Functional Bimetal/Carbon Composites Co/Zr@AC for Pesticide Atrazine Removal from Water
Abstract
1. Introduction
2. Results and Discussions
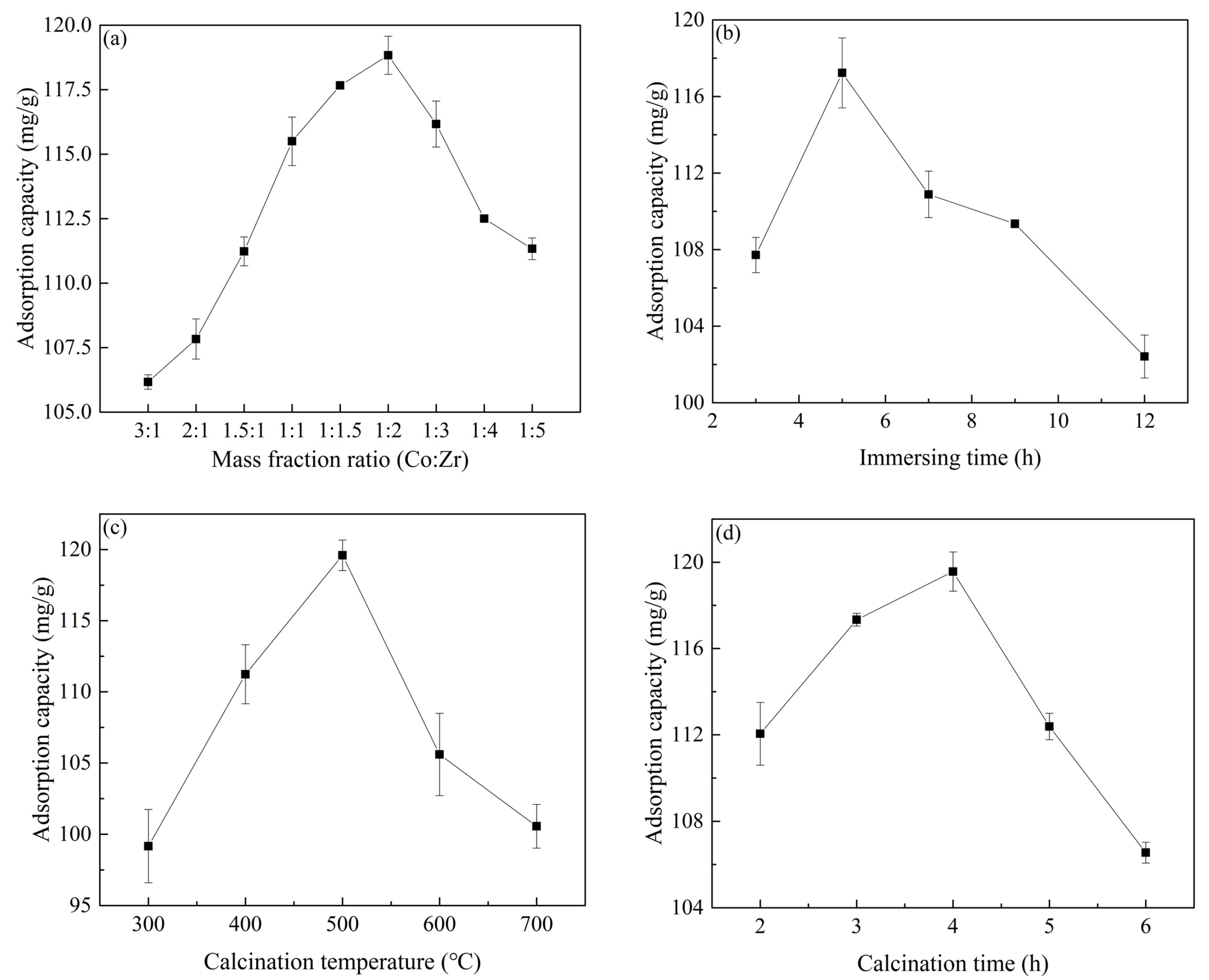
2.1. Effect of Different Preparation Conditions on Material Properties
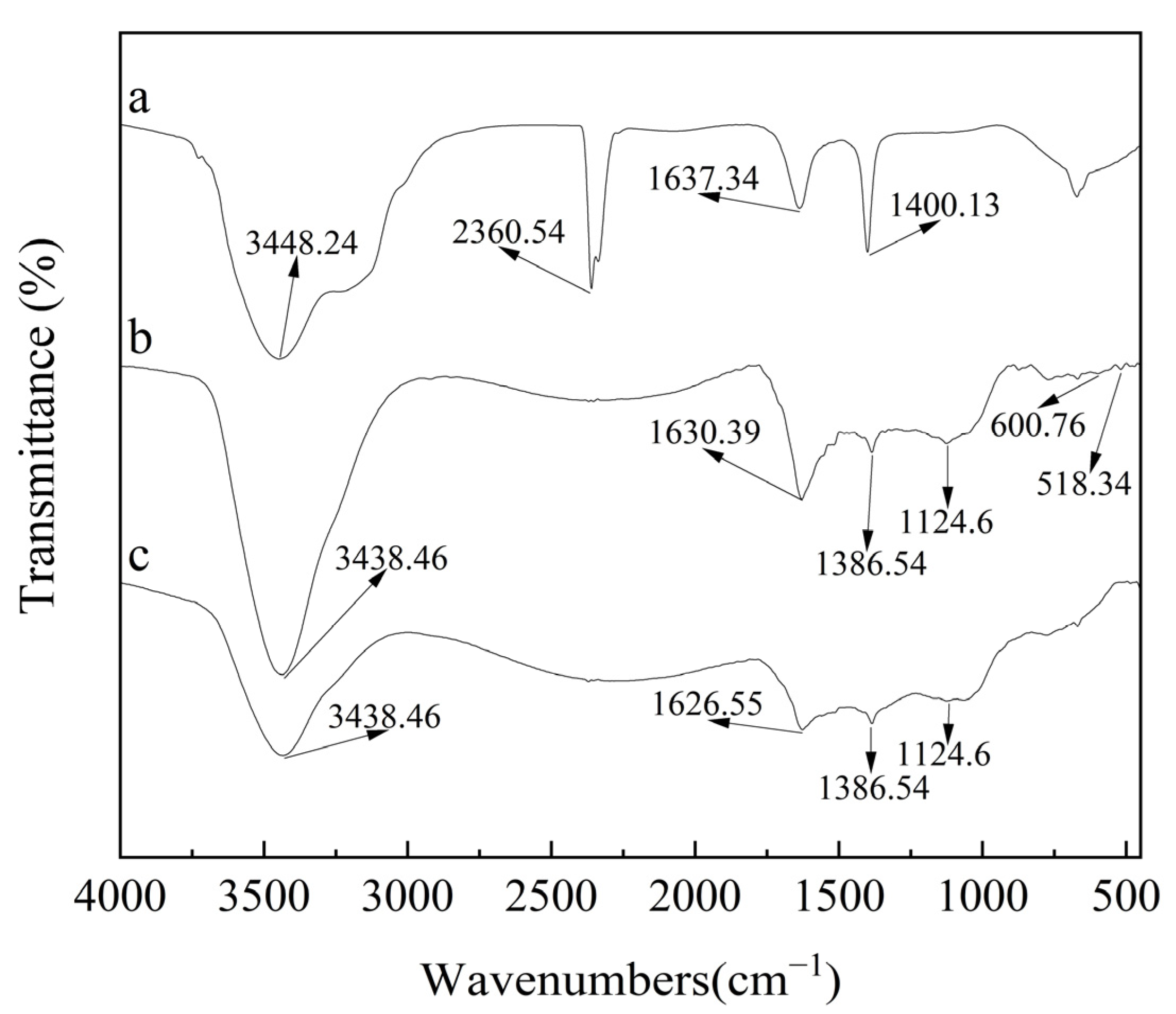
2.2. Characterization
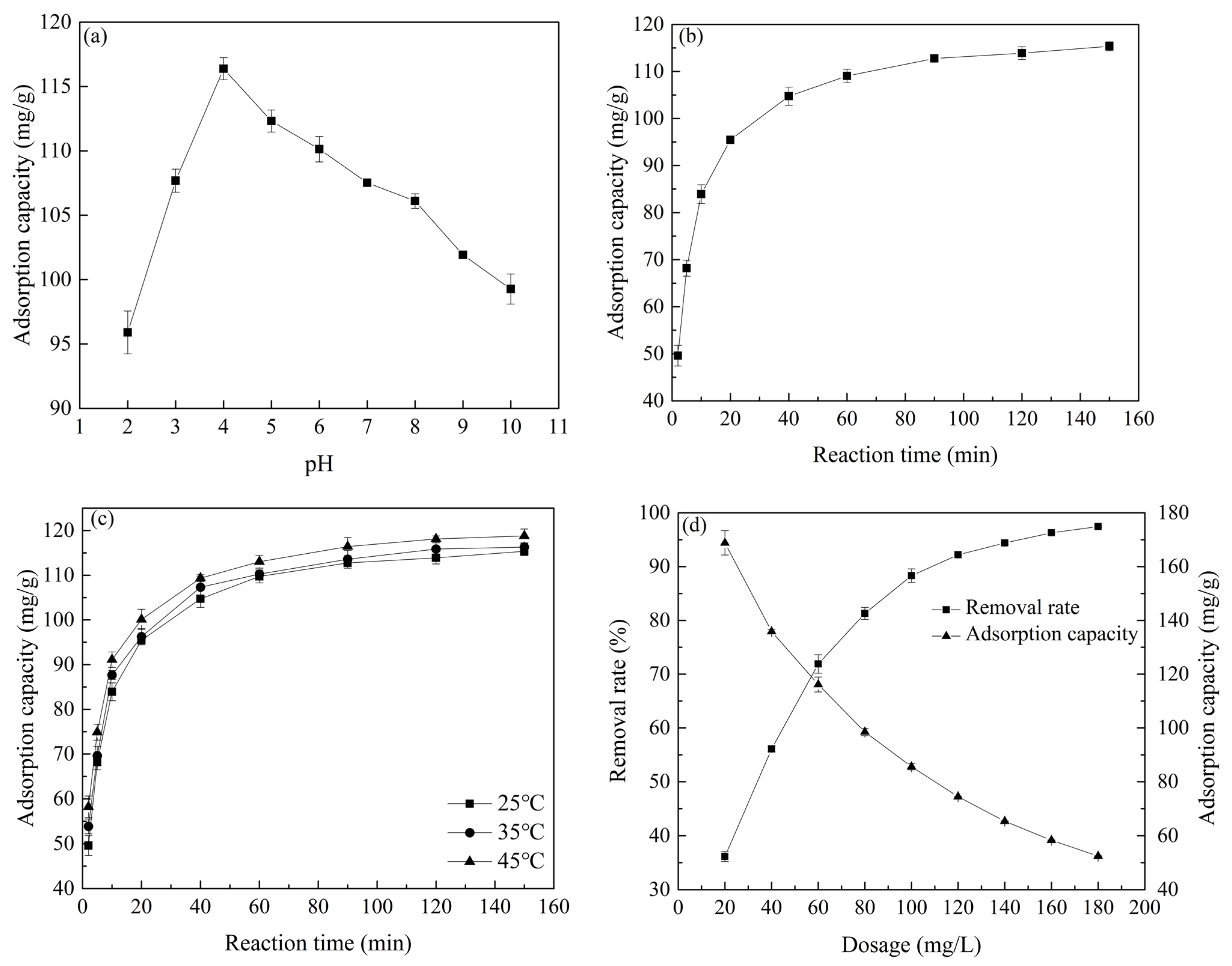
2.3. Effect of Different Adsorption Conditions on Atrazine Removal
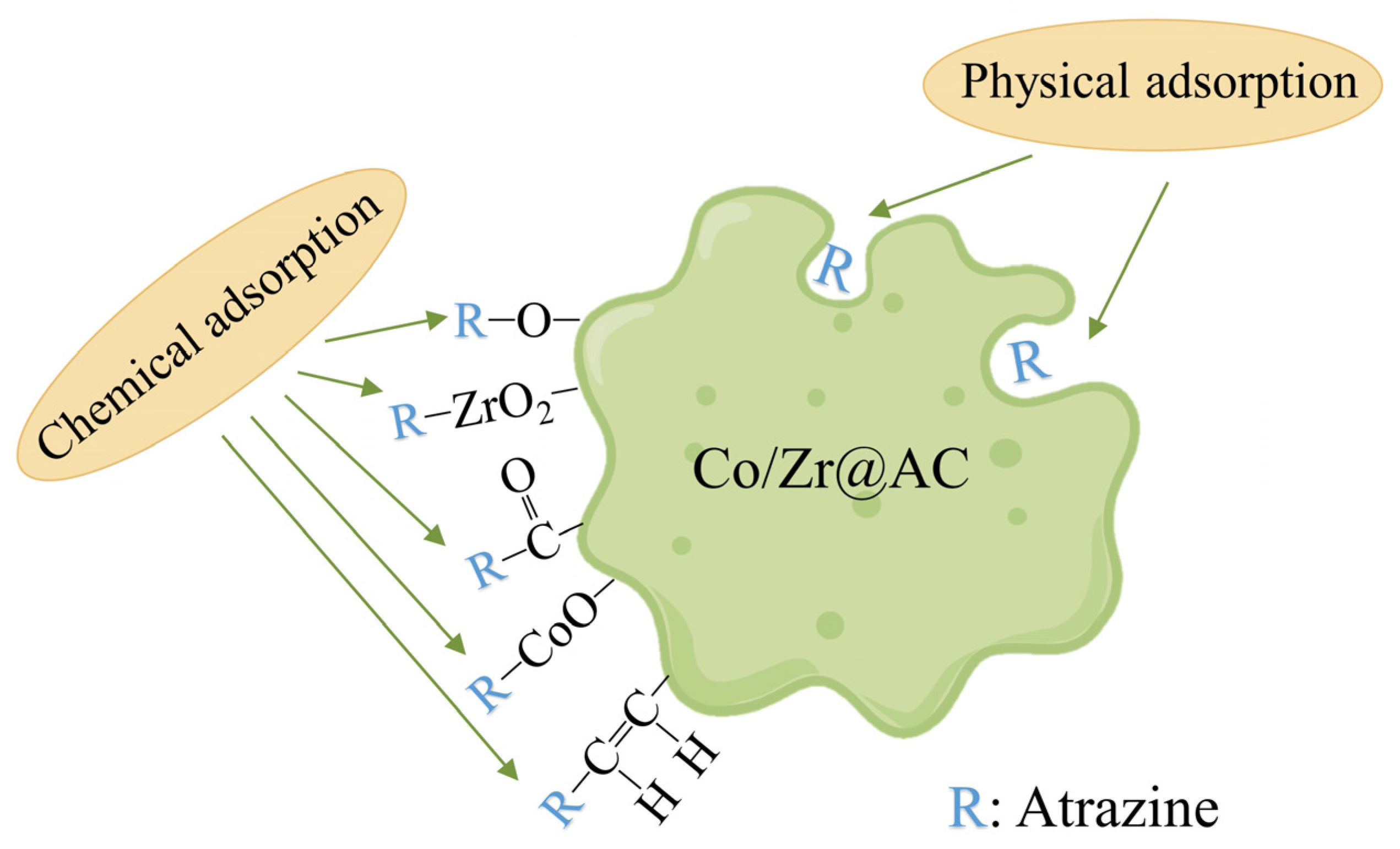
2.4. Adsorption Kinetics and Adsorption Isotherms
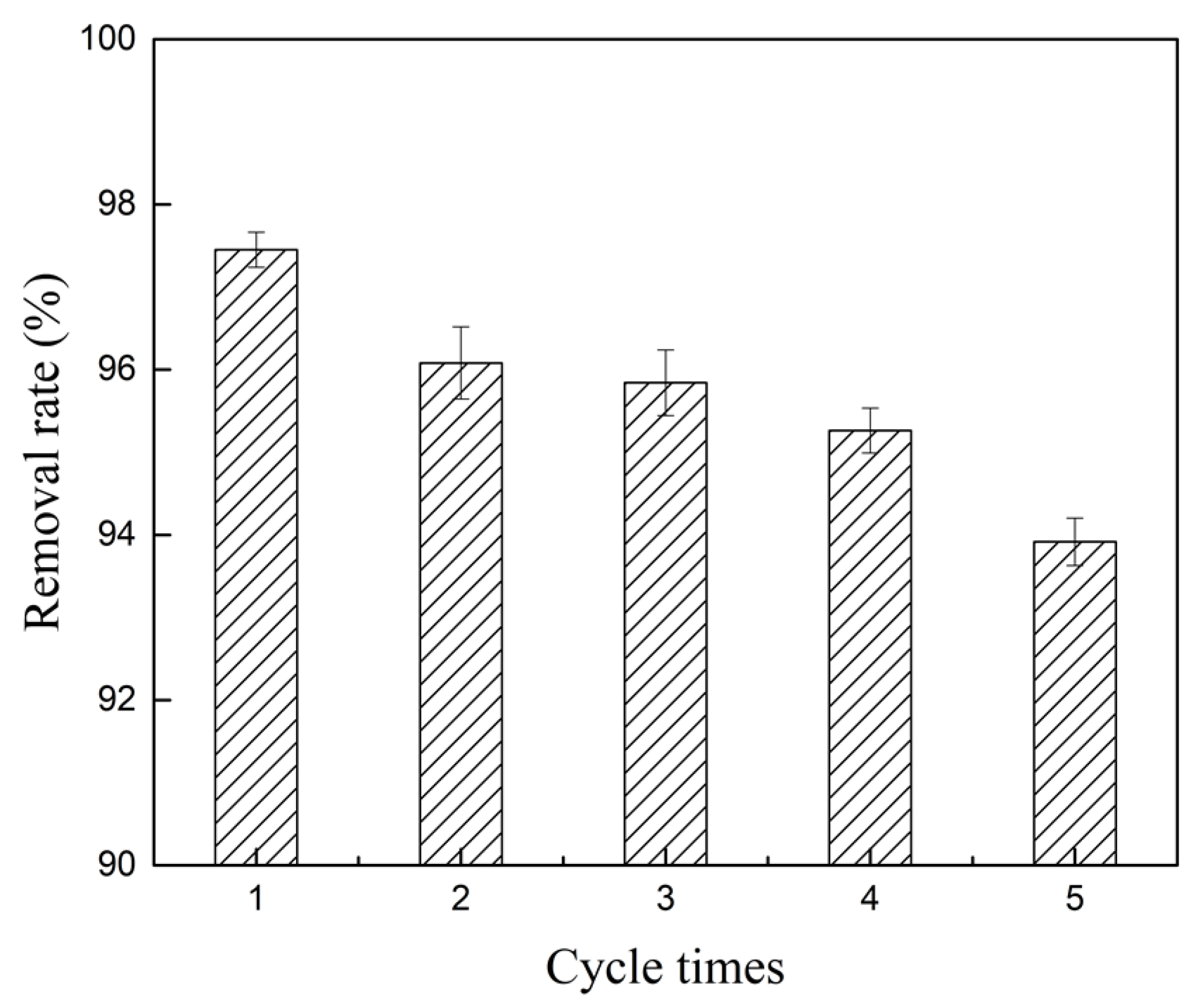
2.5. Cycle Experiments
3. Materials and Methods
3.1. Materials
3.2. Preparation of Co@AC
3.3. Atrazine Removal Experiment
3.4. Analysis Method
3.5. Characterization
3.6. Desorption and Cycle Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mac Loughlin, C.; Canosa, I.S.; Silveyra, G.R.; Lopez Greco, L.S.; Rodriguez, E.M. Effects of atrazine on growth and sex differentiation, in juveniles of the freshwater crayfish Cherax quadricarinatus. Ecotoxicol. Environ. Saf. 2016, 131, 96–103. [Google Scholar] [CrossRef]
- Salahshoor, Z.; Ho, K.V.; Hsu, S.Y.; Hossain, A.H.; Trauth, K.; Lin, C.H.; Fidalgo, M. Detection of Atrazine and Its Metabolites in Natural Water Samples Using Photonic Molecularly Imprinted Sensors. Molecules 2022, 27, 5075. [Google Scholar] [CrossRef]
- Wei, X.; Liu, C.; Li, Z.; Zhang, D.; Zhang, W.; Li, Y.; Shi, J.; Wang, X.; Zhai, X.; Gong, Y.; et al. A cell-based electrochemical sensor for assessing immunomodulatory effects by atrazine and its metabolites. Biosens. Bioelectron. 2022, 203, 114015. [Google Scholar] [CrossRef]
- Wu, X.; He, H.; Yang, W.L.; Yu, J.; Yang, C. Efficient removal of atrazine from aqueous solutions using magnetic Saccharomyces cerevisiae bionanomaterial. Appl. Microbiol. Biotechnol. 2018, 102, 7597–7610. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; Yu, B.; Wu, X.; Xu, J.; Dong, F.; Zheng, Y. Characterization of peanut-shell biochar and the mechanisms underlying its sorption for atrazine and nicosulfuron in aqueous solution. Sci. Total Environ. 2020, 702, 134767. [Google Scholar] [CrossRef] [PubMed]
- Shirmardi, M.; Alavi, N.; Lima, E.C.; Takdastan, A.; Mahvi, A.H.; Babaei, A.A. Removal of atrazine as an organic micro-pollutant from aqueous solutions: A comparative study. Process Saf. Environ. Prot. 2016, 103, 23–35. [Google Scholar] [CrossRef]
- He, H.; Liu, Y.; You, S.; Liu, J.; Xiao, H.; Tu, Z. A Review on Recent Treatment Technology for Herbicide Atrazine in Contaminated Environment. Int. J. Environ. Res. Public Health 2019, 16, 5129. [Google Scholar] [CrossRef]
- Sadeghnia, H.; Shahba, S.; Ebrahimzadeh-Bideskan, A.; Mohammadi, S.; Malvandi, A.M.; Mohammadipour, A. Atrazine neural and reproductive toxicity. Toxin Rev. 2021, 41, 1290–1303. [Google Scholar] [CrossRef]
- Krishnasamy, L.; Krishna, K.; Subpiramaniyam, S. Photocatalytic degradation of atrazine in aqueous solution using La-doped ZnO/PAN nanofibers. Environ. Sci. Pollut. Res. Int. 2022, 29, 54282–54291. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Liu, Y.; Gao, Y.; Kim, H.Y.; Ouyang, Y.; Yu, D.-G. Progresses on electrospun metal–organic frameworks nanofibers and their wastewater treatment applications. Mater. Today Chem. 2022, 25, 100974. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Song, W.; Ma, R.; Yang, J.; Zhang, X.; Huang, F.; Dong, W. Rapid degradation of atrazine by a novel advanced oxidation process of bisulfite/chlorine dioxide: Efficiency, mechanism, pathway. Chem. Eng. J. 2022, 445, 136558. [Google Scholar] [CrossRef]
- Yu, J.; He, H.; Yang, W.L.; Yang, C.; Zeng, G.; Wu, X. Magnetic bionanoparticles of Penicillium sp. yz11-22N2 doped with Fe3O4 and encapsulated within PVA-SA gel beads for atrazine removal. Bioresour. Technol. 2018, 260, 196–203. [Google Scholar] [CrossRef]
- McBeath, S.T.; Graham, N.J.D. In-situ electrochemical generation of permanganate for the treatment of atrazine. Sep. Purif. Technol. 2021, 260, 118252. [Google Scholar] [CrossRef]
- Rodriguez, C.; Leiva, E. Enhanced Heavy Metal Removal from Acid Mine Drainage Wastewater Using Double-Oxidized Multiwalled Carbon Nanotubes. Molecules 2019, 25, 111. [Google Scholar] [CrossRef]
- Yu, B.; Liu, Y.; Li, Z.; Liu, Y.; Rao, P.; Li, G. Durable substrates incorporated with MOFs: Recent advances in engineering strategies and water treatment applications. Chem. Eng. J. 2023, 455, 140840. [Google Scholar] [CrossRef]
- Eniola, J.O.; Kumar, R.; Barakat, M.A. Adsorptive removal of antibiotics from water over natural and modified adsorbents. Environ. Sci. Pollut. Res. Int. 2019, 26, 34775–34788. [Google Scholar] [CrossRef]
- Amaral, L.O.; Daniel-da-Silva, A.L. MoS2 and MoS2 Nanocomposites for Adsorption and Photodegradation of Water Pollutants: A Review. Molecules 2022, 27, 6782. [Google Scholar] [CrossRef]
- Hernandes, P.T.; Franco, D.S.P.; Georgin, J.; Salau, N.P.G.; Dotto, G.L. Investigation of biochar from Cedrella fissilis applied to the adsorption of atrazine herbicide from an aqueous medium. J. Environ. Chem. Eng. 2022, 10, 107408. [Google Scholar] [CrossRef]
- Muthusaravanan, S.; Balasubramani, K.; Suresh, R.; Ganesh, R.S.; Sivarajasekar, N.; Arul, H.; Rambabu, K.; Bharath, G.; Sathishkumar, V.E.; Murthy, A.P.; et al. Adsorptive removal of noxious atrazine using graphene oxide nanosheets: Insights to process optimization, equilibrium, kinetics, and density functional theory calculations. Environ. Res. 2021, 200, 111428. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Singh, N. Surfactant-modified bentonite clays: Preparation, characterization, and atrazine removal. Environ. Sci. Pollut. Res. Int. 2015, 22, 3876–3885. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Cheng, S.; Xia, H.; Zhang, L.; Peng, J.; Li, C.; Zhang, S. Copper loaded on activated carbon as an efficient adsorbent for removal of methylene blue. RSC Adv. 2017, 7, 14395–14405. [Google Scholar] [CrossRef]
- Mohammadi, S.Z.; Darijani, Z.; Karimi, M.A. Fast and efficient removal of phenol by magnetic activated carbon-cobalt nanoparticles. J. Alloys Compd. 2020, 832, 154942. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; Ding, Y.; Wang, F.; You, H.; Jin, C. Preparation and properties of Cu-Ni bimetallic oxide catalyst supported on activated carbon for microwave assisted catalytic wet hydrogen peroxide oxidation for biologically pretreated coal chemical industry wastewater treatment. Chemosphere 2019, 214, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, J.; Zhao, T.; Fan, S.; Zhang, C.; Hu, Q.; Hou, X. Fe3O4-CeO2 loaded on modified activated carbon as efficient heterogeneous catalyst. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 59–69. [Google Scholar] [CrossRef]
- Singhania, A.; Krishnan, V.V.; Bhaskarwar, A.N.; Bhargava, B.; Parvatalu, D.; Banerjee, S. Catalytic performance of bimetallic Ni-Pt nanoparticles supported on activated carbon, gamma-alumina, zirconia, and ceria for hydrogen production in sulfur-iodine thermochemical cycle. Int. J. Hydrogen Energy 2016, 41, 10538–10546. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; He, H.; Zhang, J.; Liu, J.; Wang, D.; Huang, L.; Tu, Z. Preparation, Performances and Mechanisms of Co@AC Composite for Herbicide Atrazine Removal in Water. Water 2021, 13, 240. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa-Fernández, E.J. Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J. Supercrit. Fluids 2018, 140, 196–206. [Google Scholar] [CrossRef]
- Marzano, M.; Pontón, P.I.; Marinkovic, B.A. Co-precipitation of low-agglomerated Y2W3O12 nanoparticles: The effects of aging time, calcination temperature and surfactant addition. Ceram. Int. 2019, 45, 20189–20196. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. Biochar efficiency in pesticides sorption as a function of production variables--a review. Environ. Sci. Pollut. Res. Int. 2015, 22, 13824–13841. [Google Scholar] [CrossRef]
- Al-Senani, G.M.; Deraz, N.M.; Abd-Elkader, O.H. Magnetic and Characterization Studies of CoO/Co3O4 Nanocomposite. Processes 2020, 8, 844. [Google Scholar] [CrossRef]
- Fakhri, A.; Behrouz, S.; Tyagi, I.; Agarwal, S.; Gupta, V.K. Synthesis and characterization of ZrO2 and carbon-doped ZrO2 nanoparticles for photocatalytic application. J. Mol. Liq. 2016, 216, 342–346. [Google Scholar] [CrossRef]
- Huang, B.Y.; Liu, Y.G.; Li, B.; Liu, S.B.; Zeng, G.M.; Zeng, Z.W.; Wang, X.H.; Ning, Q.M.; Zheng, B.H.; Yang, C.P. Effect of Cu(II) ions on the enhancement of tetracycline adsorption by Fe3O4@SiO2-Chitosan/graphene oxide nanocomposite. Carbohydr. Polym. 2017, 157, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Nuithitikul, K.; Srikhun, S.; Hirunpraditkoon, S. Kinetics and equilibrium adsorption of Basic Green 4 dye on activated carbon derived from durian peel: Effects of pyrolysis and post-treatment conditions. J. Taiwan Inst. Chem. Eng. 2010, 41, 591–598. [Google Scholar] [CrossRef]
- Aydin, H.; Gunduz, B.; Aydin, C. Surface morphology, spectroscopy, optical and conductivity properties of transparent poly(9-vinylcarbazole) thin films modified with graphene oxide. Synth. Met. 2019, 252, 1–7. [Google Scholar] [CrossRef]
- Munoz, A.J.; Espinola, F.; Ruiz, E. Biosorption of Ag(I) from aqueous solutions by Kiebsiella sp. 3S1. J. Hazard. Mater. 2017, 329, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Lin, H.; Nong, Q.; Wu, Y.; Wu, T.; He, Y. Synthesis and characterization of a ZrO2/g-C3N4 composite with enhanced visible-light photoactivity for rhodamine degradation. RSC Adv. 2014, 4, 40029–40035. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, W.L.; He, H.; Yang, C.; Yu, J.; Wu, X.; Zeng, G.; Tarre, S.; Green, M. Preparation, performances and mechanisms of magnetic Saccharomyces cerevisiae bionanocomposites for atrazine removal. Chemosphere 2018, 200, 380–387. [Google Scholar] [CrossRef]
- Sun, S.W.; He, H.J.; Yang, C.P.; Cheng, Y.; Liu, Y.P. Effects of Ca2+ and fulvic acids on atrazine degradation by nano-TiO2: Performances and mechanisms. Sci. Rep. 2019, 9, 11. [Google Scholar] [CrossRef]
- Wei, X.; Wu, Z.; Wu, Z.; Ye, B.-C. Adsorption behaviors of atrazine and Cr(III) onto different activated carbons in single and co-solute systems. Powder Technol. 2018, 329, 207–216. [Google Scholar] [CrossRef]
- Salvestrini, S.; Sagliano, P.; Iovino, P.; Capasso, S.; Colella, C. Atrazine adsorption by acid-activated zeolite-rich tuffs. Appl. Clay Sci. 2010, 49, 330–335. [Google Scholar] [CrossRef]
- Lladó, J.; Lao-Luque, C.; Ruiz, B.; Fuente, E.; Solé-Sardans, M.; Dorado, A.D. Role of activated carbon properties in atrazine and paracetamol adsorption equilibrium and kinetics. Process Saf. Environ. Prot. 2015, 95, 51–59. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Dominguez-Vargas, J.R.; Olivares-Marin, F.J.; de Heredia, J.B. On the use of carbon blacks as potential low-cost adsorbents for the removal of non-steroidal anti-inflammatory drugs from river water. J. Hazard. Mater. 2010, 177, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Sbizzaro, M.; Sampaio, S.C.; dos Reis, R.R.; de Assis Beraldi, F.; Rosa, D.M.; de Freitas Maia, C.M.; do Nascimento, C.T.; da Silva, E.A.; Borba, C.E. Effect of production temperature in biochar properties from bamboo culm and its influences on atrazine adsorption from aqueous systems. J. Mol. Liq. 2021, 343, 117667. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, S.; Zhang, Y.; Xu, J.; Qiu, L.; Wang, L. Investigation into adsorption characteristics and mechanism of atrazine on nano-MgO modified fallen leaf biochar. J. Environ. Chem. Eng. 2021, 9, 105727. [Google Scholar] [CrossRef]
- Manzotti, F.; dos Santos, O.A.A. Evaluation of removal and adsorption of different herbicides on commercial organophilic clay. Chem. Eng. Commun. 2019, 206, 1515–1532. [Google Scholar] [CrossRef]
- Liu, H.-c.; Chen, W.; Cui, B.; Liu, C. Enhanced atrazine adsorption from aqueous solution using chitosan-modified sepiolite. J. Cent. South Univ. 2015, 22, 4168–4176. [Google Scholar] [CrossRef]
- Toledo-Jaldin, H.P.; Blanco-Flores, A.; Sanchez-Mendieta, V.; Martin-Hernandez, O. Influence of the chain length of surfactant in the modification of zeolites and clays. Removal of atrazine from water solutions. Environ. Technol. 2018, 39, 2679–2690. [Google Scholar] [CrossRef]
- Tüysüz, M.; Köse, K.; Aksüt, D.; Uzun, L.; Evci, M.; Köse, D.A.; Youngblood, J.P. Removal of Atrazine Using Polymeric Cryogels Modified with Cellulose Nanomaterials. Water Air Soil Pollut. 2022, 233, 472. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; Schnorr, C.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Rhoden, C.R.B.; Dotto, G.L. Microporous activated carbon from the fruits of the invasive species Hovenia dulcis to remove the herbicide atrazine from waters. J. Mol. Liq. 2022, 364, 120014. [Google Scholar] [CrossRef]
- Lazarotto, J.S.; da Boit Martinello, K.; Georgin, J.; Franco, D.S.P.; Netto, M.S.; Piccilli, D.G.A.; Silva, L.F.O.; Lima, E.C.; Dotto, G.L. Application of araçá fruit husks (Psidium cattleianum) in the preparation of activated carbon with FeCl3 for atrazine herbicide adsorption. Chem. Eng. Res. Des. 2022, 180, 67–78. [Google Scholar] [CrossRef]
- Ateia, M.; Ceccato, M.; Budi, A.; Ataman, E.; Yoshimura, C.; Johnson, M.S. Ozone-assisted regeneration of magnetic carbon nanotubes for removing organic water pollutants. Chem. Eng. J. 2018, 335, 384–391. [Google Scholar] [CrossRef]
- Binh, Q.A.; Nguyen, V.-H.; Kajitvichyanukul, P. Influence of pyrolysis conditions of modified corn cob bio-waste sorbents on adsorption mechanism of atrazine in contaminated water. Environ. Technol. Innov. 2022, 26, 102381. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S.; et al. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on pi-pi electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef] [PubMed]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]





| Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (nm) | Pore Size (nm) | |
|---|---|---|---|---|
| AC | 881 | 0.611 | 0.371 | 2.853 |
| Co/Zr@AC | 1079 | 0.705 | 0.458 | 2.737 |
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|
| k1 | Qcal(mg/g) | R2 | k2 | Qcal(mg/g) | R2 |
| 0.0183 | 42.656 | 0.885 | 0.0021 | 117.925 | 0.999 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | Kf ((mg/g)/(mg/L)n) | n (g/L) | R2 |
| 272.26 | 14.07 | 0.997 | 31.25 | 1.82 | 0.981 |
| Adsorbent | T (K) | C0(mg/L) | SBET (m2/g) | Qm (mg/g) | Reference |
|---|---|---|---|---|---|
| MgO modified fallen leaf biochar | 298 | 2–12 | 93 | 22.40 | [45] |
| Commercial organophilic clay | 298 | 10 | 2.13 | 2.28 | [46] |
| Chitosan-modified sepiolit | 298 | 2–20 | 71 | 17.92 | [47] |
| surfactant modified clay | 298 | 5 | / | 4.24 | [48] |
| Cellulose nanofiber modified polymeric cryogels | 298 | 250 | / | 95.76 | [49] |
| Activated carbon from Hovenia dulcis | 298 | 30 | 898 | 58.00 | [50] |
| Activated carbon with FeCl3 | 298 | 5–40 | 431 | 55.85 | [51] |
| Magnetic carbon nanotubes | 298 | 1–30 | 96 | 57.80 | [52] |
| HCl modified corncob biochar | 298 | 1–25 | 350 | 19.58 | [53] |
| N-doped biochars | 298 | 5 | 700 | 103.60 | [54] |
| Co/Zr@AC | 298 | 10 | 1079 | 112.74 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Liu, Y.; He, H.; Liu, J.; Yang, X.; Zhang, L.; Tang, Y.; Zhu, H. Functional Bimetal/Carbon Composites Co/Zr@AC for Pesticide Atrazine Removal from Water. Molecules 2023, 28, 2071. https://doi.org/10.3390/molecules28052071
Liu D, Liu Y, He H, Liu J, Yang X, Zhang L, Tang Y, Zhu H. Functional Bimetal/Carbon Composites Co/Zr@AC for Pesticide Atrazine Removal from Water. Molecules. 2023; 28(5):2071. https://doi.org/10.3390/molecules28052071
Chicago/Turabian StyleLiu, Danxia, Yongpan Liu, Huijun He, Jie Liu, Xiaolong Yang, Lin Zhang, Yiyan Tang, and Hongxiang Zhu. 2023. "Functional Bimetal/Carbon Composites Co/Zr@AC for Pesticide Atrazine Removal from Water" Molecules 28, no. 5: 2071. https://doi.org/10.3390/molecules28052071
APA StyleLiu, D., Liu, Y., He, H., Liu, J., Yang, X., Zhang, L., Tang, Y., & Zhu, H. (2023). Functional Bimetal/Carbon Composites Co/Zr@AC for Pesticide Atrazine Removal from Water. Molecules, 28(5), 2071. https://doi.org/10.3390/molecules28052071







