Abstract
Many interesting applications have been found for nitroimidazoles as therapeutic agents. Among others, some of these compounds can radiosensitize hypoxic tumor cells. The introduction of a second nitroimidazole ring to the molecule can improve the level of its pharmacological effect. The aim of this article is to overview the literature concerning active compounds that contain two nitroimidazole moieties in their structures.
1. Introduction
The antibiotic azomycin was the first active nitroimidazole to be discovered [1]. Its discovery provided the major impulse for the systematic search for medicines showing activity against anaerobic protozoa. This research led to the acquisition of the 5-nitroimidazole derivative—metronidazole, which is most widely used in the treatment of anaerobic protozoan parasitic infections caused by Trichomonas vaginalis, Giardia duodenalis, and Entamoeba histolytica [2,3].
A common feature of tumors is an imbalance between oxygen (O2) supply and consumption, called hypoxia, the severity of which varies among tumor types [4,5]. In tumor tissue, which becomes larger and proliferates, oxygen demand is exceeded by oxygen supply, and the distance between cells and the existing vasculature rises, limiting oxygen distribution. Initial oxygenation of the tissue as well as the dimension and point in tumor development are connected with oxygen levels in the tumor. Main determinants of tumor hypoxia are atypical structure and function of the micro-blood vessels which supply the tumor, increased diffusion distances between the nutritional microvessels and the tumor cells, and reduced oxygen transport capacity of the blood because of the existence of disease- or treatment-related anemia. Tumor hypoxia might diminish the effectiveness of radiotherapy, some O2-dependent cytotoxic agents, and photodynamic therapy.
The inability of surrounding vasculature to keep pace with the tumor growth is a main factor leading to the development of hypoxic regions in tumors [6]. Radiosensitizers have been shown to enhance tumor damage by selectively increasing the radiosensitivity of hypoxic cells. Many nitroimidazole derivatives have been found to sensitize hypoxic cells to the lethal effect of ionizing radiation when present at the time of irradiation [7]. When a nitroimidazole derivative passes into a viable cell by diffusion, the compound undergoes a set of chemical alterations: initially, the molecule attains a single electron in the cytoplasm by an enzymatic process to create a potentially reactive species. Subsequently, the molecule is immediately reoxidized in the presence of adequate intracellular O2 levels [8]. This process is repeated until the whole compound diffuses out of the cell. In the absence of an adequate supply of O2, in hypoxic tissue, they undergo further reduction to more reactive products that bind to cell components because the decreased oxygen concentration is not able to effectively compete to reoxidize the molecule. An enzyme-mediated, single-electron reduction of the NO2 group initiates the formation of these products. The nitro group is reduced to a free radical, which exists in form of an anion at neutral pH. The reduction pathway can proceed in successive steps past the NHOH derivative to terminate at the relatively inactive amine derivative. The reoxidation does not occur with low oxygen concentration. The reactive molecules subsequently go through additional reduction pathways and remain in the cell. Reduction takes place in all tissues with viable enzymatic processes, but retention only exists in those tissues with reduced oxygen levels.
In addition to their radiosensitizing activity, nitroimidazole derivatives are selectively toxic to hypoxic cells because of their reductive activation via oxygen-inhibitable metabolic pathways [9]. One of the most investigated types of compounds are 2-nitroimidazole derivatives. They undergo enzymic, oxygen-inhibitable reduction of NO2 group, resulting in preferential metabolism in hypoxic cells to reactive metabolites [10]. The introduction of a second nitroimidazole moiety into the molecule results in obtaining the double- or bis-bioreductive agent, in which the inhibitable reduction of two centers would be required for full cytotoxicity. The aim of this review is to present the current state of knowledge about molecules that contain two nitroimidazole rings in the structure. There are two types of such compounds: double-nitroimidazoles with unsymmetrical structures and bis-nitroimidazoles, with the same two nitroimidazole moieties which form symmetrical molecules or possess two symmetrical parts.
All the available biological test results for described compounds are summarized in Table 1.

Table 1.
The activities of presented double- and bis-nitroimidazoles.
2. Literature Survey
The first publication concerning bis-nitroimidazoles appeared in 1984 [20]. The authors of that paper suggested that the introduction of two nitroimidazole rings to the molecule should significantly improve the level of pharmacological efficacy of the obtained compound. This work describes the synthesis of 1,3-bis-(1-imidazolyl)propan-2-ols via the reactions of N-(3-chloro-2-hydroxypropyl) and N-(2,3-epoxypropyl) derivatives of 2-methyl-4-nitroimidazole or 2-methyl-5-nitroimidazole with 1H-imidazoles. The products are perspective compounds with potential radiosensitizing activity (Figure 1).
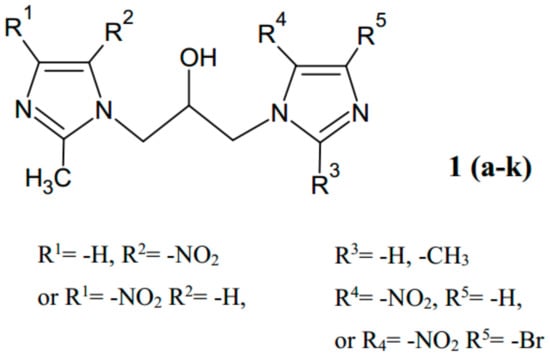
Figure 1.
The structure of hydroxyisopropyl-linked bis-nitroimidazoles.
Compound 1 (when R1 = R5 = –H, R2 = R4 = –NO2, R3 = –CH3) is the first example in the literature of the fused hybrid type of compounds among nitroimidazoles. Synthesis of this kind of hybrids, in which two molecules of well-known metronidazole are linked, has been presented in detail in Pawełczyk et al. [21] as a synthesis of “molecular consortia”.
In 1994, New Zealand scientists reported a procedure for the synthesis of double (nitroimidazolyl)alkanecarboxamides (Figure 2) [11]. These compounds were prepared by a direct coupling of side chain acid and amine components, with the use of diethyl phosphorocyanidate at room temperature. Further chemical transformations included obtaining the key intermediate, named 2-(2-methyl-5-nitro-1H-imidazolyl) ethylamine, by a set of chemical modifications, e.g., alkylation of imidazole with bromophthalimide derivative, which replaced the bromophthalimide moiety with hydrazine monohydrate to give imidazolylethylamines. The carboxamide-linked double-nitroimidazoles were obtained via a reaction of these amine derivatives with an acid component.
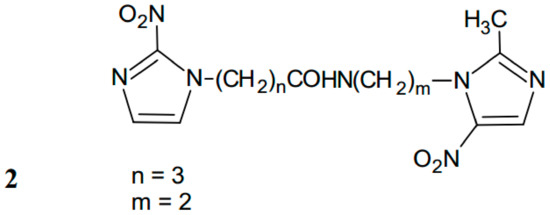
Figure 2.
The structure of carboxamide-linker double-nitroimidazole.
Linkage of the two nitroaromatic moieties was effected at 1-position through N-alkyl chains. Such linkers ensure electronic isolation, avoiding significant disorder of NO2 reduction potentials. All the substances showed aerobic toxicities corresponding to those of mononitro derivatives. Concurrently, bis-nitroimidazoles act as radiosensitizers of hypoxic cells in vitro, but they are only slightly more active than the known mononitro derivatives. One of the main limitations in biological evaluation of these carboxamide-linker compounds was their poor solubility in water. The more soluble substance (2) was active as a radiosensitizer of murine fibrosarcoma KHT tumor cells when it was administered in multiple doses (Figure 2).
The potency of 2 as a hypoxic cell radiosensitizer in vitro is no higher than that of mononitroimidazoles [9]. The kinetics of potential drug activation is consistent with the formation of a bifunctional cytotoxic species that does not act as a DNA cross-linking agent [10]. This substance was characterized by significantly higher (up to 200-fold) hypoxic selectivity than misonidazole in AA8cells and is potent against KHT and MDAH-MCa-4 tumors in in vivo tests.
Further investigation was directed at increasing aqueous solubility and obtaining higher activation under hypoxic conditions. The study was extended by the synthesis and biological evaluation of double-nitroimidazoles derivatives with an isopropyl linker group, a series with glucopyranosyl linkers, which analogues to oxamide linkers, and a succinamide chain instead of a carboxamide linker [10]. Among these derivatives, only the aminoalkyl provided substances with sufficient solubility in water so that they could be evaluated fully in cell culture (Figure 3). The amino-linked 2-nitro/5-nitro analogs (3–5) were characterized by aerobic cytotoxicity two- to seven-fold higher than that of the carboxamide-linked analogue of compound 2. The compounds with symmetrical amine linkers in 6, 7 were from six to nine times more active than 2.
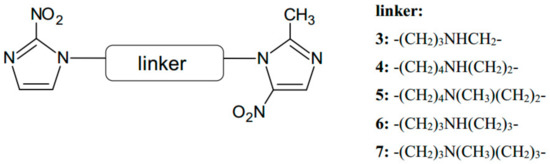
Figure 3.
The structures of the most active aminoalkyl-linked analogs (3–7).
Other authors have described a synthetic method for obtaining novel polyamine-nitroimidazole conjugates [12]. It has been suggested that polyamines can be used as vectors for the cellular delivery of different DNA targeted substances. The introduction of nitroimidazole moiety to polyamines may deliver the drug to target tumor cells by the high specific activity. Moreover, polyamine can also increase the concentration of the drug near DNA, the proposed site of action of nitroimidazoles [12]. On the basis of such assumptions, Siddiqui et al. [12] obtained a series of N1,N7- and N1,N8-bis-derivatised spermidine-nitroimidazole compounds (Figure 4), which were tested for their ability to be used as starting compounds for the polyamine uptake system in A549 lung carcinoma cells by measuring inhibition of [14C]spermidine uptake. It was found that tested conjugates were promising in the targeting of tumor cells.
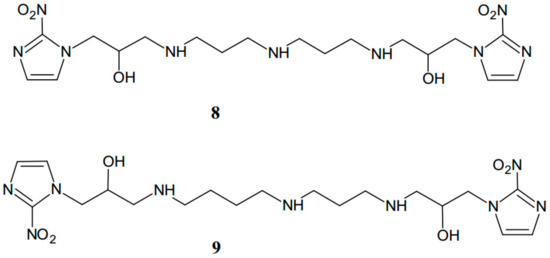
Figure 4.
Structures of N1,N7-bis-derivatised norspermidine-nitroimidazole conjugate (8) and N1,N8-bis-derivatised spermidine-nitroimidazole conjugate (9).
In 2005, a method for obtaining bis(hydroxamamide)-based tetradentate ligands for 99mTc-radiopharmaceutical was described by Xu et al. [22]. They [22] proposed the synthesis of two new compounds: N,N′-ethylene bis (1-(4-nitroimidazole-1-yl)-propanhydroxyiminoamide) (10) and N,N′-propylene bis (1-(4-nitroimidazole-1-yl)-propanhydroxyiminoamide) (11) (Figure 5).
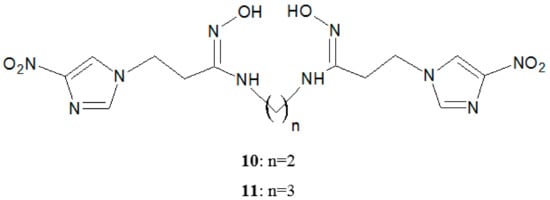
Figure 5.
Structure of tetradentate ligands (10, 11).
These bis-nitroimidazoles were labeled with 98mTc forming appropriate complexes that are electrically neutral under physiological conditions. The results suggested that the obtained products may be useful as chelating molecules for the preparation of radiopharmaceuticals.
Qian and colleagues [13,14] have demonstrated that bis-(2-nitroimidazole)-naphthalimide derivatives (12, 13) can act as fluorescent markers for hypoxia (Figure 6).
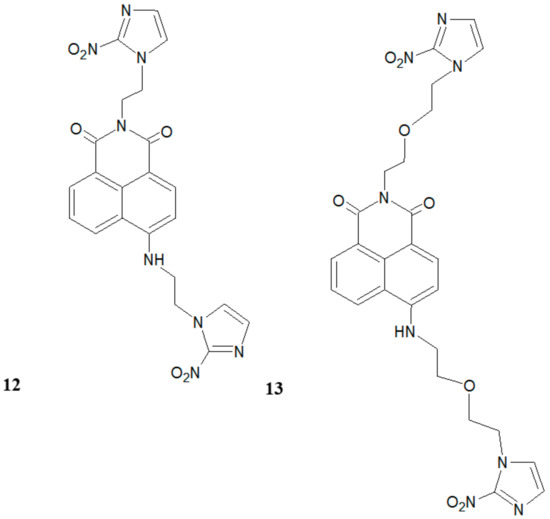
Figure 6.
The structures of two bis-(2-nitroimidazole)-naphthalimides (12, 13).
In biological tests, these compounds showed high fluorescence differences between hypoxic and normoxic cells. In particular, compound 13 was very active under hypoxic conditions.
Interestingly, some authors developed the methods of obtaining bis-/double- nitroimidazoles connected with metal atoms [17,23]. For example, Technetium-99m is used because of its properties which have ideal characteristics for medical scanning. It has a very short half-life, which is long enough to examine physiological processes, yet short enough to minimise the radiation dose to the patient. Also, it emits low energy γ radiation that reduces damage to tissues, but it can still be detected in a patient’s body by a γ-ray sensitive camera. Moreover, Technetium-99m is quickly eliminated from the organism.
Giglio and colleagues developed the novel active 99mTc-radiopharmaceuticals for imaging hypoxia [23]. These products were obtained by the formation of Tc-nitrido-complexes with two new dithiocarbamate derivatives of metronidazole (Figure 7). It was found that only compound 14 showed adequate chemical stability together with low protein binding associated with fast blood clearance and moderate lipophilicity.
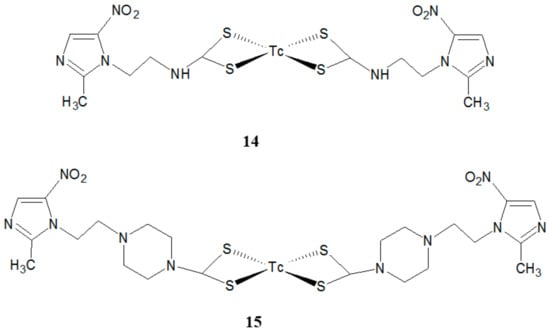
Figure 7.
The structures of two new 99mTc complexes (14, 15).
Another interesting approach to the synthesis and evaluation of bis-nitroimidazoles has been presented by Xu et al. [15], in which the authors described the method for obtaining a more expanded structure of novel nitroimidazole indocyanine dye conjugate (2-nitroimidazole-ICG) (16) (Figure 8) using a piperazine linker for tumor-targeted hypoxia fluorescence tomography [15].
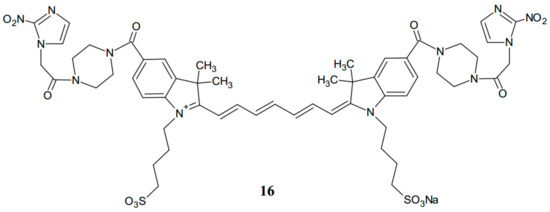
Figure 8.
The structure of 2-nitroimidazole-ICG conjugate (16).
The activity of the new compound (16) was compared with that of the earlier known analogue of ethanolamine-2-nitroimidazole-ICG. Mouse tumors, located at imaging depths of 1.5 and 2.0 cm in a turbid medium, were imaged at various time points after intravenous injection of the dyes. As a result, the measured maximum fluorescence concentration of the tumors injected with compound 16 was two-fold higher than that after injection with ethanolamine-2-nitroimidazole-ICG within 3 h. Piperazine-2-nitroimidazole-ICG (16) is a novel second-generation tumor hypoxia-targeted conjugate. The results of biological evaluation suggest that the molecules which are more rigid and planar are characterized by higher fluorescence yield, and they might release less absorbed energy [16]. The same research group has reported a new substance after further chemical modifications with an improved level of fluorescence quantum yield. This third-generation conjugate (17) has two carbon atoms less in the polyene linker, and it is prepared by coupling reaction of the new dye with 2-nitroimidazole using a piperazine linker (Figure 9).
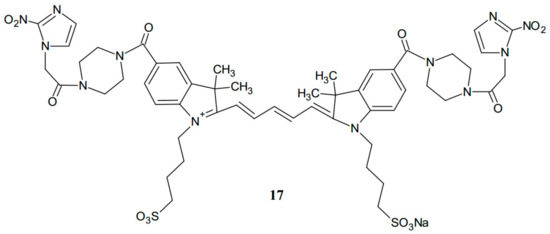
Figure 9.
The structure of the third-generation conjugate (17).
The fluorescence quantum yield is about five times higher than that measured for the second-generation hypoxia dye. Experiments in vivo were also promising. A new compound (17) showed more than two times the fluorescence intensity in the tumor as compared with a second-generation conjugate (16).
Other authors reported a method of obtaining the novel imaging agent, 99mTc ethylenedicysteinebis-misonidazole (99mTc-EC-MISO) (18) (Figure 10), for diagnosing tumor hypoxia [17]. The results of biological evaluation conducted on subcutaneous gliomal tumor-bearing mice were very promising. Single-photon emission computed tomography (SPECT/CT) imaging confirmed that the tumors can be visualized clearly with 99mTc-EC-MISO at 2 h. Introduction of a second 2-nitroimidazole moiety caused an apparent hypoxic accumulation of 99mTc-EC-MISO imaging agent in the tumor.
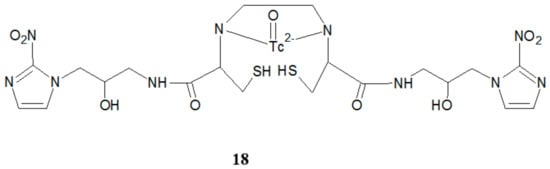
Figure 10.
The structure of 99mTc-EC-MISO.
Girhepunje and colleagues have presented a set of new 5-nitroimidazole derivatives with antibacterial and antifungal activities [18]. One of these compounds, 1,4-bis{(2-methyl-5-nitro-1H-imidazole)-2-ethyl propanoate}piperazine (19), was particularly interesting (Figure 11).
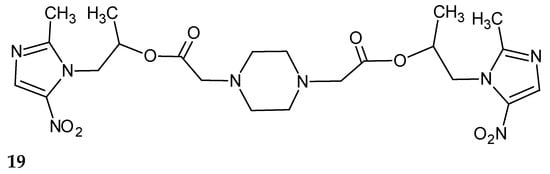
Figure 11.
The structure of the compound (19).
In biological studies, it was found to show significant antibacterial activity against Staphylococcus aureus and Escherichia coli, higher than those evaluated for other tested compounds. Moreover, it had antifungal properties, especially against Candida albicans and Aspergillus niger.
Nitroimidazole derivatives are not only used as drugs in medicine but they also have other applications. The presence of more than one nitro group makes these compounds high-energy materials. Among them is 4,4′,5,5′-tetranitro-2,2′-biimidazole (TNBI) (20), which can be used in explosive compositions because it has high detonation parameters [19]. This substance is very hygroscopic, and it can be used only in salt form (Figure 12).
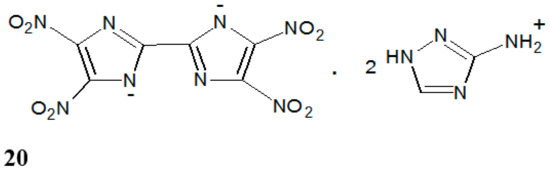
Figure 12.
The structure of 3-amino-1,2,4-triazolium 4,4′,5,5′-tetranitro-2,2′-biimidazolate (20).
As displaced Table 1 suggests, the main area of application of bis-/double- nitroimidazoles is their use in anticancer therapy. Concurrently, they have other interesting applications, e.g., antibacterial, antifungal, and nonmedical, such as explosives. Moreover, some of these compounds are used in the form of complexes with metals, especially with Technetium-99m. Such substances serve as radiopharmaceuticals.
3. Conclusions
Drugs containing a nitro group are widely used in the treatment of many illnesses and conditions e.g., hypertension, heart failure, coronary artery disease, and in the prevention of stroke [24].
Nitroimidazoles are categorized mainly as bioreductive prodrugs, characterized by both radiosensitizing and cytotoxic activities [25].
The NO2 nitrogen atom in the molecule, which has an electron affinity, is responsible for radiosensitizing activities. The radiation-caused ionization and the NO2 group undergoes reduction processes, in turn delivering oxidative damage to DNA, an oxygen-mimetic effect. Misonidazole, metronidazole, and etanidazole were placed in the group of the first nitroimidazole derivatives tested in clinical trials. Introduction of a second nitroimidazole moiety into the molecule results in the acquisition of the double/bis-nitroimidazole bioreductive agent, in which full cytotoxicity could be achieved by the inhibitable reduction of two centers. New compounds with two nitroimidazole moieties often have a more complicated structure, that is connected with the introduction to the molecule by means of biologically advantageous side chains or/and metal elements. Their desirable activities are significantly higher than the potencies of the simplest derivatives of double/bis-nitroimidazole.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maeda, K.; Osata, T.; Umezawa, H. A new antibiotic, azomycin. J. Antibiot. 1953, 6, 182. [Google Scholar] [PubMed]
- Townson, S.M.; Boreham, P.F.L.; Upcroft, P.; Upcroft, J.A. Resistance to the nitroheterocyclic drugs. Acta Trop. 1994, 56, 173–194. [Google Scholar] [CrossRef]
- Mital, A. Synthetic nitroimidazoles: Biological activities and mutagenicity relationships. Sci. Pharm. 2009, 77, 497–520. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Vaupela, P.; Harrison, L. Tumor hypoxia: Causative factors, compensatory mechanisms, and cellular response. Oncologist 2004, 9 (Suppl. 5), 4–9. [Google Scholar] [CrossRef] [PubMed]
- Tidwell, R.R.; Jones, S.K.; Geratz, J.D.; Ohemeng, K.A.; Cory, M.; Hall, J.E. Analogs of 1,5-bis(4-amidinophenoxy)pentane (pentamidine) in the treatment of experimental Pneumocystis carinii pneumonia. J. Med. Chem. 1990, 33, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.F.; Patel, K.B. Effect of lipophilicity of nitroimidazoles on radiosensitization of hypoxic bacterial cells in vitro. Br. J. Cancer 1979, 39, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Nunn, A.; Linder, K.; Strauss, H.W. Nitroimidazoles and imaging hypoxia. Eur. J. Nucl. Med. 1995, 22, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Moselen, J.W.; Hay, M.P.; Denny, W.A.; Wilson, W.R. N-[2-(2-Methyl-5-nitroimidazolyl)ethyl]-4-(2-nitroimidazolyl)butanamide (NSC 639862), a Bisnitroimidazole with enhanced selectivity as a bioreductive drug. Cancer Res. 1995, 55, 574–580. [Google Scholar] [PubMed]
- Hay, M.P.; Lee, H.H.; Wilson, W.R.; Roberts, P.B.; Denny, W.A. Hypoxia-selective antitumor agents. 10. Bis(nitroimidazoles) and related Bis(nitroheterocycles): Development of derivatives with higher rates of metabolic activation under hypoxia and improved aqueous solubility. J. Med. Chem. 1995, 38, 1928–1941. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.P.; Wilson, W.R.; Moselen, J.W.; Palmer, B.D.; Denny, W.A. Bis(nitroimidazolyl)alkanecarboxamides: A new class of hypoxia-selective cytotoxins and hypoxic cell radiosensitizers. J. Med. Chem. 1994, 37, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.Q.; Merson-Davies, L.; Cullis, P.M. The synthesis of novel polyamine-nitroimidazole conjugates designed to probe the structural specificities of the polyamine uptake system in A549 lung carcinoma cells. J. Chem. Soc. Perkin Trans. 1 1999, 22, 3243–3252. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Qian, X.; Liu, J.; Shen, L.; Li, J.; Zhang, Y. Novel fluorescent markers for hypoxic cells of naphthalimides with two heterocyclic side chains for bioreductive binding. Bioorg. Med. Chem. 2006, 14, 2935–2941. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Xiao, Y.; Xu, Y.; Guo, X.; Qian, J.; Zhu, W. “Alive” dyes as fluorescent sensors: Fluorophore, mechanism, receptor and images in living cells. Chem. Commun. 2010, 46, 6418–6436. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zanganeh, S.; Mohammad, I.; Aguirre, A.; Wang, T.; Yang, Y.; Kuhn, L.; Smith, M.B.; Zhu, Q. Targeting tumor hypoxia with 2-nitroimidazole-indocyanine green dye conjugates. J. Biomed. Opt. 2013, 18, 066009. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Zanganeh, S.; Mohammad, I.; Dietz, C.; Abuteen, A.; Smith, M.B.; Zhu, Q. Targeting tumor hypoxia: A third generation 2-nitroimidazole-indocyanine dye-conjugate with improved fluorescent yield. Org. Biomol. Chem. 2015, 13, 11220–11227. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, D.; Qian, J.; Zhang, Z.; Zhu, J.; Chen, J. Preparation and Biodistribution of Technetium-99m-Labeled Bis- Misonidazole (MISO) as an Imaging Agent for Tumour Hypoxia. Med. Chem. 2015, 11, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Girhepunje, N.S.; Kedar, P.S.; Ittadwar, A.M.; Dumore, N.G. Design, synthesis, and characterization of some 5-nitroimidazole derivatives. Ijppr. Hum. 2016, 6, 456–480. [Google Scholar]
- Lewczuk, R.; Szala, M.; Rećko, J. Otrzymywanie i badanie właściwości wysokoazotowych soli 4,4′,5,5′-tetranitro-2,2′-biimidazolu. Biul. WAT 2015, 64, 15–26. [Google Scholar] [CrossRef]
- Tułecki, J.; Zaprutko, L. Synteza związków pochodnych 2-propanolu z układem nitroimidazolu. Acta Pol. Pharm. 1984, 3, 281–292. [Google Scholar]
- Pawełczyk, A.; Sowa-Kasprzak, K.; Olender, D.; Zaprutko, L. Molecular consortia—Various structural and synthetic concepts for more effective therapeutics synthesis. Int. J. Mol. Sci. 2018, 19, 1104–1123. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Chu, T.; Wang, X.; Liu, X. Facile synthesis of bis(hydroxamamide)-based tetradentate ligands for 99mTc-radiopharmaceutical. Appl. Radiat. Isot. 2005, 62, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Giglio, J.; Fernandez, S.; Rey, A.; Cerecetto, H. Synthesis and biological characterisation of novel dithiocarbamate containing 5-nitroimidazole 99mTc-complexes as potential agents for targeting hypoxia. Bioorg. Med. Chem. Lett. 2011, 21, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Olender, D.; Żwawiak, J.; Zaprutko, L. Multidirectional efficacy of biologically active nitro compounds included in medicines. Pharmaceuticals 2018, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.K.; Wong, W.W.; Ross, H.J. Past approaches and future directions for targeting tumor hypoxia in squamous cell carcinomas of the head and neck. Crit. Rev. Oncol. Hematol. 2016, 103, 86–98. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).