In Vitro Activities of Enantiopure and Racemic 1′-Acetoxychavicol Acetate against Clinical Isolates of Mycobacterium tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Condition
2.2. Extraction and Purification of ACA
2.3. Minimum Inhibitory Concentration Determination Using Microplate Alamar Blue Assay
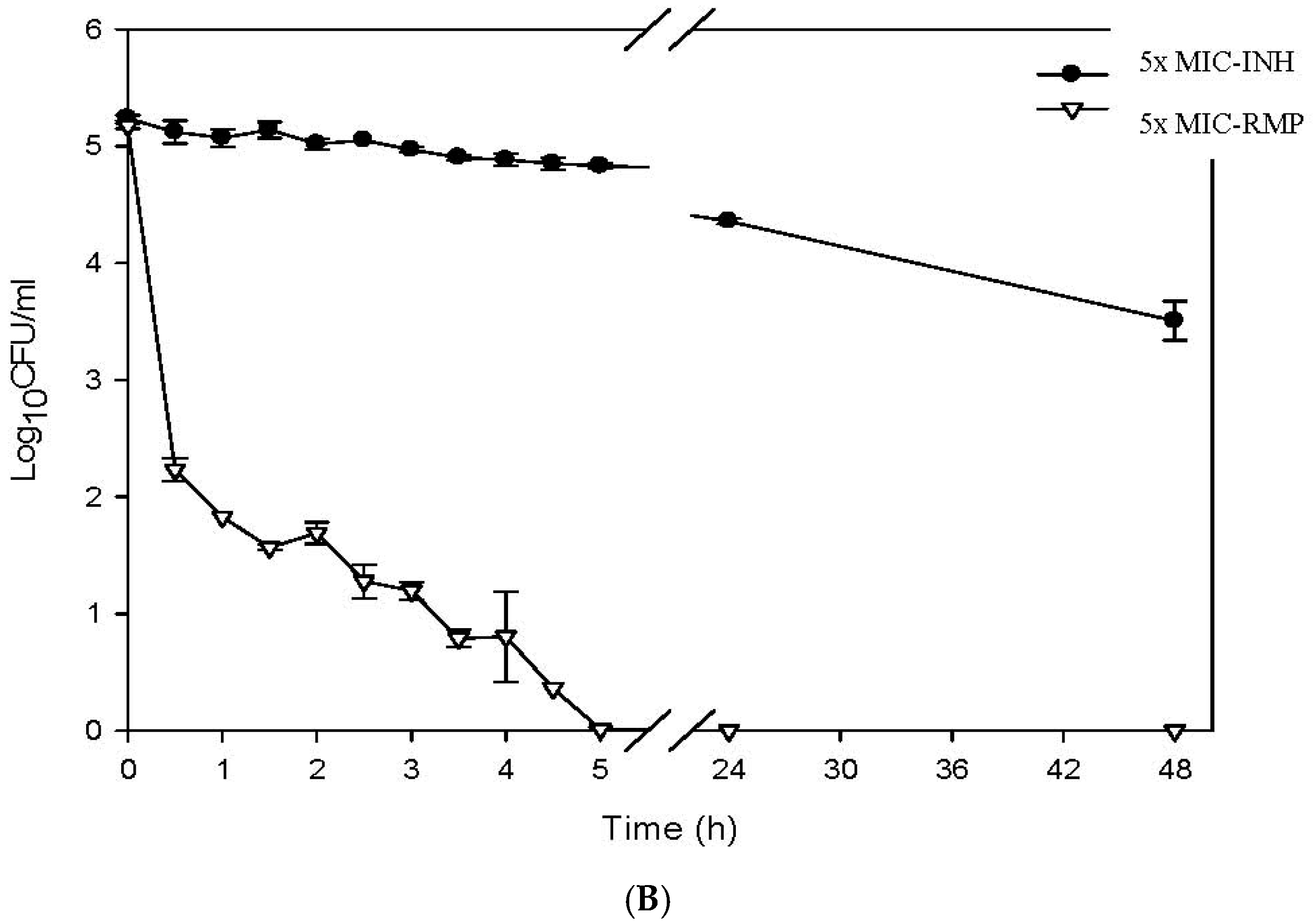
2.4. Determination of the Killing Curve of ACA against M. tuberculosis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Organization WHO. Global Tuberculosis Report 2015; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- Palittapongarnpim, P.; Kirdmanee, C.; Kittakoop, P.; Rukseree, K. 1’-Acetoxychavicol Acetate for Tuberculosis Treatment. U.S. Patent 20,020,192,262, 19 December 2002. [Google Scholar]
- Phongpaichit, S.; Vuddhakul, V.; Subhadhirasakul, S.; Wattanapiromsakul, C. Evaluation of the antimycobacterial activity of extracts from plants used as self-medication by aids patients in Thailand. Pharm. Biol. 2006, 44, 71–75. [Google Scholar] [CrossRef]
- Gautam, R.; Saklani, A.; Jachak, S.M. Indian medicinal plants as a source of antimycobacterial agents. J. Ethnopharmacol. 2007, 110, 200–234. [Google Scholar] [CrossRef] [PubMed]
- Soundhari, C.; Rajarajan, S. In vitro screening of lyophilised extracts of Alpinia galanga L. and Oldenlandia umbellata L. For antimycobacterial activity. Int. J. Biol. Pharm. Res. 2013, 4, 427–432. [Google Scholar]
- Gupta, P.; Bhatter, P.; D’souza, D.; Tolani, M.; Daswani, P.; Tetali, P.; Birdi, T. Evaluating the anti mycobacterium tuberculosis activity of Alpinia galanga (L.) Willd. Axenically under reducing oxygen conditions and in intracellular assays. BMC Complement. Altern. Med. 2014, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Uhl, J.; Kline, B.; Cockerill, F. Assessment of invasion frequencies of cultured HEp-2 cells by clinical isolates of Helicobacter pylori using an acridine orange assay. J. Clin. Pathol. 1998, 51, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Janssen, A.; Scheffer, J. Acetoxychavicol acetate, an antifungal component of Alpinia galanga. Plant. Med. 1985, 51, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Ch, B.; Narasu, L.M.; Giri, A. Antibacterial activity of Alpinia galanga (L) Willd crude extracts. Appl. Biochem. Biotechnol. 2010, 162, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Noro, T.; Sekiya, T.; Katoh, M.; Oda, Y.; Miyase, T.; Kuroyanagi, M.; Ueno, A.; Fukushima, S. Inhibitors of xanthine oxidase from Alpinia galanga. Chem. Pharm. Bull. 1988, 36, 244–248. [Google Scholar] [CrossRef]
- Oonmetta-Aree, J.; Suzuki, T.; Gasaluck, P.; Eumkeb, G. Antimicrobial properties and action of galangal (Alpinia galanga Linn.) on Staphylococcus aureus. LWT-Food Sci. Technol. 2006, 39, 1214–1220. [Google Scholar] [CrossRef]
- Yang, X.; Eilerman, R.G. Pungent principal of Alpinia galangal (L.) swartz and its applications. J. Agric. Food Chem. 1999, 47, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Jaki, B.; Franzblau, S.; Pauli, G.F. An NMR method towards the routine chiral determination of natural products. Phytochem. Anal. 2004, 15, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Li, B. 1’ s-1’-acetoxychavicol acetate isolated from Alpinia galanga inhibits human immunodeficiency virus type 1 replication by blocking rev transport. J. Gen. Virol. 2006, 87, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Yaku, K.; Matsui-Yuasa, I.; Azuma, H.; Kojima-Yuasa, A. 1’-Acetoxychavicol acetate enhances the phase II enzyme activities via the increase in intranuclear Nrf2 level and cytosolic p21 level. Am. J. Chin. Med. 2011, 39, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, H.S.; Franzblau, S. Microplate alamar blue assay (MABA) and low oxygen recovery assay (LORA) for Mycobacterium tuberculosis. Methods Mol. Biol. 2015, 1285, 281–292. [Google Scholar]
- Diacon, A.; Maritz, J.; Venter, A.; Van Helden, P.; Andries, K.; McNeeley, D.; Donald, P. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Khajuria, B.; Chopra, C. Antibacterial properties of volatile principles from Alpinia galanga and Acorus calamus. Antibiot. Chemother. 1957, 7, 378. [Google Scholar]
- Mokkhasmit, M.; Swatdimongkol, K.; Satrawaha, P. Study on toxicity of thai medicinal plants. Bull. Dept. Med. Sci. 1971, 12, 36–65. [Google Scholar]
- Mori, H.; Kubota, K.; Kobayashi, A. Potent aroma components of rhizomes from Alpinia galanga Willd. L. J. Jpn. Soc. Food Sci. Technol. 1995, 42, 989–995. [Google Scholar] [CrossRef]
- Sagawa, M.; Tabayashi, T.; Kimura, Y.; Tomikawa, T.; Nemoto-Anan, T.; Watanabe, R.; Tokuhira, M.; Ri, M.; Hashimoto, Y.; Iida, S. TM-233, a novel analog of 1’-acetoxychavicol acetate, induces cell death in myeloma cells by inhibiting both JAK/STAT and proteasome activities. Cancer Sci. 2015, 106, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, S.; Kobayashi, S.; Nagahori, H.; Ogiso, A. Constituents from seeds of Alpinia galanga wild. and their anti-ulcer activities. Chem. Pharm. Bull. 1976, 24, 2377–2382. [Google Scholar] [CrossRef] [PubMed]
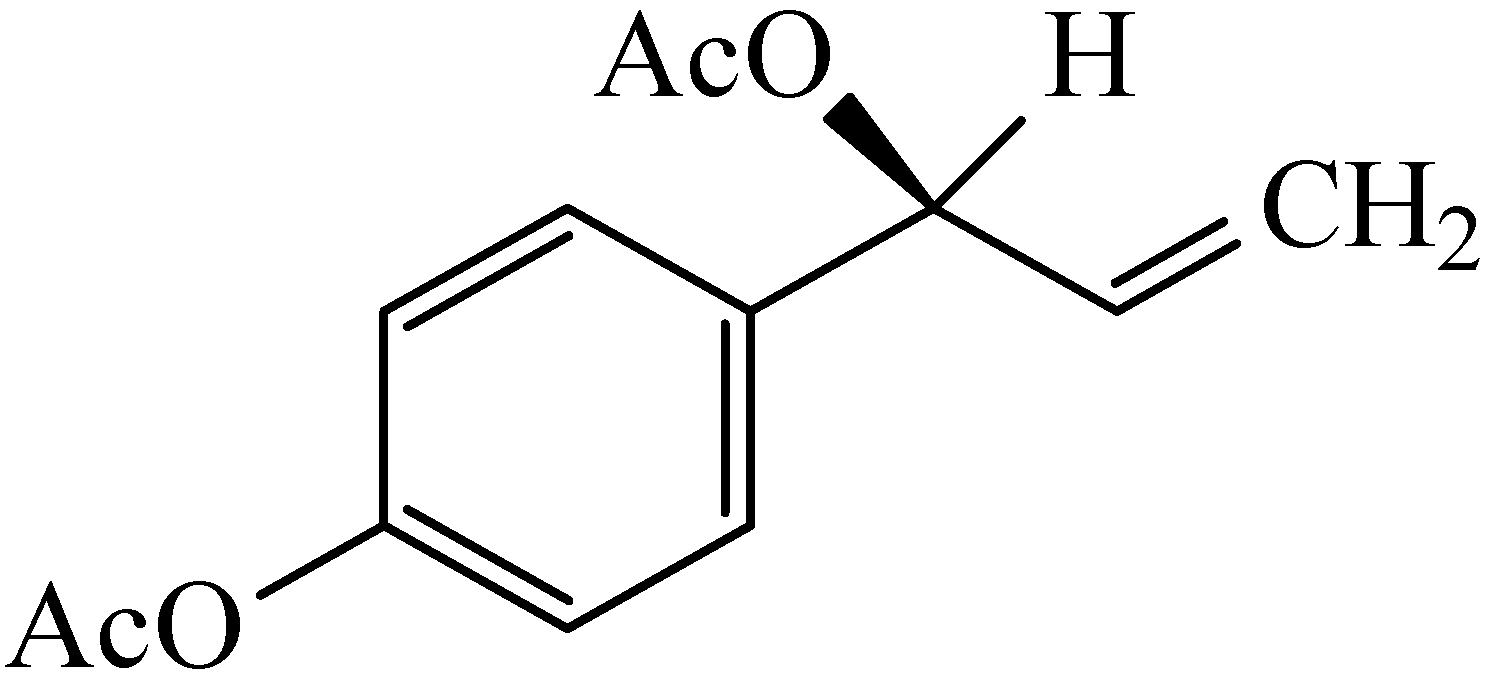

| Compound | MIC (µg/mL) | |
|---|---|---|
| H37Ra ATCC 25177 | H37Rv ATCC 27294 | |
| S-ACA | 0.2 | 0.7 |
| rac-ACA | 0.5 | 2.7 |
| RMP | 0.005 | 0.1 |
| Groups of Clinical Isolates | MIC Values of S-ACA (μg/mL) | Number of Clinical Isolates | |||
|---|---|---|---|---|---|
| 0.25 | 0.5 | 1.0 | 2.0 | ||
| Drug susceptible | 4 (4%) | 33 (33%) | 62 (62%) | 1 (1%) | 100 |
| Mono-resistant | 2 (10%) | 6 (30%) | 11 (55%) | 1 (5%) | 20 |
| MDR | 2 (6.7%) | 11 (36.7%) | 16 (53.3%) | 1 (3.3%) | 30 |
| Total | 8 (5.3%) | 50 (33.3%) | 89 (59.3%) | 3 (2%) | 150 |
| Groups of Clinical Isolates | MIC Values of rac-ACA (μg/mL) | Number of Clinical Isolates | |||||
|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1.0 | 2.0 | 4.0 | 16 | ||
| RMP-OFX-resistant | 0 | 0 | 0 | 1 (100%) | 0 | 0 | 1 |
| MDR | 0 | 1 (2.5%) | 25 (62.5%) | 13 (32.5%) | 1 (2.5%) | 0 | 40 |
| Pre-XDR | 0 | 2 (8%) | 7 (29%) | 14 (58%) | 0 | 1 (4%) | 24 |
| XDR | 0 | 4 (14%) | 12 (43%) | 12 (43%) | 0 | 0 | 28 |
| Total | 0 | 7 (8%) | 44 (47%) | 40 (43%) | 1 (1%) | 1 (1%) | 93 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warit, S.; Rukseree, K.; Prammananan, T.; Hongmanee, P.; Billamas, P.; Jaitrong, S.; Chaiprasert, A.; Jaki, B.U.; Pauli, G.F.; Franzblau, S.G.; et al. In Vitro Activities of Enantiopure and Racemic 1′-Acetoxychavicol Acetate against Clinical Isolates of Mycobacterium tuberculosis. Sci. Pharm. 2017, 85, 32. https://doi.org/10.3390/scipharm85030032
Warit S, Rukseree K, Prammananan T, Hongmanee P, Billamas P, Jaitrong S, Chaiprasert A, Jaki BU, Pauli GF, Franzblau SG, et al. In Vitro Activities of Enantiopure and Racemic 1′-Acetoxychavicol Acetate against Clinical Isolates of Mycobacterium tuberculosis. Scientia Pharmaceutica. 2017; 85(3):32. https://doi.org/10.3390/scipharm85030032
Chicago/Turabian StyleWarit, Saradee, Kamolchanok Rukseree, Therdsak Prammananan, Poonpilas Hongmanee, Pamaree Billamas, Sarinya Jaitrong, Angkana Chaiprasert, Birgit U. Jaki, Guido F. Pauli, Scott G. Franzblau, and et al. 2017. "In Vitro Activities of Enantiopure and Racemic 1′-Acetoxychavicol Acetate against Clinical Isolates of Mycobacterium tuberculosis" Scientia Pharmaceutica 85, no. 3: 32. https://doi.org/10.3390/scipharm85030032
APA StyleWarit, S., Rukseree, K., Prammananan, T., Hongmanee, P., Billamas, P., Jaitrong, S., Chaiprasert, A., Jaki, B. U., Pauli, G. F., Franzblau, S. G., & Palittapongarnpim, P. (2017). In Vitro Activities of Enantiopure and Racemic 1′-Acetoxychavicol Acetate against Clinical Isolates of Mycobacterium tuberculosis. Scientia Pharmaceutica, 85(3), 32. https://doi.org/10.3390/scipharm85030032






