1. Introduction
In developed countries with aging populations, healthcare sectors play a large role in government spending [
1,
2]. As such, the share of healthcare in the GDP increases. In terms of individual sectors and demographic trends, significant attention is paid to this development. Further, medical devices (MDs) play an important and increasing role in the delivery of healthcare [
3].
The revenue of the medical equipment and supplies manufacturing industry in 2016 was
$92.1 billion USD. The industry is relatively small, ranking at the bottom 20 percent of the manufacturing industries in terms of size. Over the past 3 years, industry revenues have been shrinking at an annual rate of −1.1 percent compared to the 0.9 percent average growth of for manufacturing industries, thus ranking at the bottom 40 percent of all manufacturing industries. Given the macroeconomic climate and industry dynamics, the forecasted revenue growth rate over the next 5 years is 4.8 percent per year for the medical equipment and supplies manufacturing industry. Further, 70.2 percent of firms in this industry are profitable, with an average net income of 12.2 percent of revenues, which ranks the industry in the top 20 percent of the manufacturing industries [
4].
Every year a considerable amount of medical technology is developed, and every year billions of crowns are invested in this development [
5,
6,
7,
8]. Different fields of medical devices are in development, owing to an increasing request for medical technology novelty (MDI).
In the Czech Republic (CR), an important role in the field of medical devices is played by the Association of Manufacturers and Suppliers of Medical Devices (AVDZP) [
9], an association of 140 companies that employ almost 9000 employees and reach a turnover of CZK 19 billion a year. The Association helps manufacturers and suppliers of medical devices to support their business and the expansion of the Czech and global markets. All members of the AVDZP declare top quality for their products and services [
9]. The main goal of the AVDZP is to implement the best solution from the viewpoint of technical excellence, quality, and cost-effectiveness. They provide support for firms to submit research projects to the Ministry of Industry and Trade or ask for EU structural funds [
10]. Innovation in healthcare must not happen in isolation; rather, it must be a multidisciplinary matter that draws on the findings, knowledge, and experience in other sectors for the mutual benefit of all parties involved. The idea is to view healthcare innovation from a comprehensive perspective and take an integrated approach to delivering value to the healthcare industry, including healthcare providers as well as care recipients, and to the society as a whole [
11,
12].
The benefit in this sector is the focus and use of, among other things, the principle of open innovation. Its principle is the use of knowledge from the external environment to accelerate innovation in the company. It is anticipated that companies may and should use external ideas, as well as internal stimuli, to develop their technologies and markets. Firms should be open to making knowledge developed in-house available to others who are able to use it or for further use. Open innovation offers ways to lower costs, risk sharing, and shorter time to market [
13]. By using and practicing on open business models, many medical device companies can capture the unique and new know-how of scientists across disciplines and also from patients [
14].
The whole product development process from its beginning to the release of the product on the market is very complex and relies significantly on the application of exact processes. They enable developers to optimally stage development, testing, validation, verification, and market release [
15,
16]. Current medical device development processes have to respond to several challenges. In the design and development process, the manufacturer must take into account all the requirements [
17,
18] and obligations arising from European or national medical device legislation. These obligations relate to the risk class of the medical device, which is a determining factor in the complexity of the design and development itself, and the marketing of the medical device. With the arrival of the new European Commission Medical Device Regulation (MDR) 2017/745 [
19], there will be further and more demanding requirements for manufacturers to enter the European market. However, it will always be crucial to correctly classify a medical device in the risk class, regardless of the country of origin of the manufacturer or the medical device.
The aim of the paper is therefore to describe the rules of classification of medical devices with regard to changes in legislation. The specification of some questions is pointed out in a selected country of the European Union, the Czech Republic. The whole problem is embedded in the concept of European legislation.
2. Theoretical Background
2.1. Classification of Economic Activities and Medical Devices in the Czech Republic
The medical devices sector is an export-led sector, and on average, 70% of its production is exported. Many firms have their subsidiaries abroad through which they gradually increase their export. The medical device portfolio is wide, with about 500,000 items ranging from dressing material to orthopedic implants or pacemakers. Manufacturers are also focusing on other fields such as nanotechnology and microsystems for instrument building, equipment for minimizing invasive methods, introducing information technology into healthcare, emergency medicine, cardiac surgery, and other fields of modern medicine [
20,
21].
The CR is a small open economy with a strongly export-oriented industry. Industry accounts for about a third of GDP, the highest percentage share among the EU-28. The manufacturing industry (MI) is a significant sector of the economy, contributing to the development of technology, knowledge, and job opportunities. In the CR, it has a long-standing tradition. Dynamic development of the MI confirms its contribution to the creation of gross value added and employment, which reached approximately 27% in 2017. In terms of revenues, the most important division of MI is the production of motor vehicles (CZ-NACE 29), the share of which is more than a quarter of the GDP. It is well ahead of other groups in the section with shares below 10%, including our observed group 26.60 (Manufacture of irradiation, electromedical, and electrotherapeutic equipment).
In 2017 group 26.60 (Manufacture of irradiation, electromedical, and electrotherapeutic equipment) shared in CZ-NACE 26 only 0.8% of added value, 0.3% of sales, 0.8% of employees and 1.8% of production units. The development of selected indicators in the period from 2008 to 2017, like sales, employed persons, as well as value-added and labor productivity, shows a development in the form of the letter “W”. The lower values of indicators were mostly at the beginning of the economic crisis in 2009, and in 2013.
Within group 26.60, the share of value added in large enterprises was 44%, medium 27% and small 29%. The shares in the number of employees were similar. The most important group in terms of sales and staffing is the second observed group, 32.50 (Manufacture of medical and dental instruments and things). Together with group 32.4 (Manufacture of games and toys), it accounts for more than 60% of the division.
As mentioned ahead, medical device production is also included in division 32 (Other manufacturing industries) and group 32.50 (Manufacture of medical and dental instruments and things). Production of CZ-NACE 32 is characterized by high material demands and individual production groups differing both in the input raw materials used and in production technologies and final products themselves. Some groups depend on the high manual skills and inventiveness of designers and workers. Many of these product groups have a long tradition (e.g., some medical devices, musical instruments).
Group 32.5 (Manufacture of medical and dental instruments and things) shared in CZ-NACE 32 for the year 2017 39.7% of added value, 32.1% of sales, 39.4% of employees, and 26.9% of production units. Most of the indicators had a positive growing tendency. Some companies of section 32 used a significant amount of targeted support from the state budget through the national programs of the Ministry of Industry and Trade (IMPULS, TIP, and TRIO) and Technology Agency of the Czech Republic (TA CR) (Alfa, Centres of Competence, and Epsilon). In the period 2004–2017, these subsidies were awarded to Medical Technologies CZ, LINET, MEDIN, BTL Medical Technology, etc. Support is divided into the introduction of enterprise innovation; product and process (48%); the creation of new, expanded or upgraded research sites (24%); the creation of new applied research results (16%); and participation in exhibitions and fairs abroad (10%). The support is mainly aimed at strengthening R&D capacities of enterprises [
20].
CZ-NACE group 32.5 recorded a favorable development in 2017. Czech companies producing medical technology are well established in the markets of Europe, America, and the Far East, and with the EGAP (Export Guarantee and Insurance Corporation) insurance support, they have the prospect of further expansion. This group accounts for more than 27% of the foreign trade turnover of the division. Among the most successful ones is LINET, which increases its turnover and the products, e.g., medical beds. From a territorial point of view, exports are largely directed to Germany (33%), through which some of the commodities from the Czech Republic are part of German exports to third countries. Germany also dominates in imports (27%), and China (13%) is in second place.
The results of the manufacturing industry in the period 2008 to 2017 were very good. Significant is the constant growth in labor productivity (except for the crisis year 2009), accompanied by an increase in the average wage and maintaining a favorable relationship between labor productivity growth and average wage growth. Detailed data for the manufacturing industry as a whole and each section, division, and group are available in an interactive table at the Ministry of Industry and Trade website [
21].
2.2. Regulation of MDs in the CR
In the CR, medical devices are defined by Act No. 268/2014 Coll., Part 1. This Act incorporates the relevant European Union regulations and regulates the treatment of medical devices and their accessories in broad terms and details. According to this national legislation, a “medical device means any instrument, apparatus, equipment, software, including software designed by its manufacturer for specific use for diagnostic or therapeutic purposes and necessary for the proper use of a medical device, material, or other object intended by the manufacturer for use in human beings”:
- (a)
Diagnosis, prevention, monitoring, treatment, or alleviation of disease;
- (b)
Diagnosis, monitoring, treatment, alleviation, or compensation of injury or disability;
- (c)
Examination, replacement, or modification of the anatomical structure or physiological process;
- (d)
Conception control.
If the general definition in the former paragraph is fulfilled, the medical devices are in particular:
- (a)
Active implantable medical devices;
- (b)
In vitro diagnostic medical devices;
- (c)
Individually made medical devices.
In 2020, the new European legislation that applies to this sector (Regulation 2017/745 on Medical Devices) will come into force (see below) and may significantly change the rules of the game. The Ministry of Industry and Trade has therefore launched consultations with leading Czech manufacturers, industry organizations, and other stakeholders to identify their needs to remain as competitive as possible within the European Union [
10].
In the CR, one of the main identified needs is to improve communication between medical device manufacturers and the Czech Notified Bodies (Electro-Technical Testing Institute and Institute for Testing and Certification), which assess the conformity of products with European regulations. It is a highly qualified activity that lacks capacity throughout the European Union. This deficiency may lead to a loss of market opportunities for medical device manufacturers.
Comprehensive legislative requirements should be discussed and clarified to suggest possibilities of supporting and developing the medical devices industry in the Czech Republic. An important condition for competitiveness with Europe’s leading countries is the
maintenance of notified bodies. The conformity assessment process will be extremely demanding and costly under the new conditions. With the decreasing number of notified bodies in the EU, the availability of this service is essential for Czech manufacturers [
10].
Medical device manufacturers cannot rely simply on delivering established products without continuously looking for ways to innovate them and deliver an improved experience to professionals using the products and enhanced health benefits to patients on the receiving end.
4. Results: Analysis in Regulatory Changes
The manufacturer has to follow the rules to decide which kind of risk class the medical device belongs to. The correct determination of the risk class depends on the rules defined in the European Regulation. These rules may not be quite clear to the manufacturer, so it was beneficial to create an intuitive decision-making procedure to help the manufacturer correctly classify their product.
This decision-making model was created on the basis of individual rules to correctly guide the manufacturer on how to classify their medical device.
In the first stage, we assume that the manufacturer, based on the definition of the medical device, has decided that they are producing the medical device.
Figure 1: The first phase of the decision—Rule 11—Software intended to provide information that is used to take decisions with diagnosis or therapeutic purposes (from class I to class III); Rule 14—devices incorporating a medicinal substance including human blood or plasma; devices used for contraception or prevention of sexually transmitted diseases; Rule 15—contraception or prevention of the transmission of sexually transmitted diseases; Rule 16—specific disinfecting, cleaning and rinsing devices; Rule 17—devices specifically intended for recording of diagnostic images generated by X-ray radiation; Rule 18—devices utilizing non-viable tissues or cells of human origin or tissues of animal or derivatives; Rule 19—devices incorporating or consisting of nanomaterial.
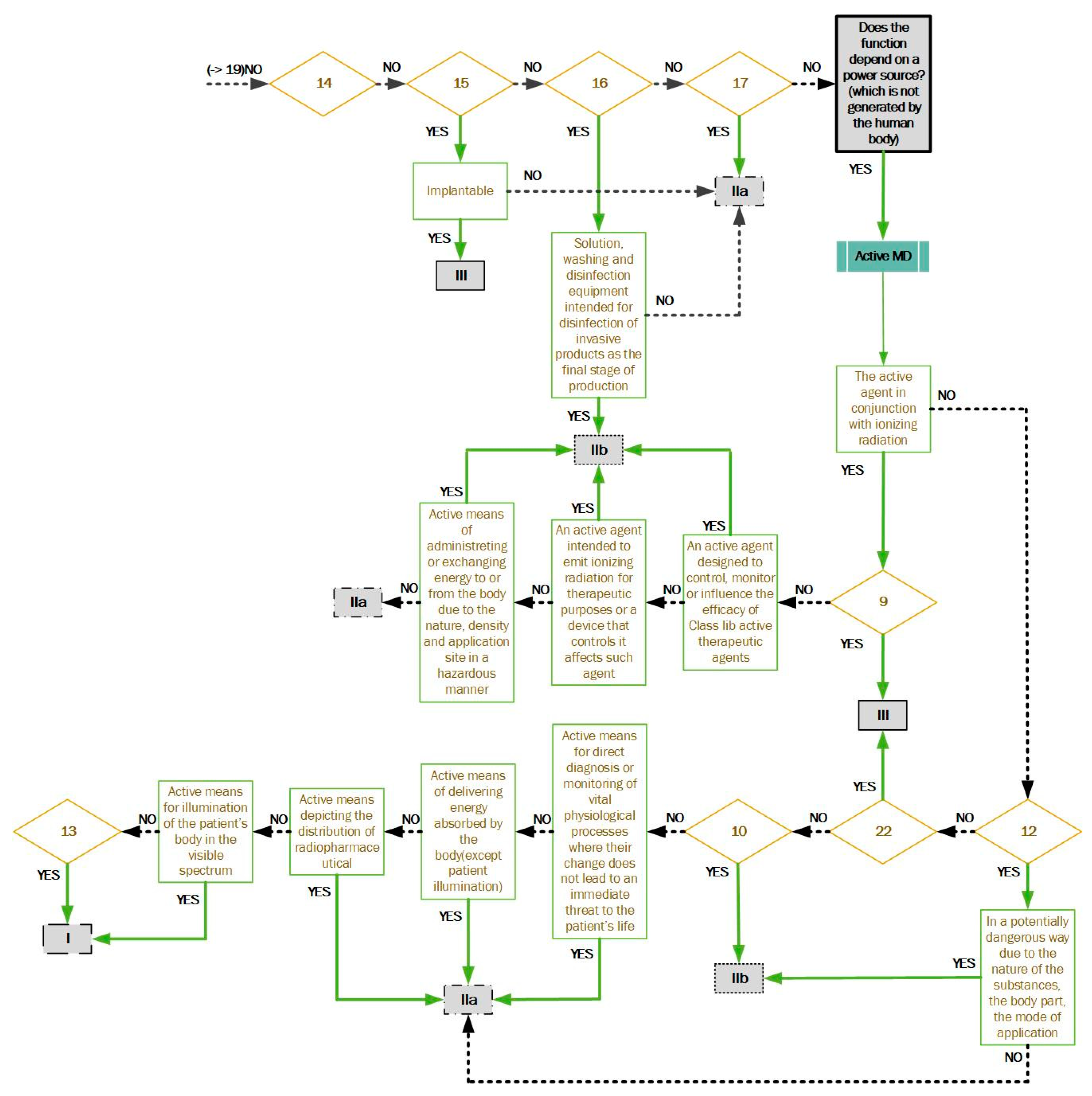
Figure 2: If the first phase does not meet the classification, the manufacturer continues to the second phase. Rule 9—active therapeutic devices intended to exchange or administer energy; Rule 10—active devices for diagnosis and monitoring, emitting ionizing radiation; Rule 12—active devices intended to administer and/or remove medicinal products, body liquids, or other substances; Rule 13—all other active devices; Rule 22—active therapeutic devices with an integrated or incorporated diagnostic function that significantly determines the patient management.
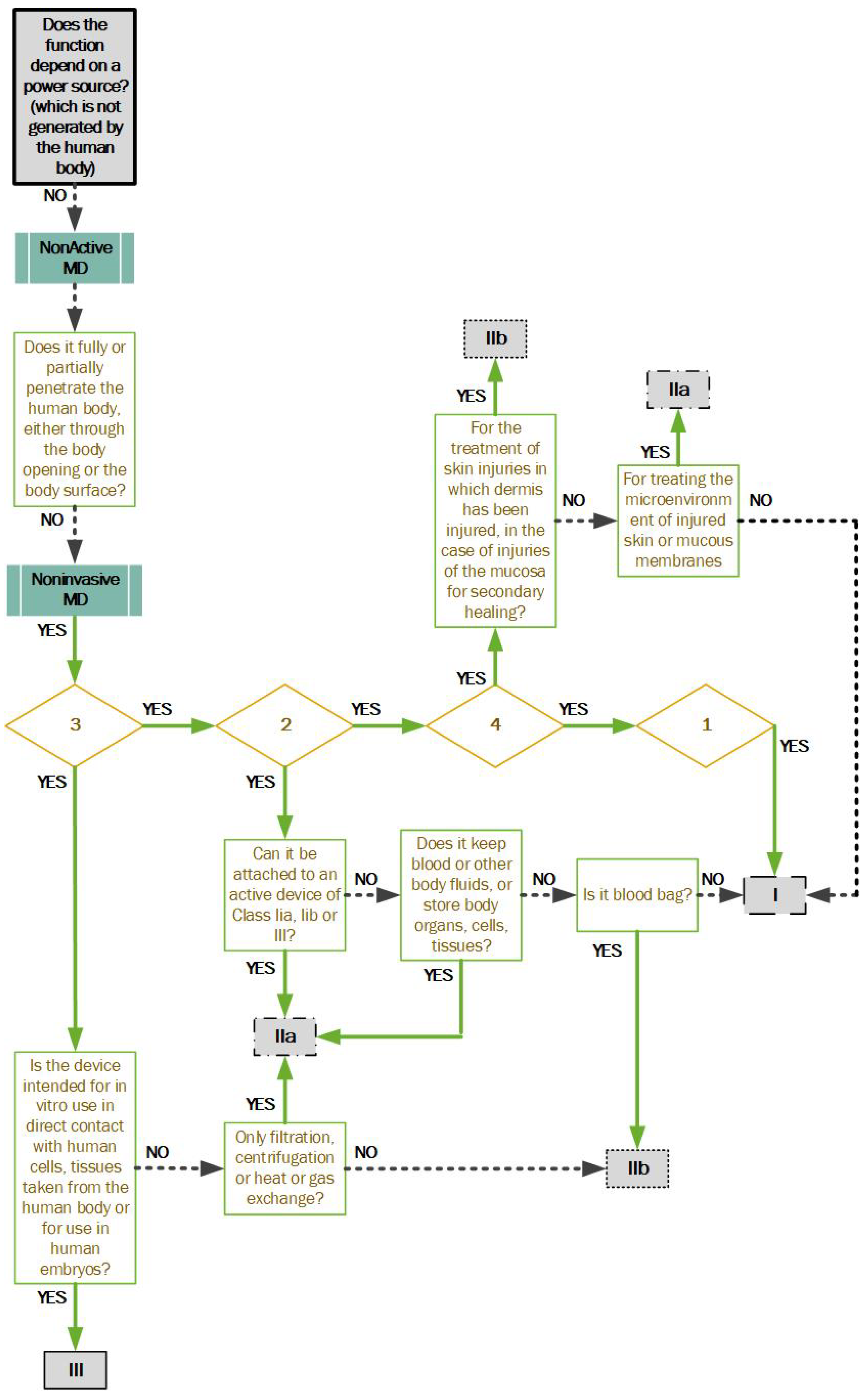
Figure 3: If the second phase does not meet the classification, the manufacturer continues to the third phase. Rule 1—non-invasive devices; Rule 2—non-invasive devices intended for channeling or storing (now includes cells); Rule 3—non-invasive devices that modify biological or chemical composition of blood, body liquids, other liquids and cells.; Rule 4—non-invasive devices in contact with injured skin or mucous membrane.
Figure 4: If the third phase does not meet the classification, the manufacturer continues to the fourth phase. Rule 5—devices invasive in body orifices; Rule 20—invasive devices with respect to body orifices to administer medicinal products by inhalation; Rule 21—substances or combinations of substances that are intended to be introduced into the human body via a body orifice or that are applied to the skin and absorbed.
Figure 5: If the fourth phase does not meet the classification, the manufacturer continues to the fifth phase. Rule 6—surgically invasive devices for transient use; Rule 7—surgically invasive devices for short term use; Rule 8—surgically invasive devices for long term use and implantable (including any device administering medicinal products, surgical mesh, or spinal disc).
It is always necessary to select the highest possible risk class. Therefore, the manufacturer is obliged to review each rule of the European regulation and decide which risk class their device falls under.
5. Discussion: Regulation and Open Innovation in Medical Devices
The relationship between regulation and innovation is based on mutual influence. Regulation clearly regulates the innovation process in a given area; conversely technological progress and changes have an impact on regulation. This interaction must be perceived for success in changing regulation [
24]. Regulatory change fully responds to changes in technical, social, and economic conditions surrounding them.
In the case of medical devices and the legislative changes currently under discussion in Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on medical devices, this is to ensure greater safety for patients. At the same time, it means an increased economic burden for medical device manufacturers in the form of conformity assessment costs. It is currently a question of whether this change will place a significant burden on small and medium-sized enterprises, which are the main innovators in the field of medical devices, to the point where production is reduced, or whether it will, as with so many examples of successfully implemented regulation, become a powerful stimulus for further innovation.
At the same time, looking at innovation itself, open innovation is a strong theme across sectors, including the medical device sector. Open innovation has become the dominant paradigm to connect new technologies and markets [
25]. The importance of open innovation in the field of medical devices is evidenced, for example, by the fact that impulses for change can come from patients themselves. Another issue is the complexity of the processes that are motionless in this industry, creating the need for maximum efficiency in all activities.
These types of user-lead innovations, which are based on the ideas of users, in this case from patients and healthcare workers, are an important source of innovation [
26]. Bernstein and Singh (2006) point out that this type of technological pressure is one of the reasons why companies in this sector also need a more comprehensive and integrated model of innovation processes. The Open Innovation Framework is also beneficial in providing a comprehensive understanding of how high-tech small firms (HTSFs) innovate in healthcare. Many studies and facts show that the medical device market can never achieve stagnation, as it inextricably develops in parallel with advances in other sectors and medical practices [
27], and these dynamics clearly point to the need to address this challenge effectively. Open innovation models bring added value through the use of many other ideas through their incorporation into a number of external concepts [
28]. The result is the use of technical potential and ensuring the growth of economic value [
14,
29,
30].
To streamline processes, optimize performance, and maximize results in any company, it is essential for all its organizational units to work as one. This requires fostering a culture of effective collaboration, openness to new knowledge, sharing experience, and driving the company toward improvement, innovation, and invention. Primarily, the fact that new rules are set up in the interest of patient safety is a challenge that could shift the development of organizations qualitatively. On the other hand, it is quite evident that the transition to the new rules, i.e., the conditions for individual classes of risk, will increase the unanimous cost increase for the company. Increasing the risk class also means a higher need for testing and control, the prices of which are in the tens and hundreds of thousands of crowns. Another related problem is the availability of notified bodies in the Czech Republic. If there are not enough of them, companies will either apply for approval abroad or their price will rise again in the Czech Republic.
The costs that will have to be paid during the existence of the product in market risk class III are also very important. For this medical device, it will be necessary to repeat the clinical trials, which are the most expensive item.
The fact is that many companies have considered how to manage this change in the past period, and this has often been a very different base of opportunity (especially for small and medium-sized companies). The variety of cost-management options include changing the medical device to fall into a lower risk class to changing the development and manufacturing and moving away from product altogether.
So far, thoughts about how Czech companies are able to fight the change are speculation. However, what will be the end result after the introduction of the new legislation will be known in the next period of 2020 to 2021.
Overall, the topic of regulation, innovation, and healthcare is a very sensitive field with an overlap into various scientific disciplines and human activities, such as economics, law, and health policy services [
25,
28,
30]. The relationship between medical device developers and national regulation agencies is critical for innovation and competitiveness in this sector. While some regulations, on the one hand, mean a reduction in activity, on the other hand, they also present an opportunity for the development of new activities and an opportunity for innovation [
31]. The process of approving innovative medical devices is generally more problematic compared to generic products. The more innovative new products are, the longer it takes to ensure their safety, check their usability, evaluate their overall benefit to the patient, and also consider many other aspects. At the same time, the burdensome impact of regulations can also be reduced within the concept of open innovation. Open innovation can be effectively utilized to make medical device development faster and cheaper [
32]. The strict requirements on the safety and reliability of medical devices also necessitate the sharing of interdisciplinary expertise, while it is almost impossible for every organization to possess the research capacity required to acquire all the capabilities internally [
33]. If the knowledge generated by scientists across research fields is more widely disseminated, then better and cheaper medical devices can be delivered faster, which increases the quality of life.
6. Conclusions
Medical devices are subjected to strict regulations to ensure the safety of the patient and eliminate as much as possible the health risks associated with using often highly technologically advanced products. The risk classification criteria are based on the intended purpose and use of the device, and their aim is to ensure that high-risk devices are subjected to a higher degree of regulation, while relatively low-risk devices are only subjected to regulations proportionate to the risk. While it is the manufacturers who must classify their devices under the appropriate risk class, regulatory authorities serve as an arbiter with respect to interpreting the requirements placed on a particular device.
International regulations and national legislature have an impact on virtually all aspects of medical device development and production: from development and design, preclinical and clinical testing, and premarket approval, through registration and certification, manufacture, and storage, to marketing and sales, export and import, and post-market control. When evaluating the level of investment effectiveness and return on investment into innovation in the sector of medical devices, the stakeholders are required to take into consideration the macroeconomic framework with specific factors in terms of their production and use. Incorporating the medical device into the correct risk class will help the manufacturer to identify and set up individual processes in the company or to prepare for all possible restrictions, rules, and regulations that must be met. All this will optimize the design and development process and help set the company’s economic growth along with competitive growth. The right approach at the outset of the design and development of a medical device will show the financial cost of placing the device on the market.