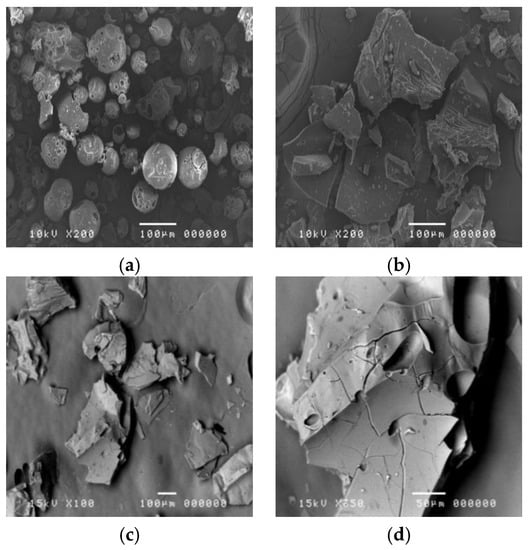
Figure 1.
Scanning electron microscope (SEM) images of (a) HPβCD powder; (b) blank HPβCD inclusion complex at magnification 200× and (c) CPSO-HPβCD inclusion complex at magnification 100× and (d) 350×.
3.3. Biological Activities of CPSO-HPβCD Inclusion Complex
The CPSO-HPβCD inclusion complex exhibited antioxidative activities, including DPPH scavenging, metal ion chelating, inhibition of lipid peroxidation activity, and tyrosinase inhibition activities with IC50 of 564.12 ± 17.14, 3587.21 ± 25.23, 464.87 ± 11.45, and 0.19 ± 0.12 mg/mL, respectively, significantly lower than free CPSO (when not incorporated in HPβCD) by about 20, 11, 8, and 4 times, respectively (Table 2). This may be due to the shielding effect of HPβCD, and when hydrophobic bioactive compounds such as CPSO were encapsulated in the bucket-shaped cavity, which may have led to the sustained release action of the active compound [29]. Other researchers have reported that CPSO can be used as a potential source for new drug development in treating various disorders caused by extreme oxidative stress, given its strong antioxidant properties [30]. Tyrosinase inhibition activity has been used to evaluate the skin-whitening effect, and kojic acid, arbutin, and ascorbic acid have been used as well-known standard whitening agents. Since our previous [7] and present study report that CPSO and CPSO-HPβCD exhibit high tyrosinase inhibition activity, the biological effect expected from this cosmetic formulation was the whitening effect, and the CPSO-HPβCD inclusion complex may have potential to develop cosmeceutical products. Moreover, one mechanism of the tyrosinase inhibitor is to chelate to the tyrosinase enzyme pocket at the copper ion. The metal ion chelation test is the preliminary test for chelation with the protein of the enzyme in the pocket [31]. If the extract shows high metal ion chelation and tyrosinase inhibition activities, it may have high whitening effects via the enzyme chelation mechanism.

Table 2.
IC50 (mg/mL) values of biological activities, including antioxidative and tyrosinase inhibition activities of CPSO-HPβCD inclusion complex, compared to CPSO and standard agents.
3.4. Physical Characteristics of CPSO-HPβCD Inclusion-Complex Formulations
All formulations containing the CPSO-HPβCD inclusion complex—including dispersion (CPSO-HPβCD dispersion), serum (CPSO-HPβCD serum), gel (CPSO-HPβCD gel), and CPSO oily solution formulations—had a yellow appearance. The CPSO oily solution formulation had a separation layer of CPSO on the surface after standing for three hours. The yellow separation layer might have occurred because the CPSO concentration was more than the maximum capacity of oil dispersion in propylene glycol. Moreover, after three months of storage, the color of the CPSO oily solution had changed from yellow to pale yellow, especially when kept at 45 °C. This might be due to the thermal instability of pigment compounds in CPSO. All other CPSO-HPβCD formulations had no significant color change with the CIELAB color analysis (data not shown) with a pH of approximately 6.5.
3.5. Chemical Characteristics of Oleic Acid in CPSO-HPβCD Inclusion Complex and CPSO-HPβCD Formulations
The chemical stability, presented as the percent remaining of oleic acid of various formulations at 4, 25, and 45 °C for three months, is shown in Figure 2. The non-inclusion complex of the oleic solution showed the lowest remaining content (64.03 ± 1.45%) of oleic acid after three months at every storage temperature. As such, the double bonds in the chemical structures of fatty acids can be oxidized and cause the rapid onset of oil rancidity when exposed to the environment [32]. This result is in agreement with a previous study, which found that the HPβCD inclusion complex can protect the inclusion substance against the environment [33]. The CPSO-HPβCD inclusion complex at 45 °C had the lowest remaining oleic acid content (88.47 ± 4.14%), whereas those at 4 °C and 25 °C had remaining oleic acid contents of 94.97 ± 1.71 and 90.54 ± 3.41%, respectively. However, no significant difference existed in the remaining content of oleic acid of the CPSO-HPβCD inclusion complex among the three storage temperatures.
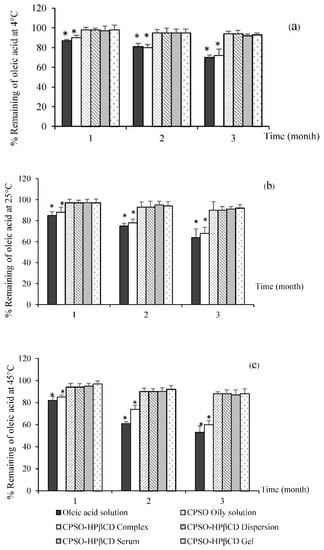
Figure 2.
Chemical stability presented as perfect remaining of oleic acid in various formulations at (a) 4 °C, (b) 25 °C, and (c) 45 °C for three months.
The chemical stability of each formulation—including CPSO-HPβCD dispersion, CPSO-HPβCD serum, CPSO-HPβCD gel, and CPSO oily solution—was also determined as the amount of oleic acid remaining under the three storage temperatures (Figure 2). The CPSO oily solution had the lowest percentages of oleic acid remaining after three months at 4, 25, and 45 °C of 72.68 ± 1.78, 68.26 ± 2.41, and 60.85 ± 3.41%, respectively. The remaining percentages’ significant decrease (p > 0.05) might be because the oleic acid in the CPSO oily solution formulation was not protected by the HPβCD structure, leading to a higher oxidation rate by the surrounding environment. This effect might convert oleic acid to ketone or aldehyde, resulting in rancidity. Furthermore, a separation layer of CPSO was found on top of the CPSO oily solution. However, the formulations that contained the CPSO-HPβCD inclusion complex (CPSO-HPβCD dispersion, serum, and gel) had remaining percentages of oleic acid over 80% when compared to the initial formulation under all storage temperatures for three months. The CPSO-HPβCD dispersion formulation exhibited the highest remaining content of oleic acid after three months at 4, 25, and 45 °C of 93.56 ± 1.87, 92.71 ± 2.34, and 88.00 ± 2.01% of the initial amount, respectively.
3.6. Skin Permeation of CPSO-HPβCD Inclusion-Complex Formulations
The rat-skin model may not be the best choice for a skin-permeation study. However, rodent skin (mice, rats, and guinea pigs) is the most commonly used skin in in vitro percutaneous permeation studies because of its availability. The advantages of these animals are their small size, uncomplicated handling, and relatively low cost [34]. Among rodents, rat skin has more structural similarities to human tissue. Except for rat skin, rodent skin generally shows higher permeation rates than human skin [35,36]. Nevertheless, rat skin was used in the skin-permeation assessment [37,38,39,40]. Moreover, the permeation kinetic parameters are frequently comparable with human skin. The cumulative amount of oleic acid used as a chemical marker of CPSO in various formulations in the whole skin and the receptor fluid within six hours is shown in Figure 3. The cumulative amount of oleic acid in all formulations gradually increased in both the whole skin and the receptor fluid throughout the experiment. The HPβCD inclusion complex containing CPSO in serum formulation (CPSO-HPβCD serum) exhibited the highest cumulative amount of oleic acid in the whole skin after six hours of 32.75 ± 1.25 µg/cm2, followed by the CPSO-HPβCD dispersion (29.28 ± 2.12 µg/cm2), and CPSO-HPβCD gel (25.25 ± 2.15 µg/cm2). The fluxes that permeated through the rat skin into the receptor fluid at six hours for the various formulations were calculated using the linear part of the correlation between the cumulative amount of oleic acid divided by the unit area (2.46 cm2) and time (six hours). The CPSO-HPβCD serum exhibited the highest oleic acid flux after six hours, which was 1.02 ± 0.15 µg/cm2/h, followed by the CPSO-HPβCD gel (0.80 ± 0.12 µg/cm2/h), and CPSO-HPβCD dispersion (0.75 ± 0.01 µg/cm2/h). The CPSO oily solution formulation exhibited the lowest cumulative amount in the whole skin and flux through the receptor fluid after six hours of 15.61 ± 2.11 µg/cm2 and 0.13 ± 0.01 µg/cm2/h, respectively. All CPSO-HPβCD formulations significantly appeared to penetrate the skin more efficiently, whereas the CPSO oily solution exhibited lower penetration with some remaining oil on the skin surface. In addition, superior skin penetration of the inclusion complex was observed in the serum and dispersion formulations (CPSO-HPβCD serum-dispersion, p > 0.05) compared to the CPSO-HPβCD inclusion complex in gel formulation (CPSO-HPβCD gel). This might be due to the viscosity of the formulations and the steric effect of the gel structure, which may retard the skin penetration of oleic acid in CPSO [41]. Moreover, the penetration enhancer effect of propylene glycol [42]—which was used in the CPSO oily solution, CPSO-HPβCD dispersion, and CPSO-HPβCD serum via pure cosolvent effect [43] or solvating the α-keratin structures of the cells [44]—showed lower influence than the HPβCD inclusion complex in skin penetration.
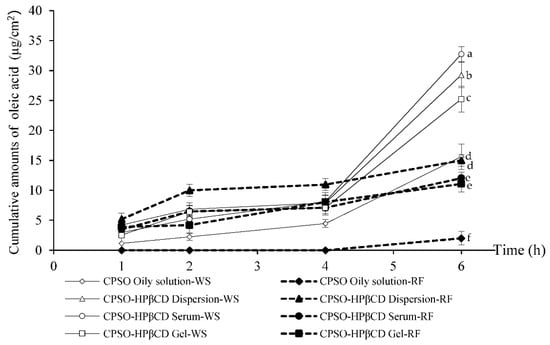
Figure 3.
Cumulative amounts of oleic acid in various formulations of the C. paniculatus seed oil (CPSO) and hydroxypropyl-β-cyclodextrin inclusion complex (HPβCD) in the whole skin (WS) and receptor fluid (RF) within six hours. At six hours, mean cumulative amounts with the same letter are not significantly different. Thus, means with different letters (e.g., ‘a’ or ‘b’) were statistically different (p < 0.05).
However, oleic acid was found in the receptor fluid in all formulations, which means that CPSO in all formulations, especially in CPSO-HPβCD formulations, could penetrate the blood system, which may be due to its small particle size. In the pharmaceutical field, the formulations of an increasing amount of marketed drugs include cyclodextrins because of their capacity to trap drugs into their cavity, enabling their use in drug delivery to facilitate the body-tissue penetration of hydrophilic or hydrophobic drugs [45,46]. Even if cyclodextrins could be harmful to the human organism only at extremely high concentrations [47], the toxicity of CPSO should be evaluated to further develop the CPSO-HPβCD product. In this study, the abilities of the CPSO oily solution and CPSO-HPβCD formulations to be removed after skin application were compared. The results showed that the CPSO-HPβCD formulation was easier to remove by water after skin application than the CPSO oily solution. This might be due to the effect of HPβCD on CPSO solubility. The aqueous solubility ability was also found in other substance studies (i.e., griseofulvin and phenothiazine cyclodextrin complexation) [48,49]. Finally, this study suggests that the inclusion complex in serum formulation may be the most suitable system for topical application, given that it had the highest skin penetration of oleic acid, proper viscosity, and could be removed after skin application. However, the systemic toxicity of this formulation should be a concern.
4. Conclusions
CPSO, which is primarily composed of oleic acid, was incorporated in HPβCD cavities to improve its stability and skin penetration. The inclusion ratio and total recovery of the CPSO-HPβCD inclusion complex were 92.4 ± 0.7% and 87.5 ± 3.4%, respectively. The CPSO-HPβCD inclusion complex in a solid state showed irregular-shaped crystals with a glossy smooth surface. The mean particle sizes of the HPβCD powder, blank HPβCD, and CPSO-HPβCD inclusion-complex solutions measured by DLS were 103.30 ± 11.74, 112.71 ± 6.97, and 240.95 ± 13.38 nm, respectively. For biological activity studies—including DPPH scavenging, metal ion chelating, inhibition of lipid, and tyrosinase inhibition activities—the CPSO-HPβCD inclusion complex exhibited lower inhibition activity than free CPSO. This inclusion complex was prepared as CPSO-HPβCD dispersion, serum, and gel formulations. All CPSO-HPβCD formulations showed no significant change in color, with a pH of approximately 6.5, and the oleic acid percentages remaining in all formulations were over 80% of the initial amount when stored at 4, 25, and 45 °C for three months. For the rat skin penetration study using Franz diffusion cells, the observant oleic acid was found in the receptor fluid, especially in CPSO-HPβCD formulations. The CPSO-HPβCD serum formulation exhibited the highest cumulative amount of oleic acid in the whole skin and flux through receptor fluid after six hours of 32.75 ± 1.25 µg/cm2 and 1.02 ± 0.15 µg/cm2/h, respectively. The CPSO oily solution formulation exhibited the lowest cumulative amount in the whole skin and flux through the receptor fluid after six hours of 15.61 ± 2.11 µg/cm2 and 0.13 ± 0.01 µg/cm2/h, respectively. This result can be applied to further develop CPSO-HPβCD products for topical application. However, the toxicity of CPSO should be evaluated for further experiments.
Author contributions
Conceptualization, W.R.; Methodology, W.R.; Formal Analysis, K.L.; Investigation, P.J.; Resources, J.S.; Writing-Original Draft Preparation, C.K.; Writing-Review and Editing, W.R. and P.J.; Supervision, W.R.; Project Administration, W.R.; Funding Acquisition, W.R.
Funding
The authors are grateful for the financial support of the Chiang Mai University Junior Research Fellowship Program 2015, the Faculty of Pharmacy, Chiang Mai University Research grant 2016 and the grant from Lanna Innovation and Digital Media Development Centre, Social Research Institute, Chiang Mai University.
Acknowledgments
The authors are grateful for the C. paniculatus seed oil from the Highland Research and Development Institute, Thailand.
Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Chatterjee, A.; Prakashi, S.C. The Treatise on Indian Medicinal Plants; Niscair: New Delhi, India, 2003; Volume 3, p. 160. [Google Scholar]
- Singh, U.; Wadhawani, A.M.; Johri, B.M. Dictionary of Economical Plants of India; Indian council of Agricultural Research: New Delhi, India, 1996. [Google Scholar]
- Nadkarni, K.M.; Nadkarni, A.K.; Nadkarni, K.M. Indian Plants and Drugs; Popular Bombay Prakashan Pvt Ltd.: Mumbai, India, 1976; Volume 1, p. 296. [Google Scholar]
- Rana, V.S.; Das, M. Fatty acid and non-fatty acid components of the seed oil of Celastrus paniculatus willd. Int. J. Fruit Sci. 2017, 17, 407–414. [Google Scholar] [CrossRef]
- Tanojo, H.; Boelsma, E.; Junginger, H.E.; Ponec, M.; Bodde, H.E. In vivo human skin barrier modulation by topical application of fatty acids. Skin Pharmacol. Appl. Skin Physiol. 1998, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Ziboh, V.A.; Miller, C.C.; Cho, Y. Metabolism of polyunsaturated fatty acids by skin epidermal enzymes: Generation of antiinflammatory and antiproliferative metabolites. Am. J. Clin. Nutr. 2000, 71 (Suppl. 1), 361S–366S. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Sringarm, K.; Jantrawut, P. Stability enhancement of Celastrus paniculatus seed oil by loading in niosomes. Asian J. Pharm. Clin. Res. 2014, 7, 186–191. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A.; Yu, S.C.; Pépin, C.; Seiller, M. Cyclodextrins and emulsions. Int. J. Pharm. 2003, 266, 85–90. [Google Scholar] [CrossRef]
- Schmid, G. Cyclodextrin glycosyltransferase production: Yield enhancement by overexpression of cloned genes. Trends Biotechnol. 1989, 7, 244–248. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Citernesi, U.; Sciacchitano, M. Cyclodextrins in functional dermocosmetics. Cosmet. Toilet. 1995, 110, 53–61. [Google Scholar]
- Sarveiya, V.; Templeton, J.F.; Benson, H.A.E. Inclusion complexation of the sunscreen 2-hydroxy-4-methoxy benzophenone (oxybenzone) with hydroxypropyl-β-cyclodextrin: Effect on membrane diffusion. J. Incl. Phenom. Macrocycl. Chem. 2004, 49, 275–281. [Google Scholar] [CrossRef]
- Radhika, P.; Nagabhushanam, M.V.; Ramana, M.V. Preparation and characterization of Lornoxicam solid systems using cyclodextrins for improved bioavailability. Bull. Pharm. Res. 2015, 5, 101–107. [Google Scholar]
- Wang, J.; Cao, Y.; Sun, B.; Wang, C. Physicochemical and release characterisation of garlic oil-β-cyclodextrin inclusion complexes. Food Chem. 2011, 127, 1680–1685. [Google Scholar] [CrossRef]
- Tachibana, Y.; Kikuzaki, H.; Hj-Lajis, N.; Nakatani, N. Antioxidant activity of carbazoles from Murraya koenigii leaves. J. Agric. Food Chem. 2001, 49, 5589–5594. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Namiki, M. A novel type of antioxidant isolated from leaf wax of eucalyptus leaves. Agric. Biol. Chem. 1981, 45, 735–739. [Google Scholar] [CrossRef]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Shimizu, K.; Kondo, R.; Sakai, K.; Lee, S.; Sato, H. The inhibitory components from Artocarpus incisus on melanin biosynthesis. Planta Med. 1998, 64, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Plessis, J.; Egbaria, K.; Weiner, N. Influence of formulation factors on the deposition of liposomal components into the different strata of the skin. J. Soc. Cosmet. Chem. 1992, 43, 93–100. [Google Scholar]
- Kabnurkar, R.B. Phytopharmaceutical studies of the topical formulations of Celastrus paniculatus Willd (CELASTRACEAE). J. Pharm. Biomed. Sci. 2012, 17, 1–4. [Google Scholar]
- Bhanumathy, M.; Chandrasekar, S.B.; Chandur, U.; Somasundaram, T. Phytopharmacology of Celastrus paniculatus: An overview. Int. J. Pharm. Sci. Drug Res. 2010, 2, 176–181. [Google Scholar]
- Arora, N.; Pandey-Rai, S. Celastrus paniculatus, an endangered indian medicinal plant with miraculous cognitive and other therapeutic properties: An overview. Int. J. Pharma Bio Sci. 2012, 3, 290–303. [Google Scholar]
- Salústio, P.J.; Feio, G.; Figueirinhas, J.L.; Pinto, J.F.; Cabral Marques, H.M. The influence of the preparation methods on the inclusion of model drugs in a β-cyclodextrin cavity. Eur. J. Pharm. Biopharm. 2009, 71, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Mourtzinos, I.; Salta, F.; Yannakopoulou, K.; Chiou, A.; Karathanos, V.T. Encapsulation of olive leaf extract in β-cyclodextrin. J. Agric. Food Chem. 2007, 55, 8088–8094. [Google Scholar] [CrossRef] [PubMed]
- Del Toro-Sánchez, C.; Ayala-Zavala, J.; Machi, L.; Santacruz, H.; Villegas-Ochoa, M.; Alvarez-Parrilla, E.; González-Aguilar, G. Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 431–441. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential oils loaded in nanosystems: A developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Pandey-Rai, S. GC–MS analysis of the essential oil of Celastrus paniculatus Willd seeds and antioxidant, anti-inflammatory study of its various solvent extracts. Ind. Crops Prod. 2014, 61, 345–351. [Google Scholar] [CrossRef]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2, 4-substituted resorcinol moiety. Bioorg. Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Kouba, M.; Mourot, J. A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids. Biochimie 2011, 93, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J.; Szemán, J.; Kató, L. Fatty acid-cyclodextrin complexes: Properties and applications. J. Incl. Phenom. Macrocycl. Chem. 1993, 16, 339–354. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Mueller, K.R. Comparisons of in vitro nitroglycerin (TNG) flux across Yucatan pig, hairless mouse, and human skins. Pharm. Res. 1990, 7, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sugibayashi, K.; Morimoto, Y. Species differences in percutaneous absorption of nicorandil. J. Pharm. Sci. 1991, 80, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Ariyoshi, S.; Ohura, K.; Sawada, T.; Nakada, Y. Expression of carboxylesterase isozymes and their role in the behavior of a fexofenadine prodrug in rat skin. J. Pharm. Sci. 2016, 105, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Karthikeyan, R.S. Penetration enhancing effect of menthol on the percutaneous flux of nicardipine hydrochloride through excised rat epidermis from hydroxypropyl cellulose gels. Pharm. Dev. Technol. 2002, 7, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kunta, J.R.; Goskonda, V.R.; Brotherton, H.O.; Khan, M.A.; Reddy, I.K. Effect of menthol and related terpenes on the percutaneous absorption of propranolol across excised hairless mouse skin. J. Pharm. Sci. 1997, 86, 1369–1373. [Google Scholar] [PubMed]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Suetake, T.; Sasai, S.; Zhen, Y.X.; Tagami, H. Effects of silicone gel sheet on the stratum corneum hydration. Br. J. Plast. Surg. 2000, 53, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Arellano, A.; Santoyo, S.; Martın, C.; Ygartua, P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur. J. Pharm. Sci. 1999, 7, 129–135. [Google Scholar] [CrossRef]
- Goodman, M.; Barry, B.W. Action of penetration enhancers on human skin as assessed by the permeation of model drugs 5-fluorouracil and estradiol I Infinite dose technique. J. Investig. Dermatol. 1988, 91, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Sarpotdar, P.P.; Zatz, J.L. Evaluation of penetration enhancement of lidocaine by nonionic surfactants through hairless mouse skin in vitro. J. Pharm. Sci. 1986, 75, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin drug carrier systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, H.J.; Schollmeyer, E. Application of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar] [PubMed]
- Veiga, M.D.; Díaz, P.J.; Ahsan, F. Interactions of griseofulvin with cyclodextrins in solid binary systems. J. Pharm. Sci. 1998, 87, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Lutka, A. Effect of cyclodextrin complexation on aqueous solubility and photostability of phenothiazine. Die Pharm. 2000, 55, 120–123. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).