Leishmanicidal Activity of Biogenic Fe3O4 Nanoparticles
Abstract
1. Introduction
2. Material and Methods
2.1. Biosynthesis of Fe3O4 Nanoparticles
2.2. Characterization Methods and Instruments
3. Results
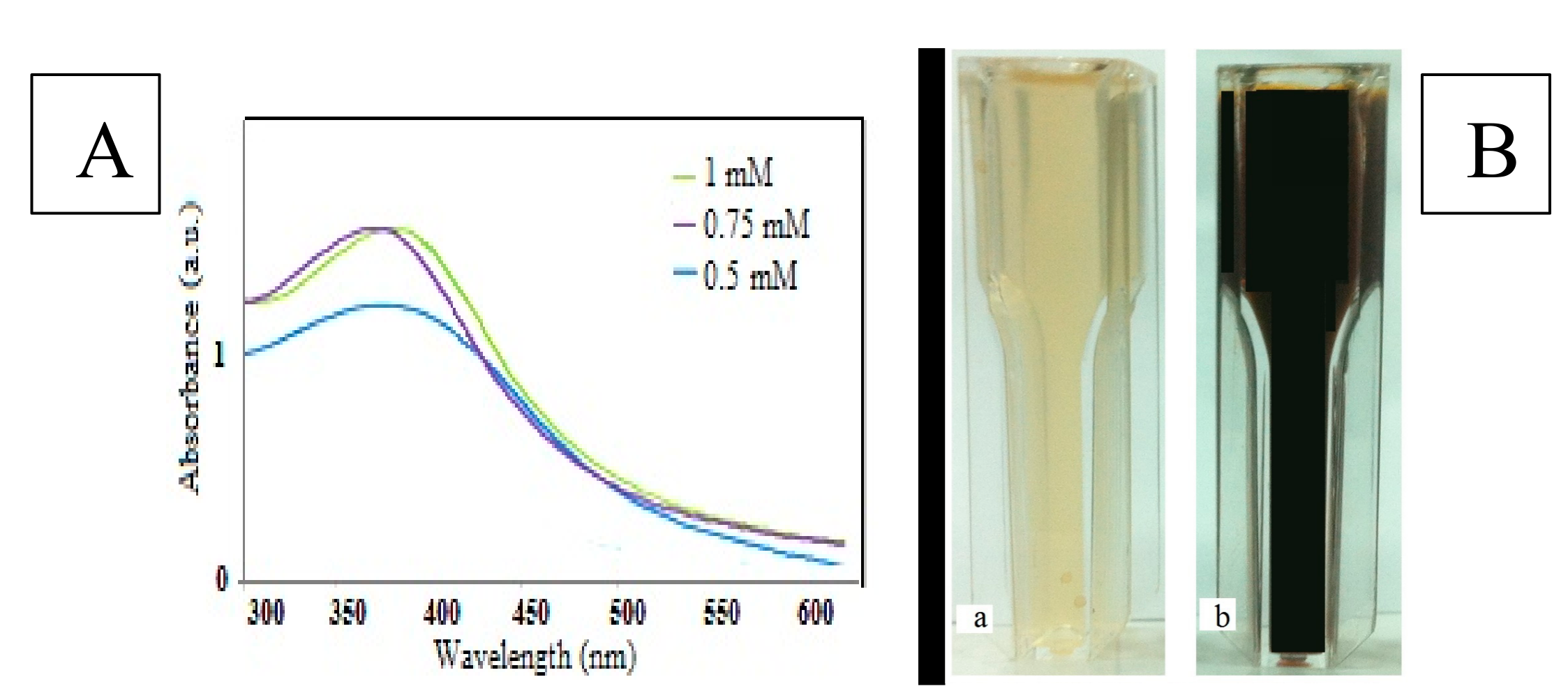
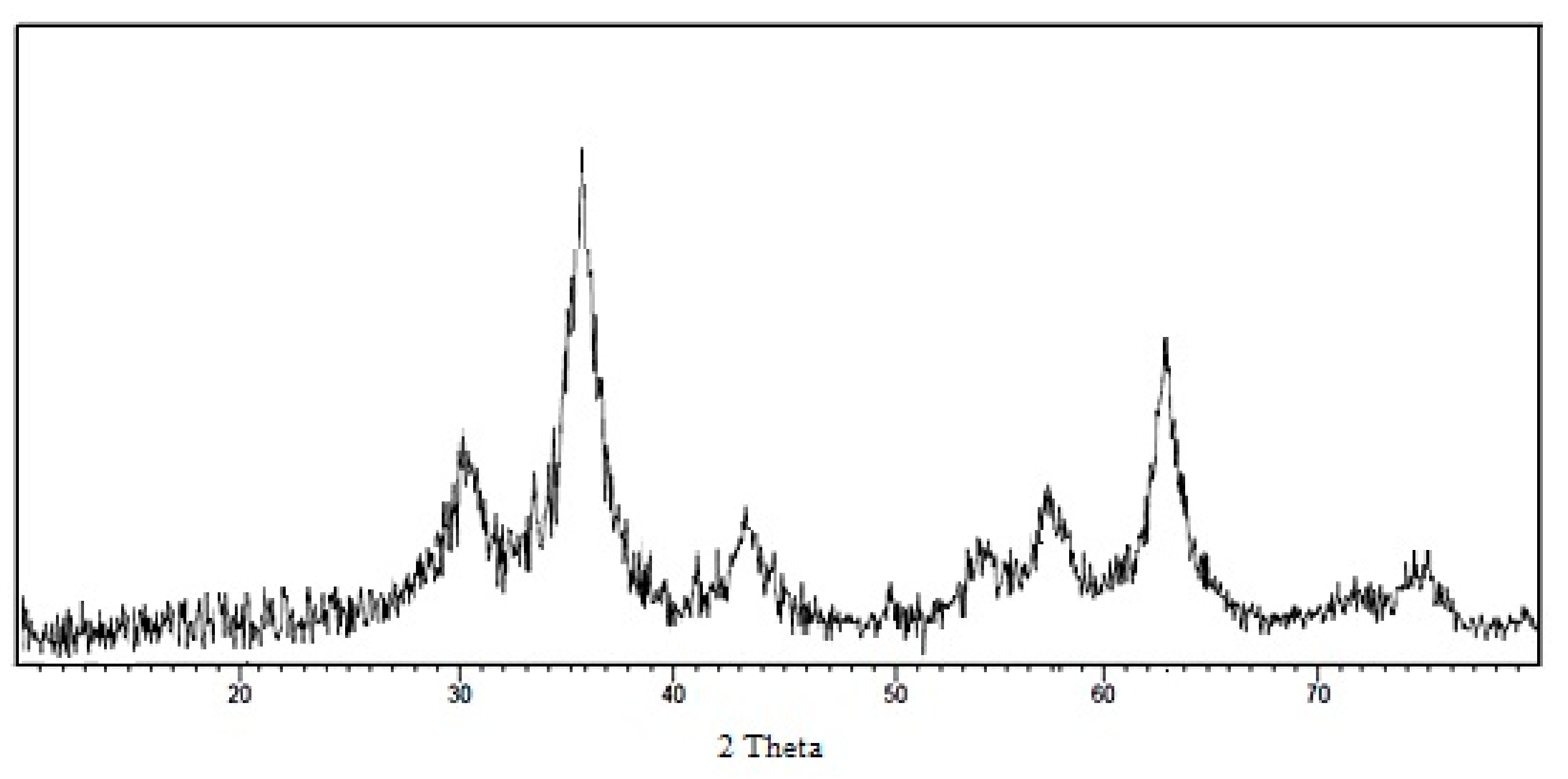
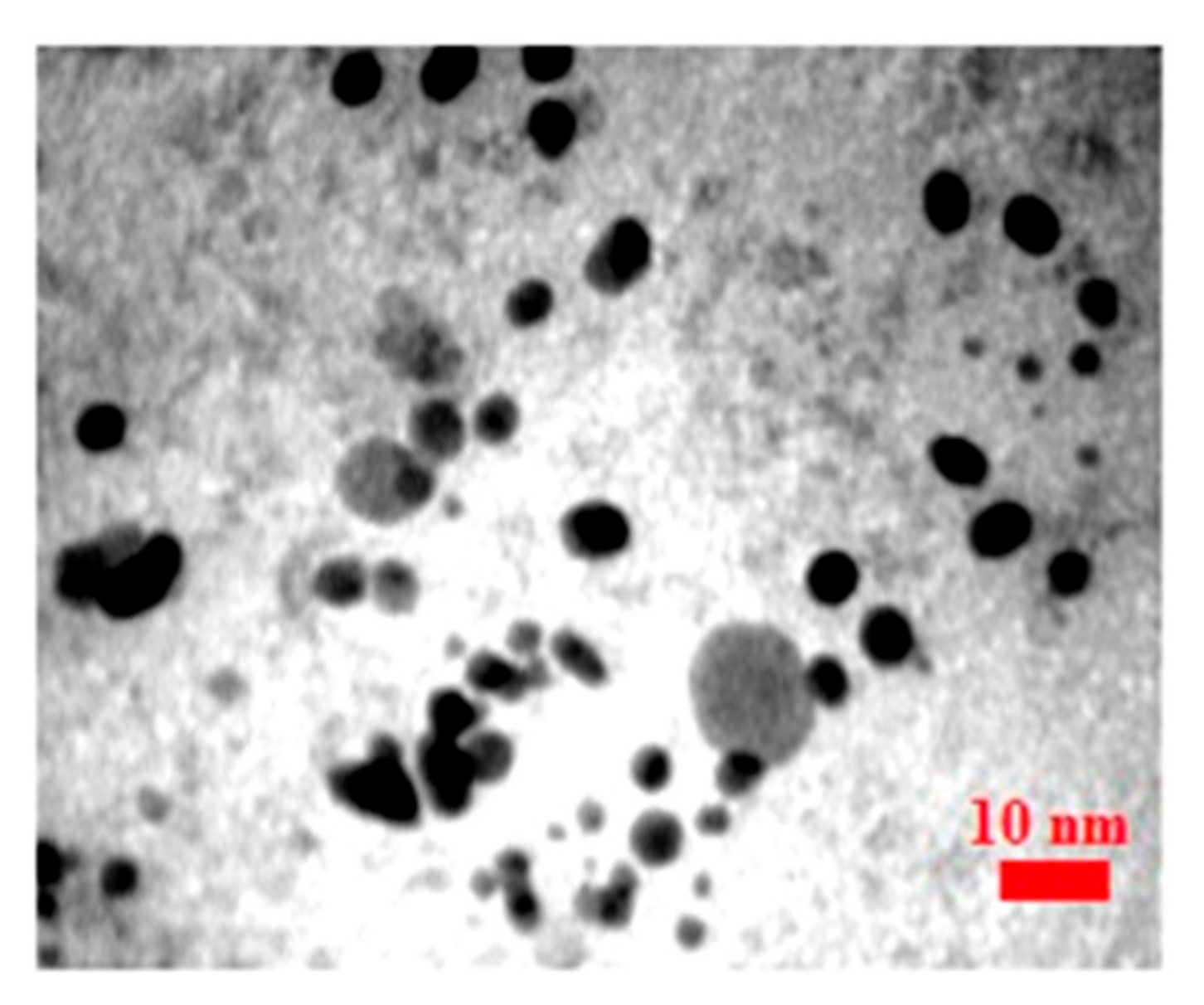
3.1. Characterization
3.2. Fourier Transform Infrared Spectroscopy Analysis
3.3. Antipromastigote Assay
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Mahmoud, W.; Elazzazy, A.M.; Danial, E.N. In Vitro Evaluation of Antioxidant, Biochemical and Antimicrobial Properties of Biosynthesized Silver Nanoparticles against Multidrug-Resistant Bacterial Pathogens. Biotechnol. Biotechnol. Equip. 2017, 31, 373–379. [Google Scholar] [CrossRef]
- Hameed, A.S.H.; Karthikeyan, C.; Ahamed, A.P.; Thajuddin, N.; Alharbi, N.S.; Alharbi, S.A.; Ravi, G. In Vitro Antibacterial Activity of ZnO and Nd Doped ZnO Nanoparticles against ESBL Producing Escherichia coli and Klebsiella pneumoniae. Sci. Rep. 2016, 6, 24312. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Tymczyna, L.; Chmielowiec-Korzeniowska, A.; Pulit-Prociak, J. Nanosilver Biocidal Properties and Their Application in Disinfection of Hatchers in Poultry Processing Plants. Bioinorg. Chem. Appl. 2016, 2016, 5214783. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Mortazavi, S.M.; Kishani-Farahani, Z.; Amini, A.; Amini, E.; Heli, H. Biosynthesis of Silver Nanoparticles Using Pine Pollen and Evaluation of the Antifungal Efficiency. Iran. J. Biotechnol. 2017, 15, 95–101. [Google Scholar] [CrossRef]
- Khatami, M.; Heli, H.; Jahani, P.M.; Azizi, H.; Nobre, M.A.L. Copper/Copper Oxide Nanoparticles Synthesis Using Stachys lavandulifolia and Its Antibacterial Activity. IET Nanobiotechnol. 2017, 11, 709–713. [Google Scholar] [CrossRef]
- Poor, M.H.S.; Khatami, M.; Azizi, H.; Abazari, Y. Cytotoxic Activity of Biosynthesized Ag Nanoparticles by Plantago Major Towards a Human Breast Cancer Cell Line. Rend. Lincei 2017, 28, 693–699. [Google Scholar] [CrossRef]
- Zare, E.; Pourseyedi, S.; Khatami, M.; Darezereshki, E. Simple Biosynthesis of Zinc Oxide Nanoparticles Using Nature’s Source, and It’s In Vitro Bio-Activity. J. Mol. Struct. 2017, 1146, 96–103. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Khatami, M.; Sharifi, I.; Heli, H.; Kaykavousi, K.; Poor, M.H.S.; Kharazi, S.; Nobre, M.A.L. Bacterial Biosynthesis of Gold Nanoparticles Using Salmonella enterica subsp. enterica serovar Typhi Isolated from Blood and Stool Specimens of Patients. J. Clust. Sci. 2017, 28, 2997–3007. [Google Scholar]
- Khatami, M.; Mehnipor, R.; Poor, M.H.S.; Jouzani, G.S. Facile Biosynthesis of Silver Nanoparticles Using Descurainia Sophia and Evaluation of Their Antibacterial and Antifungal Properties. J. Clust. Sci. 2016, 27, 1601–1612. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Banach, M. Silver Nanoparticles—A Material of the Future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Singh, P.; Singh, H.; Castro-Aceituno, V.; Ahn, S.; Kim, Y.J.; Farh, M.E.-A.; Yang, D.C. Engineering of Mesoporous Silica Nanoparticles for Release of Ginsenoside Ck and Rh2 to Enhance Their Anticancer and Anti-Inflammatory Efficacy: In Vitro Studies. J. Nanopart. Res. 2017, 19, 257. [Google Scholar] [CrossRef]
- Selot, R.; Marepally, S.; Vemula, P.K.; Jayandharan, G.R. Nanoparticle Coated Viral Vectors for Gene Therapy. Curr. Biotechnol. 2016, 5, 44–53. [Google Scholar] [CrossRef]
- Junejo, Y.; Güner, A.; Baykal, A. Synthesis and Characterization of Amoxicillin Derived Silver Nanoparticles: Its Catalytic Effect on Degradation of Some Pharmaceutical Antibiotics. Appl. Surf. Sci. 2014, 317, 914–922. [Google Scholar] [CrossRef]
- Güner, A.; Çevik, E.; Şenel, M.; Alpsoy, L. An Electrochemical Immunosensor for Sensitive Detection of Escherichia coli O157:H7 by Using Chitosan, MWCNT, Polypyrrole with Gold Nanoparticles Hybrid Sensing Platform. Food Chem. 2017, 229, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Singh, P.; Castro-Aceituno, V.; Yesmin Simu, S.; Kim, Y.-J.; Mathiyalagan, R.; Yang, D.-C. Gold Nanoparticles Synthesized Using Panax Ginseng Leaves Suppress Inflammatory-Mediators Production Via Blockade of Nf-Κb Activation in Macrophages. Artif. Cells Nanomed. Biotechnol. 2017, 45, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.C.; Zhang, Q.; Manthiram, K.; Ye, X.; Lomont, J.P.; Harris, C.B.; Weller, H.; Alivisatos, A.P. Study of Heat Transfer Dynamics from Gold Nanorods to the Environment Via Time-Resolved Infrared Spectroscopy. ACS Nano 2016, 10, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Pulit, J.; Banach, M. Preparation of Nanosilver and Nanogold Based on Dog Rose Aqueous Extract. Bioinorg. Chem. Appl. 2014, 2014, 658935. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Cui, H. Functional Nanoparticles for Magnetic Resonance Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 814–841. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Bae, Y.; Pittman, T.A.; Yokel, R.A. Alternating Magnetic Field-Induced Hyperthermia Increases Iron Oxide Nanoparticle Cell Association/Uptake and Flux in Blood-Brain Barrier Models. Pharm. Res. 2015, 32, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.A.; Dai, Q.; Sarsons, C.D.; Chen, J.; Rocheleau, J.V.; Hwang, D.M.; Zheng, G.; Cramb, D.T.; Rinker, K.D.; Chan, W.C. Tailoring Nanoparticle Designs to Target Cancer Based on Tumor Pathophysiology. Proc. Natl. Acad. Sci. USA 2016, 113, E1142–E1151. [Google Scholar] [CrossRef] [PubMed]
- Parvanian, S.; Mostafavi, S.M.; Aghashiri, M. Multifunctional Nanoparticle Developments in Cancer Diagnosis and Treatment. Sens. Bio-Sens. Res. 2017, 13, 81–87. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Sajadi, S.M. Green Synthesis of the Cu/Fe3O4 Nanoparticles Using Morinda Morindoides Leaf Aqueous Extract: A Highly Efficient Magnetically Separable Catalyst for the Reduction of Organic Dyes in Aqueous Medium at Room Temperature. Appl. Surf. Sci. 2016, 364, 636–644. [Google Scholar] [CrossRef]
- Lunge, S.; Singh, S.; Sinha, A. Magnetic Iron Oxide (Fe3O4) Nanoparticles from Tea Waste for Arsenic Removal. J. Magn. Magn. Mater. 2014, 356, 21–31. [Google Scholar] [CrossRef]
- Cheera, P.; Karlapudi, S.; Sellola, G.; Ponneri, V. A Facile Green Synthesis of Spherical Fe3O4 Magnetic Nanoparticles and Their Effect on Degradation of Methylene Blue in Aqueous Solution. J. Mol. Liq. 2016, 221, 993–998. [Google Scholar] [CrossRef]
- Tuček, J.; Nejad, M.S.; Bonjar, G.H.S.; Khatami, M.; Amini, A.; Aghighi, S. In vitro and in vivo antifungal properties of silver nanoparticles against Rhizoctonia solani, a common agent of rice sheath blight disease. IET Nanobiotechnol. 2017, 11, 236–240. [Google Scholar]
- Hufschmid, R.; Arami, H.; Ferguson, R.M.; Gonzales, M.; Teeman, E.; Brush, L.N.; Browning, N.D.; Krishnan, K.M. Synthesis of Phase-Pure and Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition. Nanoscale 2015, 7, 11142–11154. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, W.; Fellows, B.; Qi, B.; Darroudi, T.; Kitchens, C.; Ye, L.; Crawford, T.M.; Mefford, O.T. Continuous Synthesis of Iron Oxide (Fe3O4) Nanoparticles via Thermal Decomposition. Particuology 2016, 26, 47–53. [Google Scholar] [CrossRef]
- Jahani, S.; Khorasani-Motlagh, M.; Noroozifar, M. DNA Interaction of Europium(Iii) Complex Containing 2,2′-Bipyridine and Its Antimicrobial Activity. J. Biomol. Struct. Dyn. 2016, 34, 612–624. [Google Scholar] [CrossRef] [PubMed]
- El-Kassas, H.Y.; Aly-Eldeen, M.A.; Gharib, S.M. Green Synthesis of Iron Oxide (Fe3O4) Nanoparticles Using Two Selected Brown Seaweeds: Characterization and Application for Lead Bioremediation. Acta Oceanol. Sin. 2016, 35, 89–98. [Google Scholar] [CrossRef]
- Hajesmaeelzadeh, F.; Shanehsazzadeh, S.; Grüttner, C.; Daha, F.J.; Oghabian, M.A. Effect of Coating Thickness of Iron Oxide Nanoparticles on Their Relaxivity in the Mri. Iran. J. Basic Med. Sci. 2016, 19, 166–171. [Google Scholar] [PubMed]
- Zhang, Z.; Hu, Y.; Yang, J.; Xu, Y.; Zhang, C.; Wang, Z.; Shi, X.; Zhang, G. Facile Synthesis of Folic Acid-Modified Iron Oxide Nanoparticles for Targeted Mr Imaging in Pulmonary Tumor Xenografts. Mol. Imaging Biol. 2016, 18, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Tajik, S.; Jahani, S. Electrocatalytic Determination of Hydrazine and Phenol Using a Carbon Paste Electrode Modified with Ionic Liquids and Magnetic Core-Shell Fe3o4@Sio2/Mwcnt Nanocomposite. Electroanalysis 2016, 28, 1093–1099. [Google Scholar] [CrossRef]
- Genena, A.K.; Hense, H.; Junior, A.S.; de Souza, S.M. Rosemary (Rosmarinus officinalis): A Study of the Composition, Antioxidant and Antimicrobial Activities of Extracts Obtained with Supercritical Carbon Dioxide. Food Sci. Technol. 2008, 28, 463–469. [Google Scholar] [CrossRef]
- Outaleb, T.; Hazzit, M.; Ferhat, Z.; Baaliouamer, A.; Yekkour, A.; Zitouni, A.; Sabaou, N. Composition, Antioxidant and Antimicrobial Activities of Algerian Rosmarinus officinalis L. Extracts. J. Essent. Oil Bear. Plants 2015, 18, 654–665. [Google Scholar] [CrossRef]
- Khanna-Chopra, R.; Semwal, V.K. Superoxide Dismutase and Ascorbate Peroxidase Are Constitutively More Thermotolerant Than Other Antioxidant Enzymes in Chenopodium Album. Phys. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2011, 17, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Groiss, S.; Selvaraj, R.; Varadavenkatesan, T.; Vinayagam, R. Structural Characterization, Antibacterial and Catalytic Effect of Iron Oxide Nanoparticles Synthesised Using the Leaf Extract of Cynometra Ramiflora. J. Mol. Struct. 2017, 1128 (Suppl. C), 572–578. [Google Scholar] [CrossRef]
- Sharifi, F.; Sharififar, F.; Sharifi, I.; Alijani, H.; Khatami, M. Cytotoxicity, leishmanicidal, and antioxidant activity of biosynthesized zinc sulfide nanoparticles using Phoenix dactylifera. IET Nanobiotechnol. 2017. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Ezzatkhah, F.; Sharififar, F.; Sharifi, I.; Dezaki, E.S. Antileishmanial and Cytotoxic Effects of Essential Oil and Methanolic Extract of Myrtus communis L. Korean J. Parasitol. 2015, 53, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Arakha, M.; Pal, S.; Samantarrai, D.; Panigrahi, T.K.; Mallick, B.C.; Pramanik, K.; Mallick, B.; Jha, S. Antimicrobial Activity of Iron Oxide Nanoparticle Upon Modulation of Nanoparticle-Bacteria Interface. Sci. Rep. 2015, 5, 14813. [Google Scholar] [CrossRef] [PubMed]
- Barapatre, A.; Aadil, K.R.; Jha, H. Synergistic Antibacterial and Antibiofilm Activity of Silver Nanoparticles Biosynthesized by Lignin-Degrading Fungus. Bioresour. Bioprocess. 2016, 3, 8. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. Green Synthesis of Silver Nanoparticles Using Azadirachta Indica Aqueous Leaf Extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Nejad, M.S.; Khatami, M.; Bonjar, G.H.S. Extracellular Synthesis Gold Nanotriangles Using Biomass of Streptomyces microflavus. IET Nanobiotechnol. 2016, 10, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, M.; Mir, N.; Mousavi-Kamazani, M.; Bagheri, S.; Salavati-Niasari, M. Biosynthesis and Characterization of Silver Nanoparticles Prepared from Two Novel Natural Precursors by Facile Thermal Decomposition Methods. Sci. Rep. 2016, 6, 32539. [Google Scholar] [CrossRef] [PubMed]
- Lateef, A.; Akande, M.A.; Ojo, S.A.; Folarin, B.I.; Gueguim-Kana, E.B.; Beukes, L.S. Paper Wasp Nest-Mediated Biosynthesis of Silver Nanoparticles for Antimicrobial, Catalytic, Anticoagulant, and Thrombolytic Applications. 3 Biotech 2016, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Dabirzadeh, M.; Sharifi, I.; Babaei, Z. Leishmania Major: Genetic Profiles of the Parasites Isolated from Chabahar, Southeastern Iran by Ppip-Pcr. Iran. J. Parasitol. 2016, 11, 290–295. [Google Scholar] [PubMed]
- Sundaram, P.A.; Augustine, R.; Kannan, M. Extracellular Biosynthesis of Iron Oxide Nanoparticles by Bacillus Subtilis Strains Isolated from Rhizosphere Soil. Biotechnol. Bioprocess Eng. 2012, 17, 835–840. [Google Scholar] [CrossRef]
- Behera, S.S.; Patra, J.K.; Pramanik, K.; Panda, N.; Thatoi, H. Characterization and Evaluation of Antibacterial Activities of Chemically Synthesized Iron Oxide Nanoparticles. J. Nano Sci. Eng. 2012, 2, 196–200. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Magnetic Properties and Biological Activity Evaluation of Iron Oxide Nanoparticles. J. Nanomater. 2013, 2013, 893970. [Google Scholar] [CrossRef]
- Mahdavi, M.; Namvar, F.; Ahmad, M.; Mohamad, R. Green Biosynthesis and Characterization of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using Seaweed (Sargassum muticum) Aqueous Extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Mir, A.; Mallik, D.; Sinha, A.; Nayar, S.; Webster, T.J. Bactericidal Effect of Iron Oxide Nanoparticles on Staphylococcus aureus. Int. J. Nanomed. 2010, 5, 277–283. [Google Scholar]
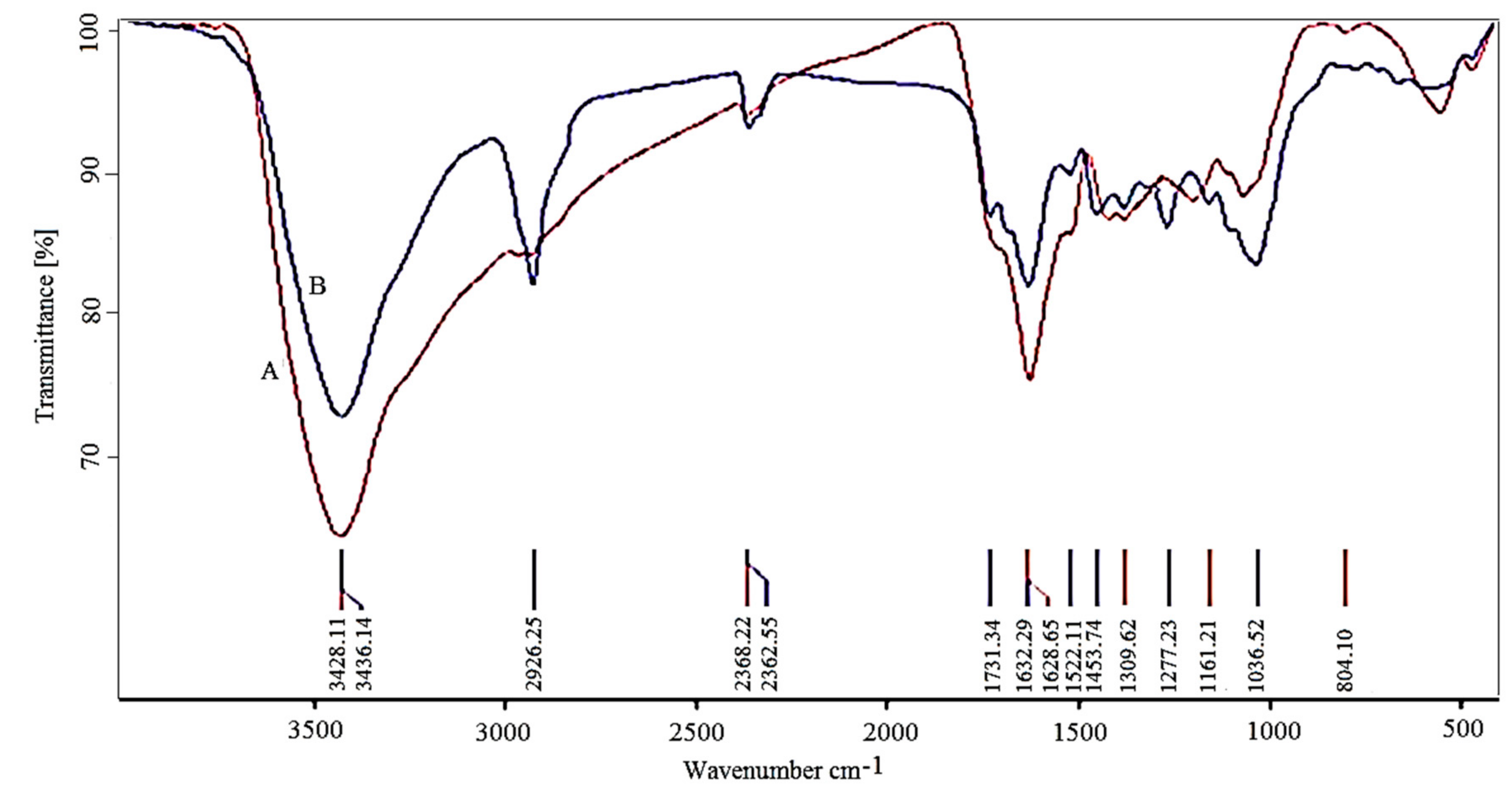

| Band (cm−1) | Assigned or Associated | Reference |
|---|---|---|
| 556 and 472 | Metal oxygen (Fe–O) | [40] |
| 1036 | C–O | [10] |
| 1277 | C–N aromatic and aliphatic amines | [41] |
| 1632 | Carbonyl (–C=O) group stretching vibration | [42] |
| 2362 | CH stretching | [43] |
| 2926 | Asymmetric stretching of C–H | [44] |
| 3426 | N–H stretching vibration of group NH2 and O–H | [42,45] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatami, M.; Alijani, H.; Sharifi, I.; Sharifi, F.; Pourseyedi, S.; Kharazi, S.; Lima Nobre, M.A.; Khatami, M. Leishmanicidal Activity of Biogenic Fe3O4 Nanoparticles. Sci. Pharm. 2017, 85, 36. https://doi.org/10.3390/scipharm85040036
Khatami M, Alijani H, Sharifi I, Sharifi F, Pourseyedi S, Kharazi S, Lima Nobre MA, Khatami M. Leishmanicidal Activity of Biogenic Fe3O4 Nanoparticles. Scientia Pharmaceutica. 2017; 85(4):36. https://doi.org/10.3390/scipharm85040036
Chicago/Turabian StyleKhatami, Mehrdad, Hajar Alijani, Iraj Sharifi, Fatemeh Sharifi, Shahram Pourseyedi, Sam Kharazi, Marcos Augusto Lima Nobre, and Manouchehr Khatami. 2017. "Leishmanicidal Activity of Biogenic Fe3O4 Nanoparticles" Scientia Pharmaceutica 85, no. 4: 36. https://doi.org/10.3390/scipharm85040036
APA StyleKhatami, M., Alijani, H., Sharifi, I., Sharifi, F., Pourseyedi, S., Kharazi, S., Lima Nobre, M. A., & Khatami, M. (2017). Leishmanicidal Activity of Biogenic Fe3O4 Nanoparticles. Scientia Pharmaceutica, 85(4), 36. https://doi.org/10.3390/scipharm85040036







