Dehydroepiandrosterone (DHEA) Feeding Protects Liver Steatosis in Obese Breast Cancer Rat Model
Abstract
:1. Introduction
2. Results
2.1. Body and Liver Weights
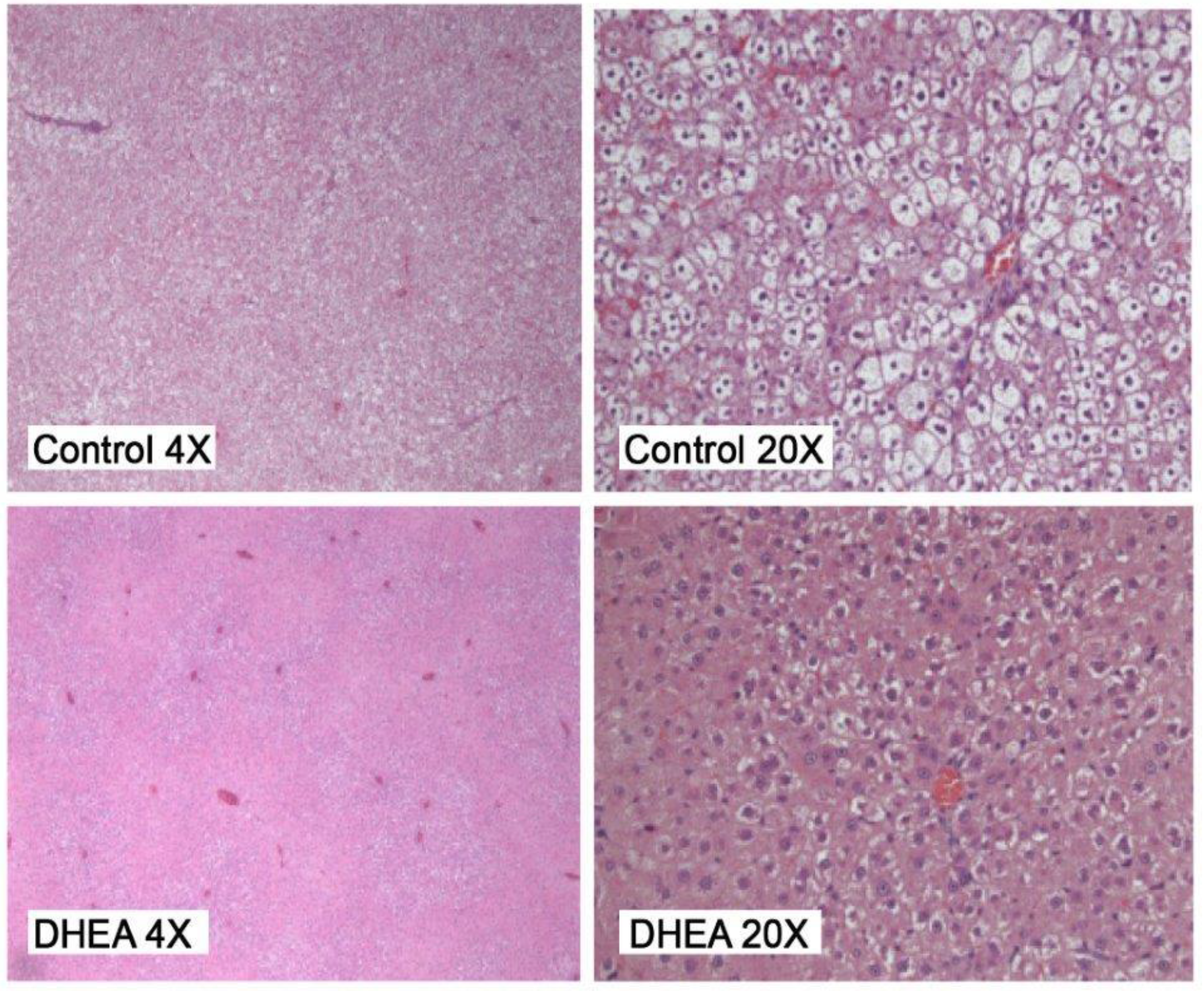
2.2. Liver Pathology
2.3. Serum Measurements for IGF-1, IGFBP-3, DHEA, and DHEA-S
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Liver Histology
4.3. Serum Measurements
4.4. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the united states, 2011–2012. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 22 December 2016).
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Picciano, M.F.; McGuire, M.K. Use of dietary supplements by pregnant and lactating women in north america. Am. J. Clin. Nutr. 2009, 89, 663S–667S. [Google Scholar] [CrossRef] [PubMed]
- Haffner, S.M.; Valdez, R.A.; Mykkanen, L.; Stern, M.P.; Katz, M.S. Decreased testosterone and dehydroepiandrosterone sulfate concentrations are associated with increased insulin and glucose concentrations in nondiabetic men. Metab. Clin. Exp. 1994, 43, 599–603. [Google Scholar] [CrossRef]
- Haffner, S.M.; Valdez, R.A.; Stern, M.P.; Katz, M.S. Obesity, body fat distribution and sex hormones in men. Int. J. Obes. Relat. Metab. Disord. 1993, 17, 643–649. [Google Scholar] [PubMed]
- Barrett-Connor, E.; Ferrara, A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: The rancho bernardo study. J. Clin. Endocrinol. Metab. 1996, 81, 59–64. [Google Scholar] [PubMed]
- Cleary, M.P.; Zisk, J.F. Anti-obesity effect of two different levels of dehydroepiandrosterone in lean and obese middle-aged female zucker rats. Int. J. Obes. 1986, 10, 193–204. [Google Scholar] [PubMed]
- Hansen, P.A.; Han, D.H.; Nolte, L.A.; Chen, M.; Holloszy, J.O. DHEA protects against visceral obesity and muscle insulin resistance in rats fed a high-fat diet. Am. J. Phys. 1997, 273, R1704–R1708. [Google Scholar] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the united states: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Younossi, Z.M. Epidemiology and natural history of NAFLD and NASH. Clin. Liver Dis. 2007, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef] [PubMed]
- Hakkak, R.; Shaaf, S.; Jo, C.H.; Macleod, S.; Korourian, S. Effects of high-isoflavone soy diet vs. casein protein diet and obesity on dmba-induced mammary tumor development. Oncol. Lett. 2011, 2, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, M.; Bradley, K.M.; Dong, F.; Anversa, P.; Ren, J. IGF-1 alleviates high-fat diet-induced myocardial contractile dysfunction: Role of insulin signaling and mitochondrial function. Hypertension 2012, 59, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Melian, E.; Velasco, B.; Barrios, R.; Sanchez-Franco, F. Basal and growth hormone-induced hepatic messenger ribonucleic acid expression of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 is independent of hyperinsulinemia and increased energy status in the genetically obese Zucker rat. Endocrinology 1997, 138, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Runchey, S.S.; Boyko, E.J.; Ioannou, G.N.; Utzschneider, K.M. The relationship between serum circulating IGF-1 and liver fat in the United States. J. Gastroenterol. Hepatol. 2014, 29, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Arturi, F.; Succurro, E.; Procopio, C.; Pedace, E.; Mannino, G.C.; Lugara, M.; Procopio, T.; Andreozzi, F.; Sciacqua, A.; Hribal, M.L.; et al. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J. Clin. Endocrinol. Metab. 2011, 96, E1640–E1644. [Google Scholar] [CrossRef] [PubMed]
- Hakkak, R.; Shaaf, S.; Jo, C.H.; MacLeod, S.; Korourian, S. Dehydroepiandrosterone intake protects against 7,12-dimethylbenz(a)anthracene-induced mammary tumor development in the obese Zucker rat model. Oncol. Rep. 2010, 24, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the united states: The third national health and nutrition examination survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Abadie, J.M.; Malcom, G.T.; Porter, J.R.; Svec, F. Dehydroepiandrosterone alters lipid profiles in Zucker rats. Lipids 2000, 35, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.; Cleary, M.P. Metabolic alterations after dehydroepiandrosterone treatment in Zucker rats. Am. J. Physiol. 1984, 246, E123–E128. [Google Scholar] [PubMed]
- Imai, K.; Koyama, M.; Kudo, N.; Shirahata, A.; Kawashima, Y. Increase in hepatic content of oleic acid induced by dehydroepiandrosterone in the rat. Biochem. Pharmacol. 1999, 58, 925–933. [Google Scholar] [CrossRef]
- Hakkak, R.; Al-Dwairi, A.; Fuchs, G.J.; Korourian, S.; Simmen, F.A. Dietary soy protein induces hepatic lipogenic enzyme gene expression while suppressing hepatosteatosis in obese female zucker rats bearing dmba-initiated mammary tumors. Genes Nutr. 2012, 7, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Nestler, J.E.; Barlascini, C.O.; Clore, J.N.; Blackard, W.G. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J. Clin. Endocrinol. Metab. 1988, 66, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Han, D.H.; Hansen, P.A.; Chen, M.M.; Holloszy, J.O. DHEA treatment reduces fat accumulation and protects against insulin resistance in male rats. J. Gerontol. Ser. A Biolog. Sci. Med. Sci. 1998, 53, B19–B24. [Google Scholar] [CrossRef]
- Kajita, K.; Ishizuka, T.; Miura, A.; Ishizawa, M.; Kanoh, Y.; Yasuda, K. The role of atypical and conventional PKC in dehydroepiandrosterone-induced glucose uptake and dexamethasone-induced insulin resistance. Biochem. Biophys. Res. Commun. 2000, 277, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Safiulina, D.; Peet, N.; Seppet, E.; Zharkovsky, A.; Kaasik, A. Dehydroepiandrosterone inhibits complex I of the mitochondrial respiratory chain and is neurotoxic in vitro and in vivo at high concentrations. Toxicol. Sci. 2006, 93, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Korourian, S.; Hakkak, R.; Ronis, M.J.; Shelnutt, S.R.; Waldron, J.; Ingelman-Sundberg, M.; Badger, T.M. Diet and risk of ethanol-induced hepatotoxicity: Carbohydrate-fat relationships in rats. Toxicol. Sci. 1999, 47, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Hakkak, R.; Zeng, H.; Dhakal, I.B.; Korourian, S. Short- and long-term soy diet versus casein protects liver steatosis independent of the arginine content. J. Med. Food 2015, 18, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
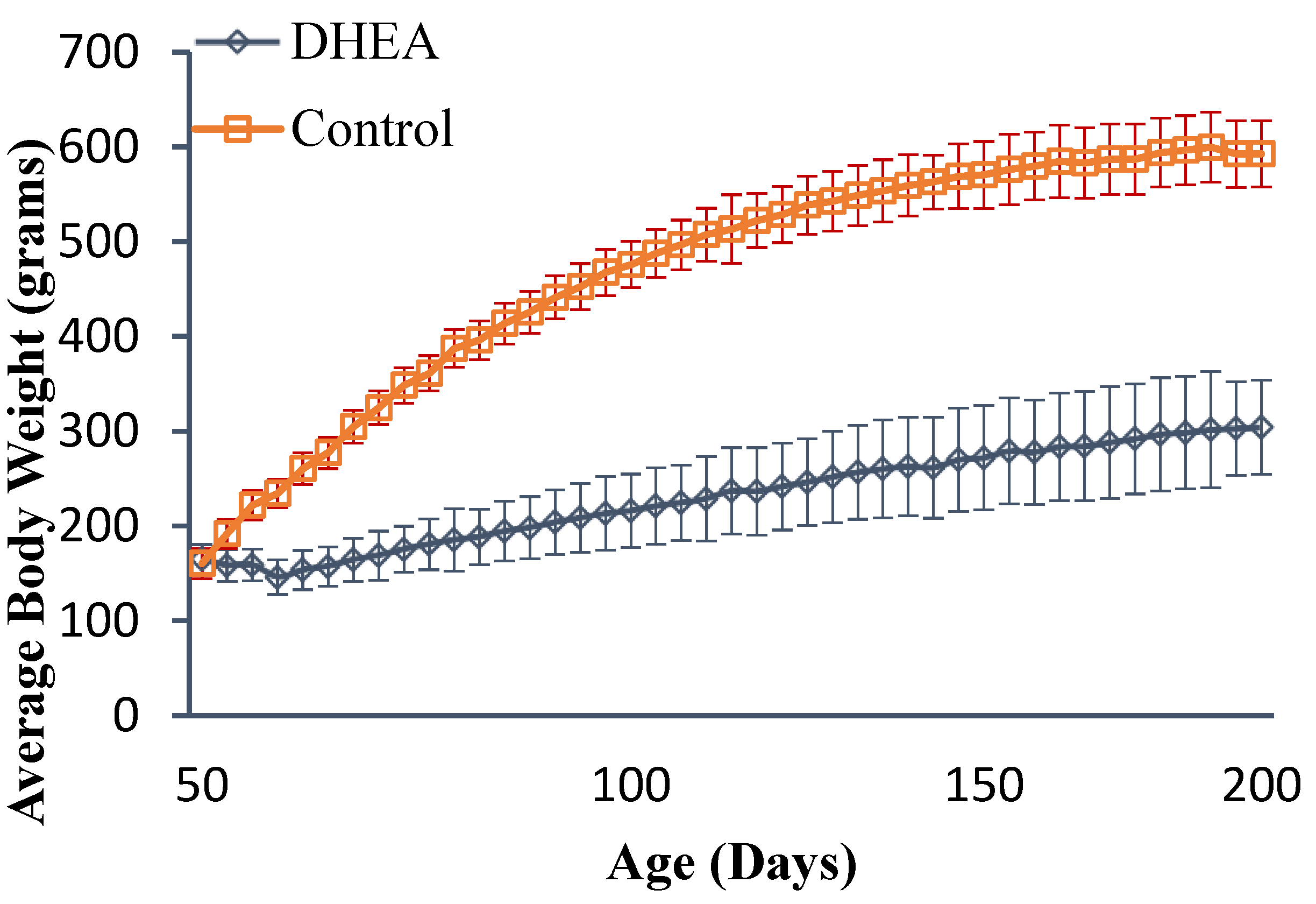
| Groups | Body Wt (g) | Liver Wt (g) Absolute | Liver Wt % of BW | Steatosis Score |
|---|---|---|---|---|
| Control | 593 ± 49 | 21.78 ± 2.88 | 3.928 ± 0.61 | 3.21 ± 1.28 |
| DHEA | 304 ± 35 a | 18.17 ± 2.55 a | 6.57 ± 0.74 a | 1.36 ± 0.44 a |
| Groups | DHEA | DHEA-S | IGF-1 | IGFBP-3 |
|---|---|---|---|---|
| Control | 3.55 ± 0.38 | 0.16 ± 0.01 | 1,295.82 ± 16.65 | 217.41 ± 2.74 |
| DHEA | 748.40 ± 15.15 a | 30.80 ± 0.55 a | 771.45 ± 7.83 a | 168.58 ± 1.73 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakkak, R.; Bell, A.; Korourian, S. Dehydroepiandrosterone (DHEA) Feeding Protects Liver Steatosis in Obese Breast Cancer Rat Model. Sci. Pharm. 2017, 85, 13. https://doi.org/10.3390/scipharm85010013
Hakkak R, Bell A, Korourian S. Dehydroepiandrosterone (DHEA) Feeding Protects Liver Steatosis in Obese Breast Cancer Rat Model. Scientia Pharmaceutica. 2017; 85(1):13. https://doi.org/10.3390/scipharm85010013
Chicago/Turabian StyleHakkak, Reza, Andrea Bell, and Soheila Korourian. 2017. "Dehydroepiandrosterone (DHEA) Feeding Protects Liver Steatosis in Obese Breast Cancer Rat Model" Scientia Pharmaceutica 85, no. 1: 13. https://doi.org/10.3390/scipharm85010013
APA StyleHakkak, R., Bell, A., & Korourian, S. (2017). Dehydroepiandrosterone (DHEA) Feeding Protects Liver Steatosis in Obese Breast Cancer Rat Model. Scientia Pharmaceutica, 85(1), 13. https://doi.org/10.3390/scipharm85010013





