Inhibitory Effects and Killing Kinetics of Lactic Acid Rice Gel Against Pathogenic Bacteria Causing Bovine Mastitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial Strains
2.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Determination
2.4. Preparation of Modified Rice Gel Containing Lactic Acid
2.5. Characterization of the Gel Formulation
2.6. The Time-Killed Analysis Determination
2.7. Detection of Bacterial Morphology by Scanning Electron Microscope (SEM)
2.8. Statistical Analysis
3. Results and Discussion
3.1. MIC and MBC Determination
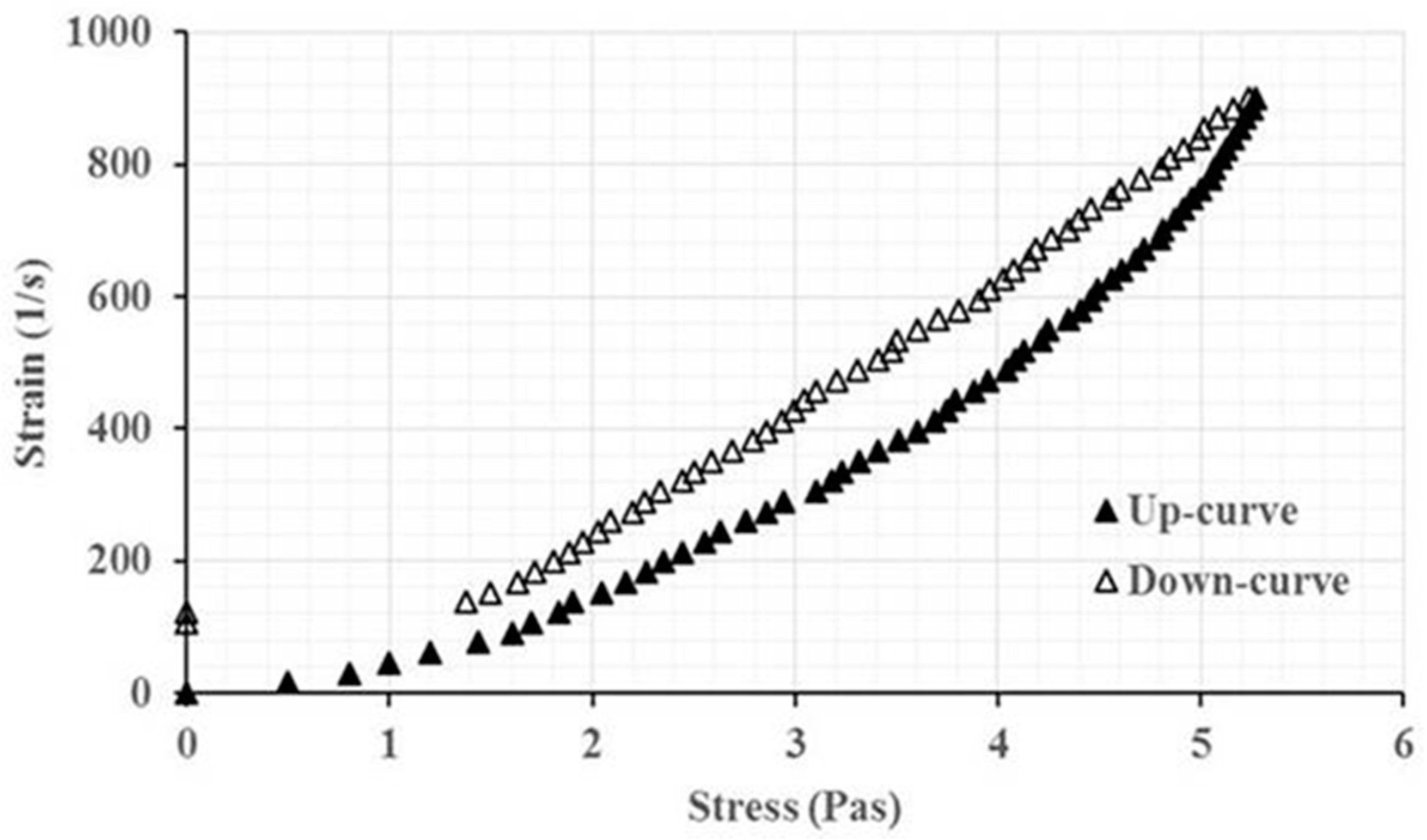
3.2. Preparation and Characterization of Modified Rice Gel Containing Lactic Acid
3.3. Time-Killed Analysis
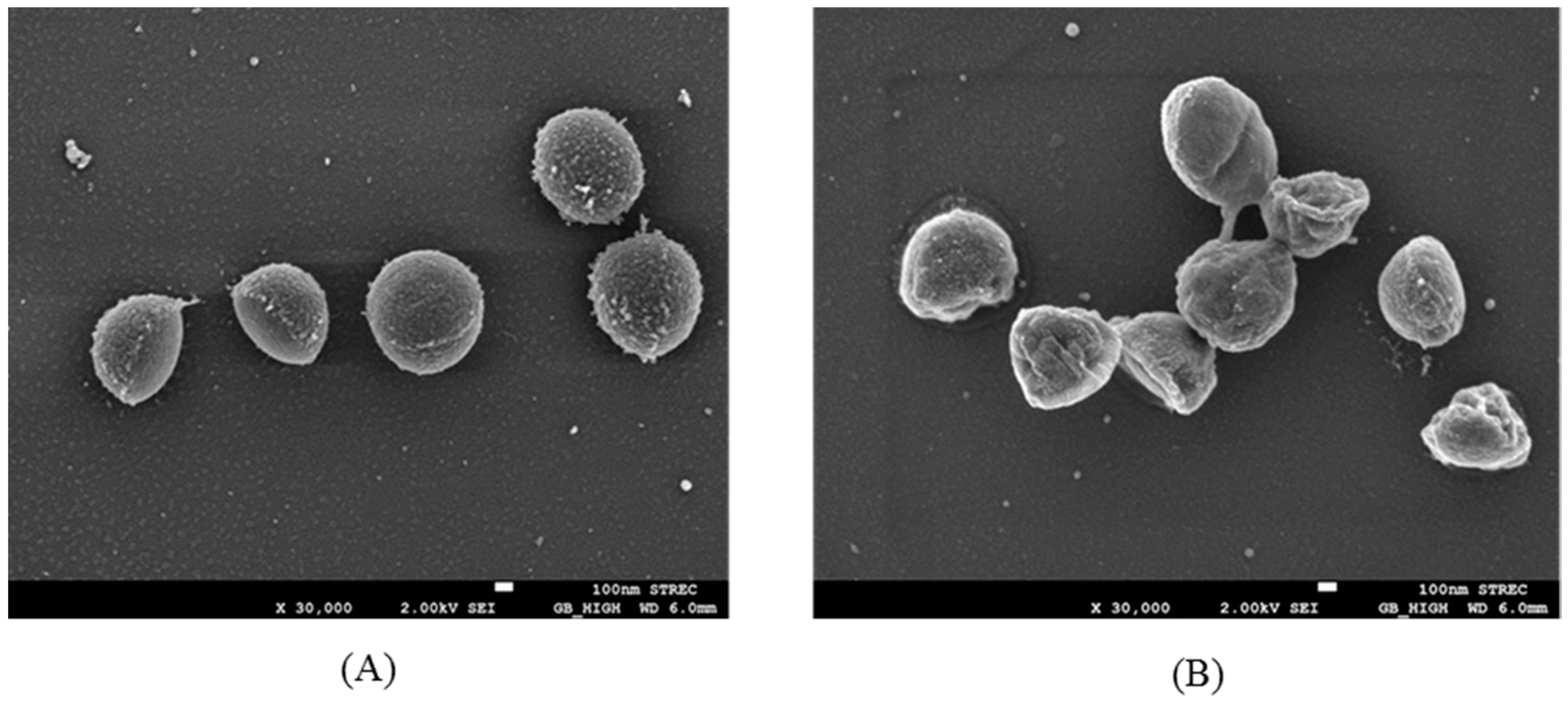
3.4. Bacterial Morphology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nagase, N.; Sasaki, A.; Yamashita, K.; Shimizu, A.; Wakita, Y.; Kitai, S.; Kawano, J. Isolation and species distribution of staphylococci from animal and human skin. J. Vet. Med. Sci. 2002, 64, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Nishikawa, A.; Suto, H.; Tsuboi, R.; Ogawa, H. Comparative study of Staphylococcus aureus isolated from the lesional and non-lesional skin of atopic dermatitis. Microbiol. Immunol. 2000, 44, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Stryjewski, M.E.; Corey, G.R. Methicillin-resistant Staphylococcus aureus: An evolving pathogen. Clin. Infect. Dis. 2014, 58, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ünal, N.; Yıldırım, M. Antibiotic resistance profiles of staphylococci species isolated from milk, teat skins and noses mucous of cows. Kafkas Univ. Vet. Fak. Derg. 2010, 16, 389–396. [Google Scholar] [CrossRef]
- Ajariyakhajorn, K.; Samngamnim, S.; Boonserm, T.; Inchaisri, C.; Thirapatsakun, T.; Farnsworth, R.J. Mastitis in small dairy holders of Nakhonpathom province, Thailand. In Proceedings of the 11th International Symposium of the World Association of Veterinary Laboratory Diagnosticians and OIE Seminar on Biotechnology, Bangkok, Thailand, 3 November 2003; pp. 122–123. [Google Scholar]
- Boonyayatra, S.; Chaisri, W. Incidence and prevalence of subclinical mastitis in small holder dairy farms of Chiang Mai province, Thailand. CMV J. 2004, 2, 25–30. [Google Scholar]
- Pumipuntu, N.; Kulpeanprasit, S.; Santajit, S.; Witawat, T.; Thida, K.; Woranich, H.; Nitaya, I. Screening method for Staphylococcus aureus identification in subclinical bovine mastitis from dairy farms. Vet. World 2017, 10, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Thorberg, B.M.; Danielsson-Tham, M.L.; Emanuelson, U.; Persson Waller, K. Bovine subclinical mastitis caused by different types of coagulase-negative staphylococci. J. Dairy Sci. 2009, 92, 4962–4970. [Google Scholar] [CrossRef] [PubMed]
- Björk, S.; Båge, R.; Kanyima, B.M.; André, S.; Nassuna-Musoke, M.G.; Owiny, D.O.; Persson, Y. Characterization of coagulase negative staphylococci from cases of subclinical mastitis in dairy cattle in Kampala, Uganda. Irish Vet J. 2014, 67, 12. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.; Cobb, E.; Donachie, P.; Judah, G.; Curtis, V.; Schmidt, W.P. The effect of handwashing with water or soap on bacterial contamination of hands. Int. J. Environ. Res. Public Health 2011, 8, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Mechanisms of bacterial resistance to biocides. Int. Biodeterior. Biodegrad. 1995, 36, 247–265. [Google Scholar] [CrossRef]
- Alsaheb, R.A.A.; Aladdin1, A.; Othman, N.Z.; Malek, R.A.; Leng, O.M.; Aziz, R.; Enshasy, H.A.E. Lactic acid applications in pharmaceutical and cosmeceutical industries. J. Chem. Pharm. Res. 2015, 7, 729–735. [Google Scholar]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium and sodium glycolates, methyl, ethyl, propyl and butyl glycolates and lactic acid, ammonium, calcium, potassium, sodium and TEA-lactates, methyl, ethyl, isopropyl and butyl lactates and lauryl, myristyl and cetyl lactates. Int. J. Toxicol. 1998, 17, 1–241. [Google Scholar]
- Rehman, K.; Zulfakar, M.H. Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Kaewpinta, A.; Yotsawimonwat, S.; Khongkhunthian, S. Preparation and characterization of lidocaine rice gel for oral application. Drug Discov. Ther. 2015, 9, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Okonogi, S.; Kaewpinta, A.; Khongkhunthian, S.; Yotsawimonwat, S. Effect of rice variety on the physicochemical properties of the modified rice powders and their derived mucoadhesive gels. Drug Discov. Ther. 2015, 9, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Ramalivhana, J.N.; Obi, C.L.; Samie, A.; Iweriebor, B.C.; Uaboi-Egbenni, P.; Idiaghe, J.E.; Momba, M.N.B. Antibacterial activity of honey and medicinal plant extracts against gram-negative microorganisms. Afr. J. Biotechnol. 2014, 13, 616–625. [Google Scholar] [CrossRef]
- Ren, C.; Fang, L.; Ling, L.; Wang, Q.; Liu, S.; Zhao, L.G.; He, Z. Design and evaluation of indapamide transdermal patch. Int. J. Pharm. 2009, 370, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kaloud, j.; Pokludova, L.; Novotna, R.; Cizek, A. Evaluation by conductance assay of shiga toxin producing Escherichia coli (STEC) O157 and O26 and their sensitivity to selected disinfectants. Acta Vet. Brno. 2003, 72, 101–109. [Google Scholar] [CrossRef]
- Haas, W.; Pillar, C.M.; Hesje, C.K.; Sanfilippo, C.M.; Morris, T.W. Bactericidal activity of besifloxacin against staphylococci, Streptococcus pneumoniae and Haemophilus influenza. J. Antimicrob. Chemother. 2010, 65, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghazaleh, B.M. Effects of ascorbic acid, citric acid, lactic acid, NaCl, potassium sorbate and Thymus vulgaris extract on Staphylococcus aureus and Escherichia coli. Afr. J. Microbiol. Res. 2013, 7, 7–12. [Google Scholar]
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial activity of lactic acid against pathogen and spoilage microorganisms. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Pundir, R.K.; Jain, P. Evaluation of five chemical food preservatives for their antibacterial activity against bacterial isolates from bakery products and mango pickles. J. Chem. Pharm. Res. 2011, 3, 24–31. [Google Scholar]
- Okonogi, S.; Khongkhunthien, S.; Jaturasitha, S. Development of mucoadhesive buccal films from rice for pharmaceutical delivery systems. Drug Discov. Ther. 2014, 8, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Neelam, K.; Vijay, S.; Lalit, S. Various techniques for the modification of starch and the applications of its derivatives. IRJP 2012, 3, 25–31. [Google Scholar]
- Lawal, O.S.; Lechner, M.D.; Kulicke, W.M. Single and multi-step carboxymethylation of water yam (Dioscorea alata) starch: Synthesis and characterization. Int. J. Biol. Macromol. 2008, 42, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.H.; Tang, J.; Paulson, A.T. Texture profile and turbidity of gellan/gelatin mixed gels. Food Res. Int. 2000, 33, 665–671. [Google Scholar] [CrossRef]
- Blazek, J.; Gilbert, E.P. Effect of enzymatic hydrolysis on native starch granule structure. Biomacromolecules 2010, 11, 3275–3289. [Google Scholar] [CrossRef] [PubMed]
- Smart, J.D. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 3, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Ahmad, S.; Mallick, M.N.; Manzoor, N.; Talegaonkar, S.; Iqbal, Z. Development of a novel synergistic thermosensitive gel for vaginal candidiasis: An in vitro, in vivo evaluation. Colloids Surf. B Biointerfaces 2013, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.B.; Bhaduri, I. Development and evaluation of Assam Bora rice starch-carbopol based oral mucoadhesive gel of irinotecan for mouth cancer. J. Pharm. Sci. Res. 2017, 9, 1139–1146. [Google Scholar]
- Verma, A.; Singh, S.; Kaur, R.; Jain, U.K. Topical gels as drug delivery systems: A review. Int. J. Pharm. Sci. Rev. Res. 2013, 23, 374–382. [Google Scholar]
- Chansiripornchai, P.; Pongsamart, S. Treatment of infected open wounds on two dogs using a film dressing of polysaccharide extracted from the hulls of durian (Durio zibethinus Murr.): Case report. Thai J. Vet. Med. 2008, 38, 55–61. [Google Scholar]
- Chansiripornchai, P.; Pramatwinai, C.; Rungsipipat, A. The efficiency of polysaccharide gel extracted from fruit-hulls of durian (Durio zibethinus L.) for wound healing in pig skin. Acta Hortic. 2005, 679, 37–43. [Google Scholar] [CrossRef]
- Khamlue, R.; Naksupan, N.; Ounaroon, A.; Saelim, N. Skin wound healing promoting effect of polysaccharides extracts from Tremella fuciformis and Auricularia auricula on the ex-vivo porcine skin wound healing model. In Proceedings of the 2012 4th International Conference on Chemical, Biological and Environmental Engineering, Phket, Thailand, 1–2 September 2012; Volume 43, pp. 93–98. [Google Scholar]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Microbiol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Warnecke, T.; Gill, R.T. Organic acid toxicity, tolerance and production in Escherichia coli biorefining applications. Microb. Cell Fact. 2005, 4, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [PubMed]



| Bacterial Strains | MIC (% v/v) | MBC (% v/v) |
|---|---|---|
| S. aureus ATCC 25923 | 0.5% | 0.5% |
| S. aureus (K14-001) | 0.5% | 0.5% |
| S. aureus (M15-166) | 0.5% | 0.5% |
| S. aureus (M16-066) | 0.5% | 0.5% |
| S. epidermidis (K14-004) | 0.5% | 0.5% |
| S. epidermidis (M15-028) | 0.5% | 0.5% |
| S. epidermidis (M15-031) | 0.5% | 0.5% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotigarpa, R.; Na Lampang, K.; Pikulkaew, S.; Okonogi, S.; Ajariyakhajorn, K.; Mektrirat, R. Inhibitory Effects and Killing Kinetics of Lactic Acid Rice Gel Against Pathogenic Bacteria Causing Bovine Mastitis. Sci. Pharm. 2018, 86, 29. https://doi.org/10.3390/scipharm86030029
Chotigarpa R, Na Lampang K, Pikulkaew S, Okonogi S, Ajariyakhajorn K, Mektrirat R. Inhibitory Effects and Killing Kinetics of Lactic Acid Rice Gel Against Pathogenic Bacteria Causing Bovine Mastitis. Scientia Pharmaceutica. 2018; 86(3):29. https://doi.org/10.3390/scipharm86030029
Chicago/Turabian StyleChotigarpa, Rinrada, Kannika Na Lampang, Surachai Pikulkaew, Siriporn Okonogi, Kittisak Ajariyakhajorn, and Raktham Mektrirat. 2018. "Inhibitory Effects and Killing Kinetics of Lactic Acid Rice Gel Against Pathogenic Bacteria Causing Bovine Mastitis" Scientia Pharmaceutica 86, no. 3: 29. https://doi.org/10.3390/scipharm86030029
APA StyleChotigarpa, R., Na Lampang, K., Pikulkaew, S., Okonogi, S., Ajariyakhajorn, K., & Mektrirat, R. (2018). Inhibitory Effects and Killing Kinetics of Lactic Acid Rice Gel Against Pathogenic Bacteria Causing Bovine Mastitis. Scientia Pharmaceutica, 86(3), 29. https://doi.org/10.3390/scipharm86030029





