Modeling, Simulation, Optimization, and Experimental Verification of Mercury Removal onto Natural and Sulfur-Impregnated Zeolite Clinoptilolite—Assessment of Feasibility for Remediation of Mercury-Contaminated Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sorption Experiments
2.2.1. Equilibrium Study
2.2.2. Kinetic Study
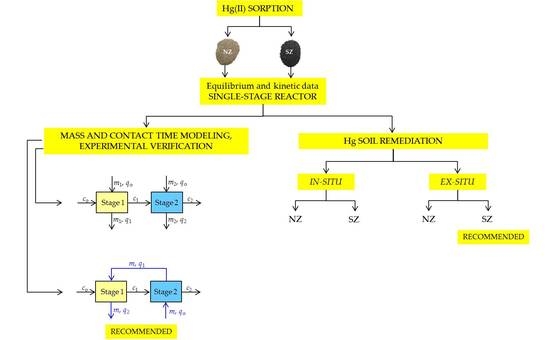
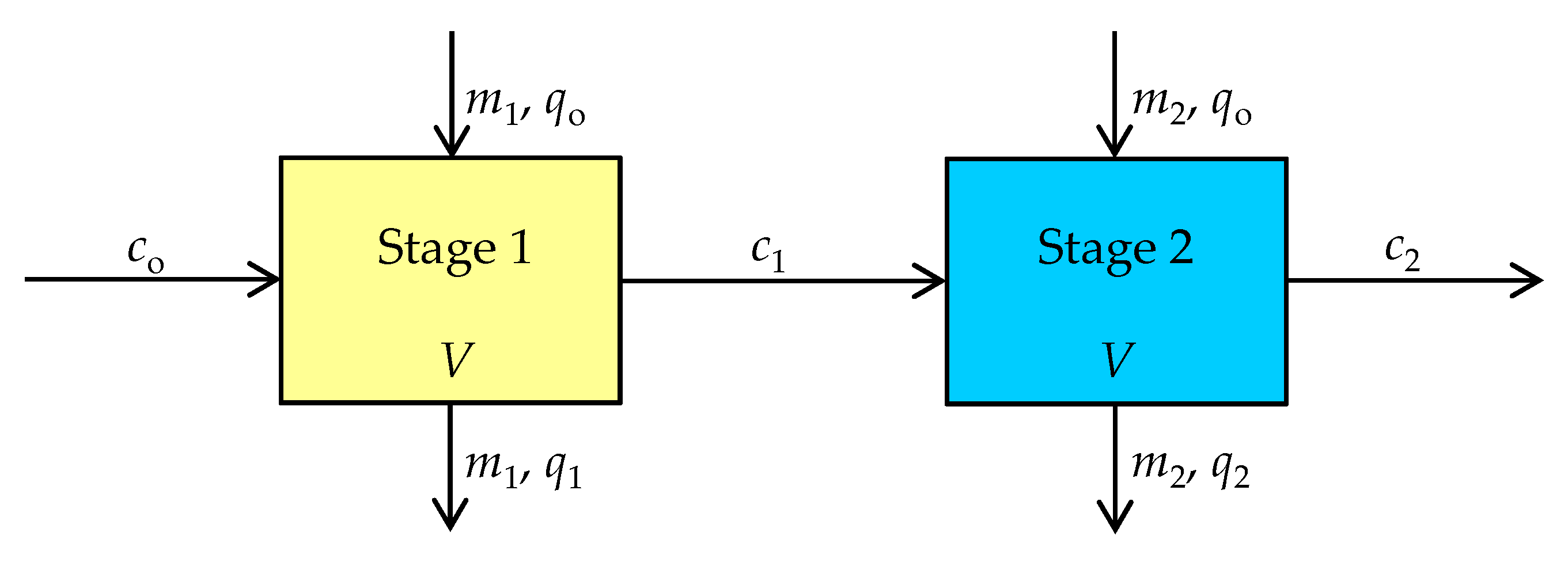
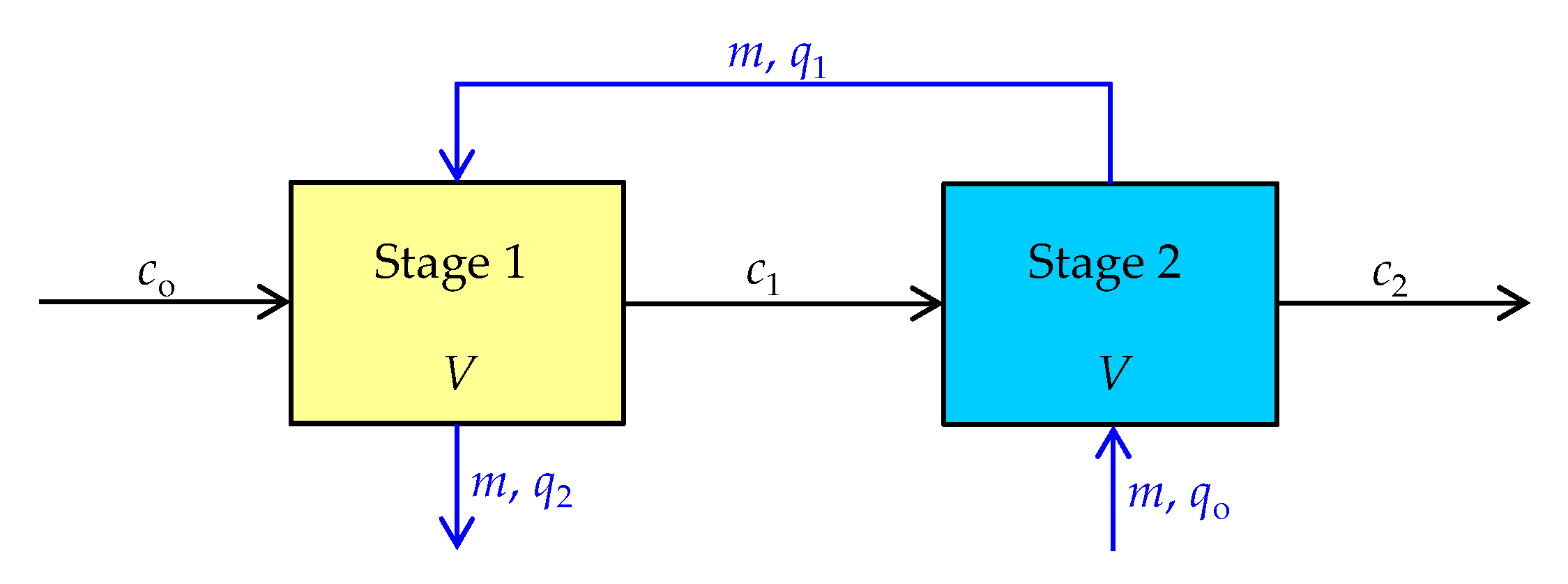
2.2.3. Kinetic Study in the Two-Stages Reactors
2.3. Experiments with a Mercury-Contaminated Soil
2.3.1. Soil Sample Preparation
2.3.2. Leaching Experiments According to the Toxicity Characteristic Leaching Procedure
2.4. Calculation of Sorption and Leaching Parameters
2.5. Isotherm and Kinetic Sorption Models
2.5.1. Isotherm Models
2.5.2. Kinetic Models
2.6. Statistical Data Analysis
3. Results and Discussion
3.1. Modeling of Equilibrium Data
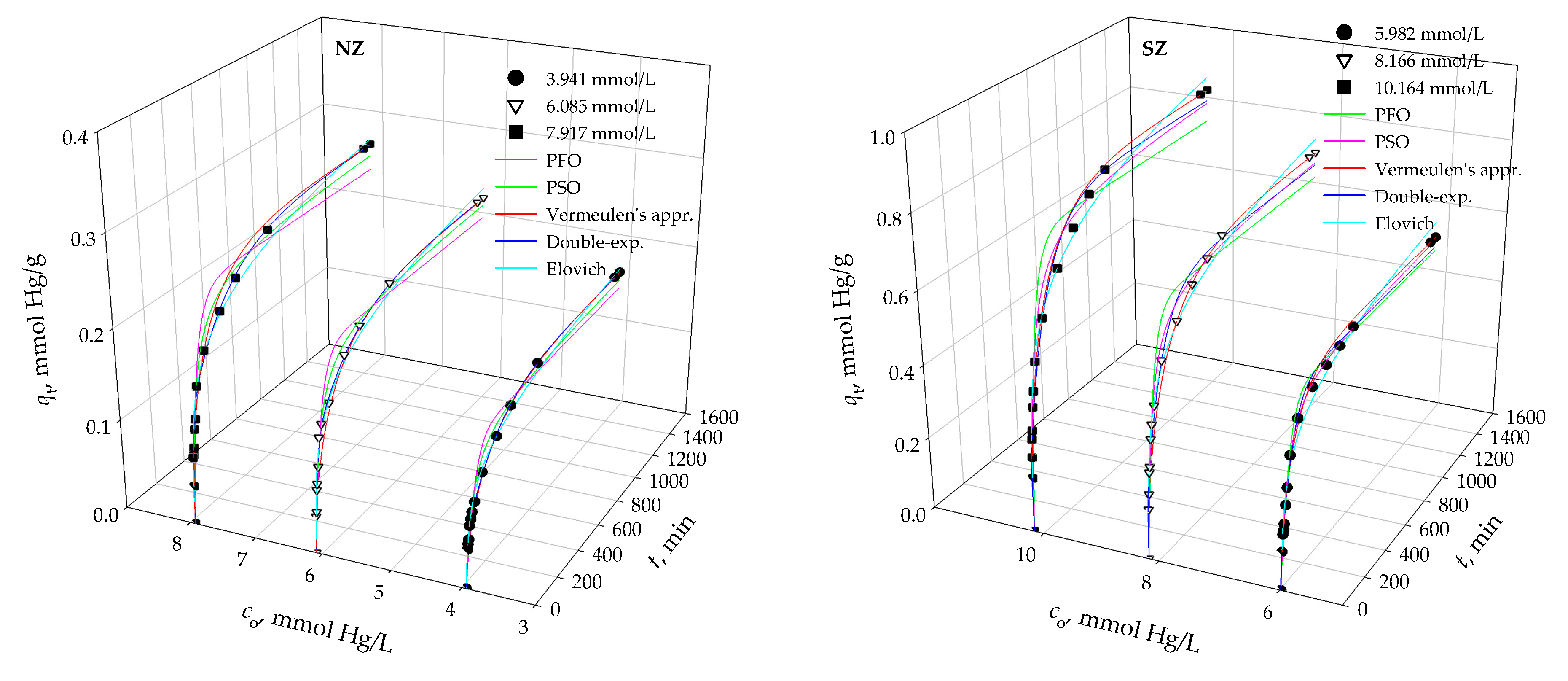
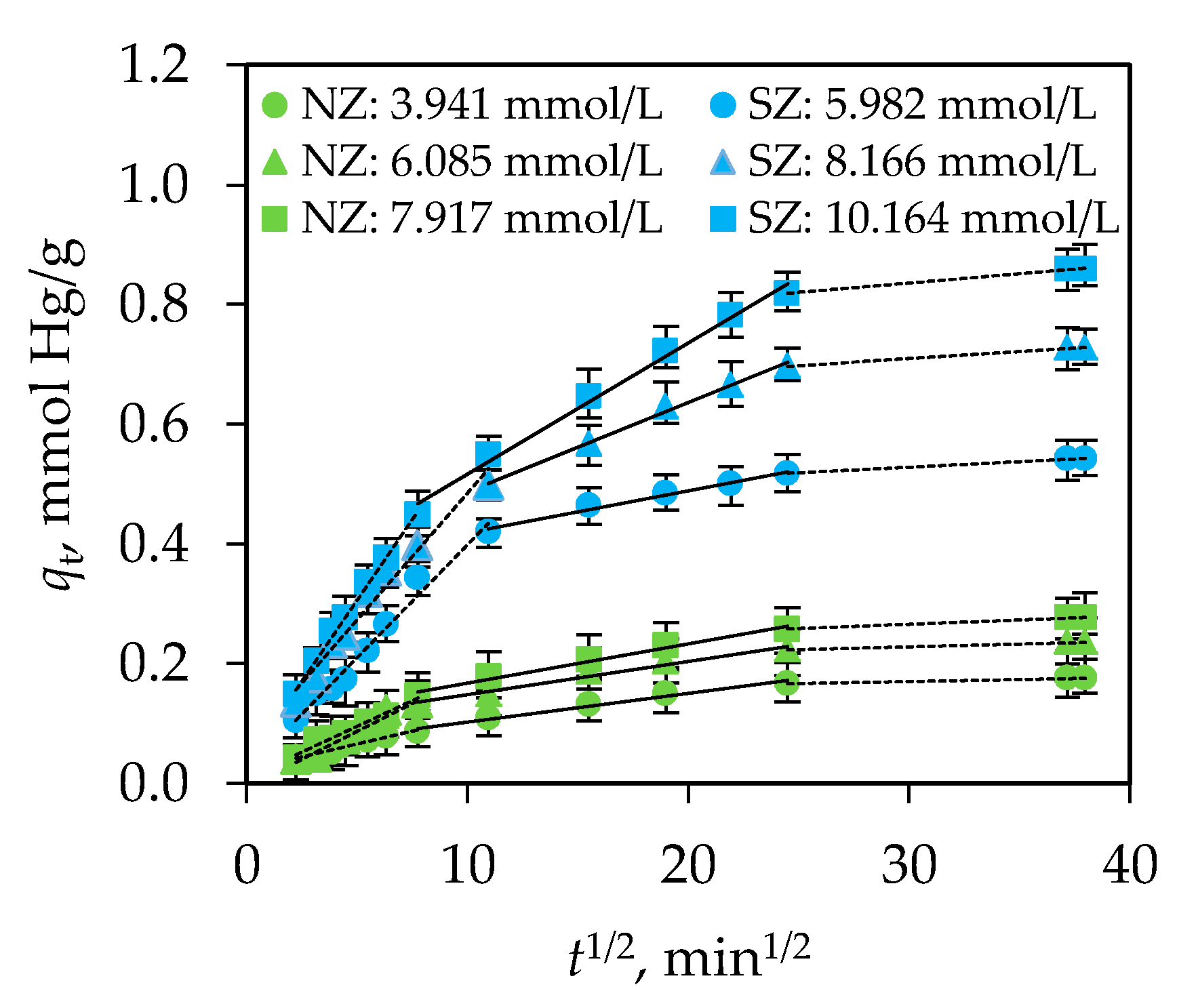
3.2. Modeling of Kinetic Data
3.3. Experimental Verification of the Model Application
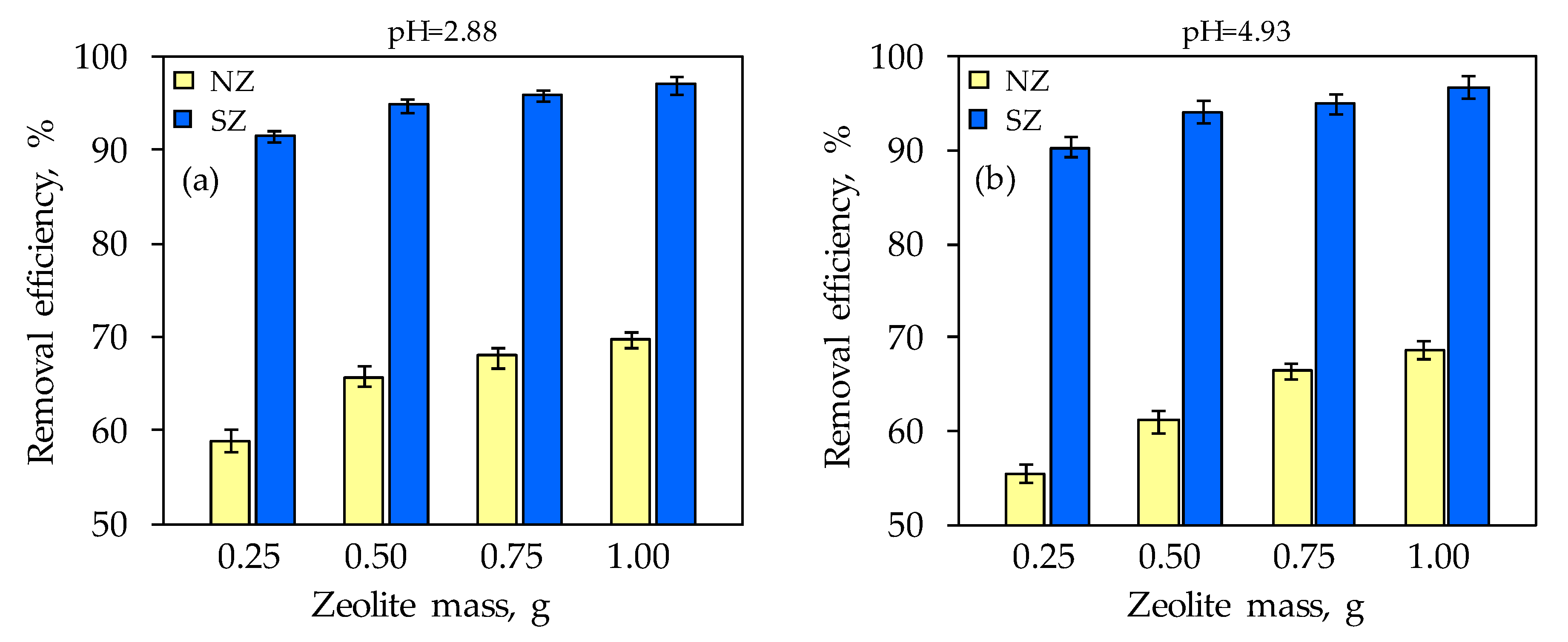
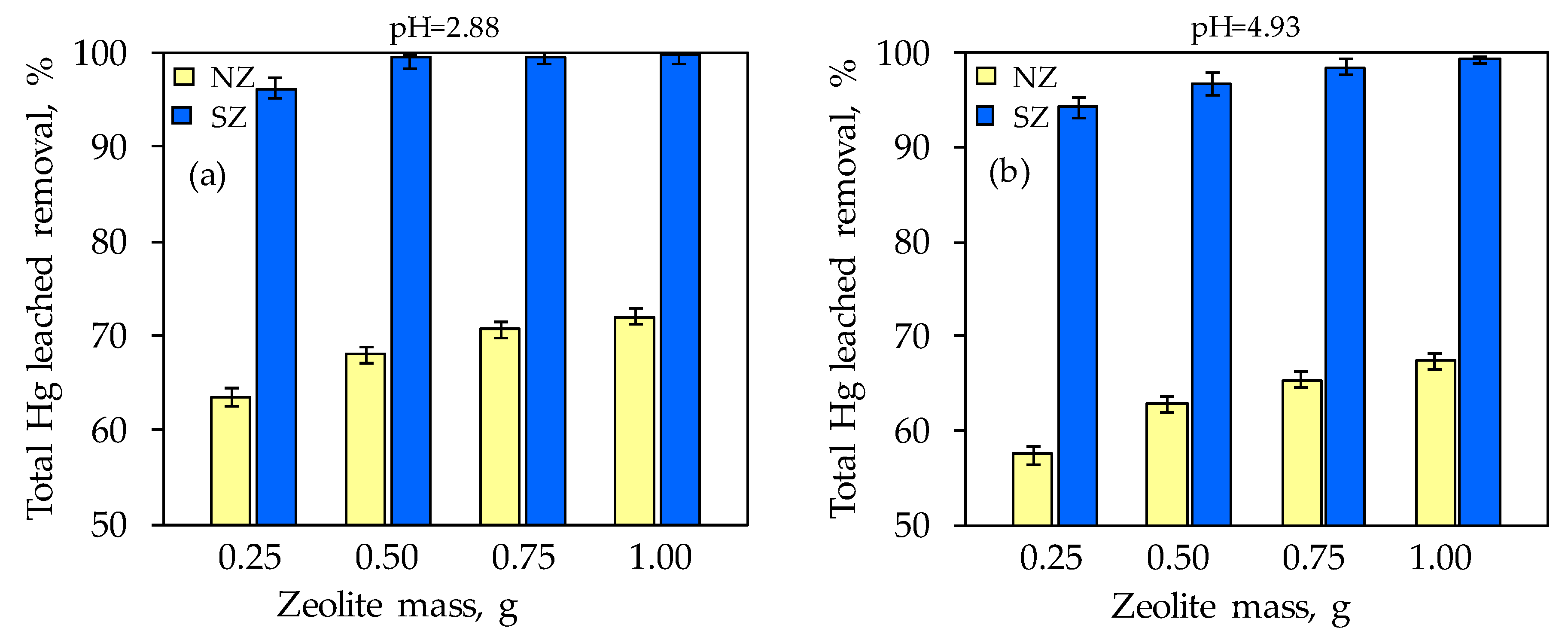
3.4. Mercury Leaching from the Mercury-Contaminated Soil and Its Sorption onto Natural and Sulfur-Impregnated Zeolite
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AAS | Atomic Absorption Spectrophotometer |
| B1 | sorbed Hg(II) concentration in the rapid step (mmol/L) |
| B2 | sorbed Hg(II) concentration in the slow step (mmol/L) |
| BT | heat of sorption obtained from the Temkin isotherm (kJ/mol) |
| c1 | Hg(II) concentration after first stage (mmol/L |
| c2 | Hg(II) concentration after second stage (mmol/L) |
| ce | equilibrium Hg(II) concentration (mmol/L) |
| cHg, raw soil | concentration of total leached Hg from raw soil (mg/L) |
| cHg, treated soil | concentration of total leached Hg from raw soil treated with zeolites (mg/L) |
| co | initial Hg(II) concentration (mmol/L) |
| dp | zeolite particle diameter (cm) |
| DV | intraparticle diffusion coefficient obtained from the Vermeulen’s approximation (cm2/min) |
| DWM | diffusion coefficient obtained from the Weber-Morris model (cm2/min) |
| E | mean free sorption energy (kJ/mol) |
| I | thickness of the boundary layer (mmol/g) |
| ICP-ES | inductively coupled plasma emission spectrometry |
| k1 | rate constant of the PFO (1/min) |
| k2 | rate constant of the PSO [g/(mmol∙min)] |
| kB1 | double exponential rapid rate constants (1/min) |
| kB2 | double exponential slow rate constants (1/min) |
| KBS | Brouers-Sotolongo isotherm constant (L/mmol) |
| KDR | Dubinin-Raduskevich isotherm constant (mol2/kJ) |
| KF | Freundlich constant (L/mmol) |
| KK | Khan isotherm constant (L/mmol) |
| KL | Langmuir constant (L/mmol) |
| KLF | Langmuir-Freundlich isotherm constant (L/mmol) |
| KT | Temkin isotherm equilibrium binding constant (L/mmol) |
| kWM | Weber-Morris diffusion constant [mmol/(g∙min1/2)] |
| kWM | Weber-Morris diffusion constant (cm2/min) |
| m | zeolite mass (g) |
| m1 | zeolite mass in the first stage (g) |
| m2 | zeolite mass in the second stage (g) |
| nF | Freundlich exponent constant indicative to the sorption intensity and surface heterogeneity (-) |
| NZ | natural zeolite |
| PFO | pseudo-first order kinetic model |
| PSO | pseudo-second order kinetic model |
| q1 | amount of Hg(II) sorbed on zeolite in the first stage of the two-stage reactor (mmol/g) |
| q2 | amount of Hg(II) sorbed on zeolite in the second stage of the two-stage reactor (mmol/g) |
| qe | amount of Hg(II) sorbed on zeolite in equilibrium (mmol/g) |
| qm | maximum sorption capacity obtained from the model |
| qt | amount of Hg(II) sorbed on zeolites in time t (mmol/g) |
| R | gas constant [8.314 J/(mol∙K)] |
| r | overall sorption rate [mmol/(g∙min)] |
| r1 | sorption rate of the rapid step [mmol/(g∙min)] |
| R1 | percentage of Hg(II) removal in the first stage (%) |
| r2 | sorption rate of the slow step [mmol/(g∙min)] |
| R2 | linear correlation coefficients |
| r2 | non-linear correlation coefficients |
| R2 | percentage of Hg(II) removal in the second stage (%) |
| RC | relative coefficient (%) |
| RF | proportion of Hg(II) sorbed in rapid step (%) |
| RL | Langmuir separation factor (-) |
| RMSE | root mean square error |
| rp | zeolite particle radius (cm) |
| rpm | revolutions per minute |
| Rtotal | total percentage of Hg(II9 removal in the first and second stages (%) |
| SEM-EDS | scanning electron microscopy with energy dispersive X-ray spectroscopy |
| SF | proportion of Hg(II) sorbed in slow step (%) |
| SZ | sulfur-impregnated zeolite |
| T | absolute temperature (K) |
| t | time (min) |
| TCLP | toxicity characteristic leaching procedure |
| V | volume of the solution (L) and m is the mass of zeolite (g) |
| XRPD | X-ray powder diffraction |
| αe | sorption efficiency in equilibrium (%) |
| αE | initial chemisorption rate of the Elovich model [mmol/(g·min)] |
| αleach | removal efficiency of leached Hg (%) |
| βE | Elovich constant related to the extent of surface coverage (g/mmol) |
| βBS | Brouers-Sotolongo isotherm parameter related with energy distribution and surface heterogeneity |
| βK | Khan isotherm exponent |
| βLF | Langmuir-Freundlich isotherm exponent (-) |
| βT | Temkin constant |
| ε | Polany potential (kJ/mol) |
| χ2 | non-linear chi-square test |
References
- Talabi, A.O.; Kayode, T.J. Groundwater Pollution and Remediation. J. Water Resour. Prot. 2019, 11, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Tomiyama, S.; Igarashi, T. The potential threat of mine drainage to groundwater resources. Curr. Opin. Environ. Sci. Health 2022, 27, 100347. [Google Scholar] [CrossRef]
- Khobragade, K. Impact of Mining Activity on environment: An Overview. Int. J. Sci. Res. Public 2020, 10, 784–791. [Google Scholar] [CrossRef]
- Bavec, Š.; Gosar, M. Introduction to geochemical studies in the Idrija urban environment. In Environmental Influences of Mercury Ore Processing—Case Studies Selected at Slovenian, Mexican, Hungarian Group Meeting in Idrija in July 2012; Gosar, M., Dizdarevič, T., Miler, M., Eds.; Geological Survey of Slovenia & Idrija Mercury Mine, Ltd.: Ljubljana, Slovenia, 2012; pp. 28–34. [Google Scholar]
- Miklavčič, A.; Mazej, D.; Jaćimović, R.; Dizdarevič, T.; Horvat, M. Mercury in food items from the Idrija Mercury Mine area. Environ. Res. 2013, 125, 61–68. [Google Scholar] [CrossRef]
- Río Segade, S.; Dias, T.; Ramalhosa, E. Mercury methylation versus demethylation: Main processes involved. In Methylmercury: Formation, Sources and Health Effects; Clampet, A.P., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 1–32. [Google Scholar]
- Gosar, M.; Teršić, T. Environmental geochemistry studies in the area of Idrija mercury mine, Slovenia. Environ. Geochem. Health 2012, 34, 27–41. [Google Scholar] [CrossRef]
- Teršič, T.; Biester, H.; Gosar, M. Leaching of mercury from soils at extremely contaminated historical roasting sites (Idrija area, Slovenia). Geoderma 2014, 226–227, 213–222. [Google Scholar] [CrossRef]
- Teršič, T.; Gosar, M.; Biester, H. Environmental impact of ancient small-scale mercury ore processing at Pšenk on soil (Idrija area, Slovenia). Appl. Geochem. 2011, 26, 1867–1876. [Google Scholar] [CrossRef]
- Teršič, T.; Gosar, M.; Biester, H. Distribution and speciation of mercury in soil in the area of an ancient mercury ore roasting site, Frbejžene trate (Idrija area, Slovenia). J. Geochem. Explor. 2011, 110, 136–145. [Google Scholar] [CrossRef]
- Randall, P.M.; Chattopadhyay, S. Mercury contaminated sediment sites—An evaluation of remedial options. Environ. Res. 2013, 125, 131–149. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Sik Ok, Y.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.G.; Rinklebe, J. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef]
- Wang, L.; Hou, D.; Cao, Y.; Ok, Y.S.; Tack, F.M.G.; Rinklebe, J.; O’Connor, D. Remediation of mercury contaminated soil, water, and air: A review of emerging materials and innovative technologies. Environ. Int. 2020, 134, 105281. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef]
- Xu, J.; Garcia Bravo, A.; Lagerkvist, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Y.; Hua, Y.; Peng, B.; Deng, M.; Yan, N.; Qu, Z. A sulfur-resistant CuS-modified active coke for mercury removal from municipal solid waste incineration flue gas. Environ. Sci. Pollut. Res. Int. 2019, 26, 24831–24839. [Google Scholar] [CrossRef]
- Abdelouahab-Reddam, Z.; Wahby, A.; El Mail, R.; Silvestre-Albero1, J.; Rodríguez-Reinoso, R.; Sepúlveda-Escribano, A. Activated Carbons Impregnated with Na2S and H2SO4: Texture, Surface Chemistry and Application to Mercury Removal from Aqueous Solutions. Adsorp. Sci. Technol. 2014, 32, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Asasian, N.; Kaghazchi, T. Sulfurized activated carbons and their mercury adsorption/desorption behavior in aqueous phase. Int. J. Environ. Sci. Technol. 2015, 12, 2511–2522. [Google Scholar] [CrossRef] [Green Version]
- Silva, H.S.; Ruiz, S.V.; Granados, D.L.; Santángelo, J.M. Adsorption of Mercury (II) from Liquid Solutions Modified Activated Carbons. Mater. Res. 2010, 13, 129–134. [Google Scholar] [CrossRef]
- Tan, G.; Sun, W.; Xu, Y.; Wang, H.; Xu, N. Sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef]
- Yuan, C.-S.; Lin, H.-Y.; Wu, C.-H.; Liu, M.-H.; Hung, C.-H. Preparation of Sulfurized Powdered Activated Carbon from Waste Tires Using an Innovative Compositive Impregnation Process. J. Air Waste Manag. Assoc. 2004, 54, 862–870. [Google Scholar] [CrossRef] [Green Version]
- Kede, C.M.; Ndibewu, P.P.; Kalumba, M.M.; Panichev, N.A.; Ngomo, H.M.; Ketcha, J.M. Adsorption of Mercury(II) onto activated carbons derived from Theobroma cacao pod husk. S. Afr. J. Chem. 2015, 68, 226–235. [Google Scholar] [CrossRef]
- Habila, M.A.; AlOthman, Z.A.; Ghfar, A.A.; Al-Zaben, M.I.M.; Alothman, A.A.S.; Abdeltawab, A.A.; El-Marghany, A.; Sheikh, M. Phosphonium-based Ionic Liquid Modified Activated Carbon from Mixed Recyclable Waste for Mercury(II) Uptake. Molecules 2019, 24, 570. [Google Scholar] [CrossRef] [Green Version]
- Chojancki, A.; Chojancka, K.; Hoffmann, J.; Górecki, H. The application of natural zeolites for mercury removal: From laboratory tests to industrial scale. Miner. Eng. 2004, 17, 933–937. [Google Scholar] [CrossRef]
- Gebremedhin-Haile, T.; Olguín, M.T.; Solache-Ríos, M. Removal of mercury ions from mixed aqueous metal solutions by natural and modified zeolitic minerals. Water Air Soil Pollut. 2003, 148, 179–200. [Google Scholar] [CrossRef]
- Misealides, P.; Godelitsas, A. Removal of heavy metals from aqueous solutions using pretreated natural zeolitic materials: The case of mercury(II). Toxicol. Environ. Chem. 1995, 51, 21–29. [Google Scholar] [CrossRef]
- Ugrina, M.; Gaberšek, M.; Daković, A.; Nuić, I. Preparation and Characterization of the Sulfur-Impregnated Natural Zeolite Clinoptilolite for Hg(II) Removal from Aqueous Solutions. Processes 2021, 9, 217. [Google Scholar] [CrossRef]
- Praus, P.; Motáková, M.; Ritz, M. Montmorillonite ion exchanged by mercury (II). Acta Geodyn. Geomater. 2012, 9, 63–70. [Google Scholar]
- Brigatti, M.F.; Colonna, S.; Malferrari, D.; Medici, L.; Poppi, L. Mercury adsorption by montmorillonite and vermiculite: A combined XRD, TG-MS, and EXAFS study. Appl. Clay Sci. 2005, 28, 1–8. [Google Scholar] [CrossRef]
- Trakarnpruk, W.; Chirandorn, N. Treated Clay for Adsorption of Mercury(II) Ions. J. Sci. Res. Chula. Univ. 2005, 30, 137–150. [Google Scholar]
- Margeta, K.; Zabukovec Logar, N.; Šiljeg, M.; Farkaš, M. Natural Zeolites in Water Treatment—How Effective is Their Use. In Water Treatment; Elshorbagy, W., Chowdhury, R.K., Eds.; IntechOpen: London, UK, 2013; pp. 81–112. [Google Scholar] [CrossRef] [Green Version]
- Busca, G. Acidity and basicity of zeolites. A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metals from wastewaters: A review. J Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Hu, X.; Xue, Y.; Liu, L.; Zeng, Y.; Long, L. Preparation and characterization of Na2S-modified biochar for nickel removal. Environ. Sci. Pollut. Res. 2018, 25, 9887–9895. [Google Scholar] [CrossRef]
- Wajima, T.; Sugawara, K. Adsorption behaviors of mercury from aqueous solution using sulfur-impregnated adsorbent developed from coal. Fuel Process. Technol. 2011, 92, 1322–1327. [Google Scholar] [CrossRef]
- Al-Ghouti, M.; Da’ana, D.; Abu-Dieyeh, M.; Khraisheh, M. Adsorptive removal of mercury from water by adsorbents derived from daze pits. Sci. Rep. 2019, 9, 15327–15340. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Vidyarthi, S.R. Enhanced sorption of mercury from compact fluorescent bulbs and contaminated water streams using functionalized multiwalled carbon nanotubes. J. Hazard. Mater. 2014, 275, 132–144. [Google Scholar] [CrossRef]
- Cai, J.H.; Jia, C.Q. Mercury Removal from Aqueous Solution Using Coke-Derived Sulfur-Impregnated Activated Carbons. Ind. Eng. Chem. Res. 2010, 49, 2716–2721. [Google Scholar] [CrossRef]
- Blais, J.F.; Djedidi, Z.; Ben Cheikh, R.; Tyagi, R.D.; Mercier, G. Metals Precipitation from Effluents: Review. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2008, 12, 135–149. [Google Scholar] [CrossRef]
- Torres, E. Biosorption: A Review of the Latest Advances. Processes 2020, 8, 1584. [Google Scholar] [CrossRef]
- Bazargan, A.; Shek, T.-H.; Hui, C.-W.; McKay, G. Optimising batch adsorbers for the removal of zinc from effluents using a sodium diimidoacetate ion exchange resin. Adsorption 2017, 23, 477–489. [Google Scholar] [CrossRef]
- Cooney, D.O. Adsorption Design for Wastewater Treatment; Lewis Publishers: Boca Raton, FL, USA, 1999. [Google Scholar]
- Di Martino, M.; Sannino, F.; Pirozzi, D. Removal of pesticide from wastewater: Contact time optimization for a two-stage batch stirred adsorber. J. Environ. Chem. Eng. 2015, 3, 365–372. [Google Scholar] [CrossRef]
- Pirozzi, D.; Sannino, F. Design of a multi-stage stirred adsorber using mesoporous metal oxides for herbicide removal from wastewaters. J. Environ. Chem. Eng. 2014, 2, 211–219. [Google Scholar] [CrossRef]
- Torres-Perez, J.; Huang, Y.; Bazargan, A.; Khoshand, A.; McKay, G. Two-stage optimization of Allura direct red dye removal by treated peanut hull waste. SN Appl. Sci. 2020, 2, 475. [Google Scholar] [CrossRef] [Green Version]
- Ugrina, M.; Vukojević Medvidović, N.; Daković, A. Characterization and environmental application of iron-modified zeolite from the Zlatokop deposit. Desalin. Water Treat. 2015, 53, 3557–3569. [Google Scholar] [CrossRef]
- USEPA. Method 1311: Toxicity Characteristic Leaching Procedure; US Environmental Protection Agency: Washington, DC, USA, 2004.
- Foo, K.Y.; Hameed, B.H. Insight into the modelling of adsorption isotherm systems, review. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and interpretation of adsorption isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Ramadoss, R.; Subramaniam, D. Removal of divalent nickel from aqueous solution using blue-green marine algae: Adsorption modeling and applicability of various isotherm models. Sep. Sci. Technol. 2019, 54, 943–961. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica, and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Uber die adsorption in losungen. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I.; Pyzhev, V. Kinetic of Ammonia Synthesis on Promoted Iron Catalyst. Acta Phys. Chem. 1940, 12, 327–356. [Google Scholar]
- Xu, H.; Zhu, S.; Xia, M.; Wang, F.; Ju, X. Three-dimension hierarchical composite via in-situ growth of Zn/Al layered double hydroxide plates onto polyaniline-wrapped carbon sphere for efficient naproxen removal. J. Hazard. Mater. 2022, 423, 127192. [Google Scholar] [CrossRef] [PubMed]
- Dubinin, M.M.; Radushkevich, L.V. The equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk Sssr. 1947, 55, 331–337. [Google Scholar]
- Radushkevich, L.V. Potential theory of sorption and structure of carbons. Zhurnal Fiz. Khimii 1949, 23, 1410–1420. [Google Scholar]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically non-uniform surface. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Khan, A.R.; Ataullah, R.; Al–Haddad, A. Equilibrium adsorption studies of some aromatic pollutants from dilute aqueous solutions on activated carbon at different temperatures. J. Colloid Interface Sci. 1997, 194, 154–156. [Google Scholar] [CrossRef]
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Brouers, F.; Sotolongo, O.; Marquez, F.; Pirard, J.P. Microporous and heterogeneous surface adsorption isotherms arising from Levy distributions. Physica A 2005, 349, 271–282. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue onto activated carbon. J. Hazard. Mater. B 2006, 137, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gaur, J.P. Chemical reaction- and particle diffusion-based kinetic modeling of metal biosorption by a phormidium sp.-dominated cyanobacterial mat. Bioresour. Technol. 2011, 102, 633–640. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larinov, O.G. Theory of adsorption from solutions of non-electrolytes on solid (I) equation adsorption from solutions and the analysis of its simplest form, (II) verification of the equation of adsorption isotherm from solutions. Izvestiya Akademii Nauk. SSSR Otd. Khim. Nauk. 1962, 2, 209–216. [Google Scholar]
- Sparks, D.L. Kinetics of sorption/release reactions on natural particles. In Structure and Surface Reactions of Soil Particles; Huang, P.M., Senesi, N., Buffle, J., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 1998; pp. 413–448. [Google Scholar]
- Xu, H.; Zhu, S.; Lu, K.; Jia, H.; Xia, M.; Wang, F. Preparation of hierarchically floral ZIF-8 derived carbon@polyaniline@Ni/Al layered double hydroxides composite with outstanding removal phenomenon for saccharin. Chem. Eng. J. 2022, 450, 138127. [Google Scholar] [CrossRef]
- Apiratikul, R.; Pavasant, P. Sorption of Cu2+, Cd2+, and Pb2+ using modified zeolite from coal fly ash. Chem. Eng. J. 2008, 144, 245–258. [Google Scholar] [CrossRef]
- Wilczak, A.; Keinath, T.M. Kinetics of sorption and desorption of copper(II) and lead(II) on activated carbon. Water Environ. Res. 1993, 65, 238–244. [Google Scholar] [CrossRef]
- Chiron, N.; Guilet, R.; Deydier, E. Adsorption of Cu(II) and Pb(II) onto a grafted silica: Isotherms and kinetic models. Water Res. 2003, 37, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Tosun, I. Ammonium removal from aqueous solutions by clinoptilolite: Determination of isotherm and thermodynamic parameters and comparison of kinetics by the Double exponential model and conventional kinetic models. Int. J. Environ. Res. Public Health 2012, 9, 970–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helferich, F. Ion Exchange; Mc Graw-Hill Inc.: New York, NY, USA, 1962; pp. 250–322. [Google Scholar]
- Vijayaraghavan, K.; Padmesh, T.V.N.; Palanivelu, K.; Velan, M. Biosorption of nickel(II) ions onto Sargassum wightii: Application of two-parameter and three-parameter isotherm models. J. Hazard. Mater. B1 2006, 33, 304–308. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, R.; Gao, W.; Li, M. Sulfur-modified chitosan hydrogel as an adsorbent for removal of Hg(II) from effluents. Fibers Polym. 2017, 18, 1229–1234. [Google Scholar] [CrossRef]
- Kara, A.; Demirbel, E. Kinetic, Isotherm and Thermodynamic Analysis on Adsorption of Cr(VI) Ions from Aqueous Solutions by Synthesis and Characterization of Magnetic-Poly(divinylbenzene-vinylimidazole) Microbeads. Water Air Soil Pollut. 2012, 223, 2387–2403. [Google Scholar] [CrossRef] [Green Version]
- Mishima, K.; Du, X.; Miyamoto, N.; Kano, N.; Imaizumi, H. Experimental and Theoretical Studies on the Adsorption Mechanisms of Uranium (VI) Ions on Chitosan. J. Funct. Biomater. 2018, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Ugrina, M.; Čeru, T.; Nuić, I.; Trgo, M. Comparative Study of Mercury(II) Removal from Aqueous Solutions onto Natural and Iron-Modified Clinoptilolite Rich Zeolite. Processes 2020, 8, 1523. [Google Scholar] [CrossRef]
- Xu, J.; Kleja, D.B.; Biester, H.; Lagerkvist, A.; Kumpiene, J. Influence of particle size distribution, organic carbon, pH and chlorides on washing of mercury contaminated soil. Chemosphere 2014, 109, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Allen, H.E.; Li, Y.; Huang, C.P.; Sanders, P.F. Adsorption of Mercury(II) by Soil: Effects of pH, Chloride, and Organic Matter. J. Environ. Qual. 1996, 25, 837–844. [Google Scholar] [CrossRef]
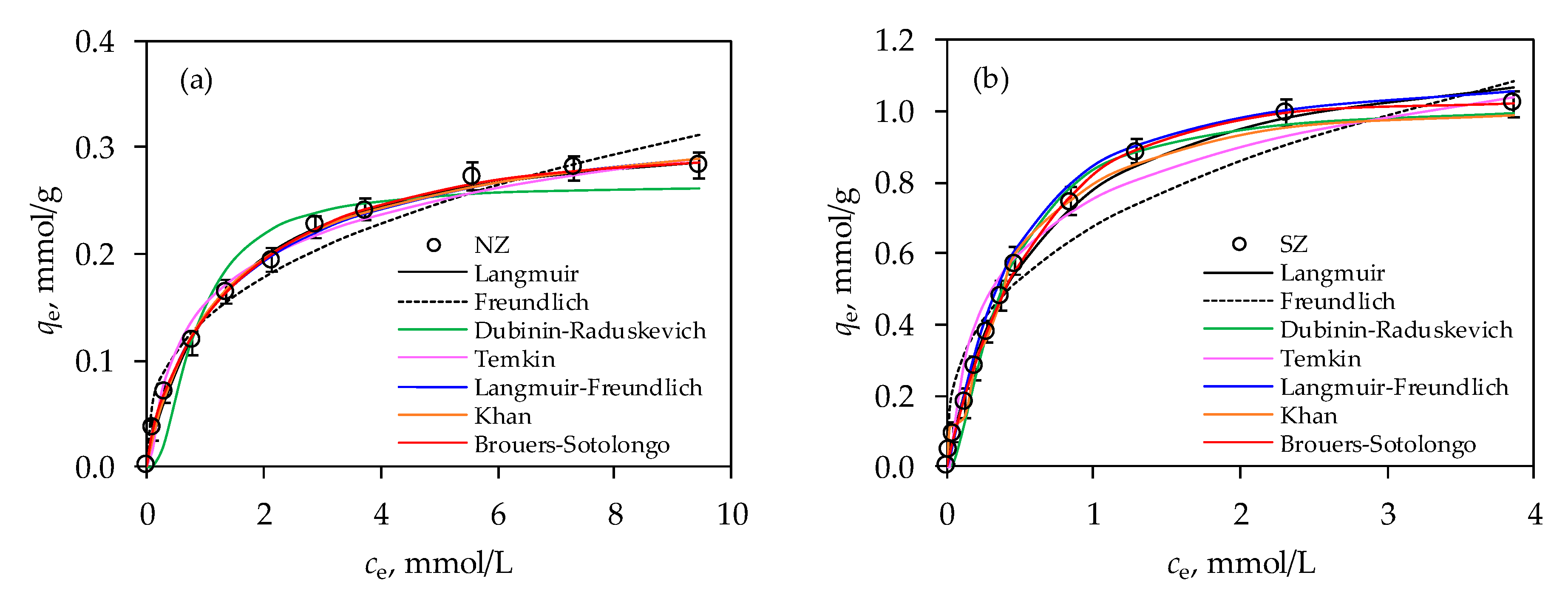


| Isotherm Model | Parameters | NZ | SZ |
|---|---|---|---|
| qe (mmol/g) | 0.282 | 1.024 | |
| Langmuir | qm (mmol/g) KL (L/mmol) RL r2 RMSE χ2 | 0.325 0.761 0.279 0.996 6.322∙10−3 4.839∙10−5 | 1.227 1.710 0.168 0.998 0.026 1.669∙10−3 |
| Freundlich | KF (L/mmol) nF r2 RMSE χ2 | 0.138 2.760 0.959 0.018 2.863∙10−3 | 0.673 2.848 0.960 0.102 0.018 |
| Temkin | KT (L/g) βT BT (kJ/mol) r2 RMSE χ2 | 12.157 40.586 0.061 0.983 0.011 2.269·10−4 | 31.882 11.643 0.213 0.971 0.094 2.131·10−4 |
| Dubinin-Radushkevich | qm (mmol/g) E (kJ/mol) KDR (mol2/kJ) r2 RMSE χ2 | 0.264 1.623 0.190 0.940 0.025 1.640∙10−3 | 1.017 2.711 0.068 0.991 0.041 8.334∙10−4 |
| Langmuir-Freundlich | qm (mmol/g) KS (L/mmol) βS r2 RMSE χ2 | 0.352 0.626 0.762 0.997 4.940∙10−3 2.128∙10−4 | 1.124 2.466 0.911 0.999 0.015 3.205∙10−4 |
| Khan | qm (mmol/g) KK (L/mmol) βK r2 RMSE χ2 | 0.271 1.001 0.929 0.995 6.044∙10−3 2.169∙10−4 | 1.010 1.326 0.847 0.994 9.012∙10−3 4.091∙10−4 |
| Brouers-Sotolongo | qm (mmol/g) KBS (L/mmol) βBS r2 RMSE χ2 | 0.297 0.627 0.738 0.998 3.492∙10−3 6.552∙10−5 | 1.025 1.558 0.950 0.999 0.011 4.776∙10−4 |
| co [mmol/L] | ce × 102 [mmol/L] | NZ | SZ |
|---|---|---|---|
| mtotal [g] | mtotal [g] | ||
| 0.460 | 0.046 | 717.30 | 426.27 |
| 1.000 | 0.100 | 879.87 | 443.40 |
| 1.950 | 0.195 | 1049.39 | 458.90 |
| 3.000 | 0.300 | 1176.15 | 469.39 |
| 4.060 | 0.406 | 1274.55 | 477.04 |
| 5.150 | 0.515 | 1357.89 | 483.25 |
| 6.140 | 0.614 | 1423.17 | 487.98 |
| 8.280 | 0.828 | 1541.92 | 496.34 |
| 10.100 | 1.010 | 1626.65 | 502.14 |
| 12.260 | 1.226 | 1714.15 | 508.04 |
| co [mmol/L] | c2 × 102 [mmol/L] | NZ | SZ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| c1 [mmol/L] | m1 [g] | m2 [g] | mtotal [g] | c1 [mmol/L] | m1 [g] | m2 [g] | mtotal [g] | ||
| 0.460 | 0.046 | 0.021 | 41.55 | 32.06 | 73.61 | 0.016 | 14.35 | 14.41 | 28.76 |
| 1.000 | 0.100 | 0.045 | 52.20 | 38.75 | 90.95 | 0.034 | 15.50 | 14.65 | 30.15 |
| 1.950 | 0.195 | 0.090 | 62.25 | 47.43 | 109.68 | 0.066 | 16.54 | 15.09 | 31.63 |
| 3.000 | 0.300 | 0.140 | 70.48 | 53.76 | 124.24 | 0.100 | 17.64 | 15.19 | 32.83 |
| 4.060 | 0.406 | 0.190 | 77.51 | 58.43 | 135.94 | 0.140 | 17.88 | 15.99 | 33.87 |
| 5.150 | 0.515 | 0.258 | 80.07 | 66.60 | 146.67 | 0.170 | 19.34 | 15.48 | 34.82 |
| 6.140 | 0.614 | 0.280 | 90.79 | 63.54 | 154.33 | 0.200 | 20.22 | 15.42 | 35.64 |
| 8.280 | 0.828 | 0.370 | 102.47 | 67.43 | 169.90 | 0.280 | 20.99 | 16.30 | 37.29 |
| 10.100 | 1.010 | 0.450 | 110.61 | 70.92 | 181.53 | 0.340 | 22.24 | 16.41 | 38.65 |
| 12.260 | 1.226 | 0.550 | 118.80 | 75.26 | 194.06 | 0.410 | 23.73 | 16.50 | 40.23 |
| co [mmol/L] | c2 × 102 [mmol/L] | NZ | SZ | ||
|---|---|---|---|---|---|
| c1 [mmol/L] | mtotal [g] | c1 [mmol/L] | mtotal [g] | ||
| 0.460 | 0.046 | 0.025 | 38.32 | 0.016 | 14.65 |
| 1.000 | 0.100 | 0.055 | 47.42 | 0.036 | 15.37 |
| 1.950 | 0.195 | 0.108 | 57.30 | 0.070 | 16.14 |
| 3.000 | 0.300 | 0.169 | 65.03 | 0.110 | 16.78 |
| 4.060 | 0.406 | 0.231 | 71.31 | 0.151 | 17.33 |
| 5.150 | 0.515 | 0.296 | 76.84 | 0.195 | 17.86 |
| 6.140 | 0.614 | 0.357 | 81.33 | 0.243 | 17.88 |
| 8.280 | 0.828 | 0.491 | 89.92 | 0.329 | 19.28 |
| 10.100 | 1.010 | 0.608 | 96.46 | 0.414 | 20.09 |
| 12.260 | 1.226 | 0.752 | 103.53 | 0.520 | 21.07 |
| Kinetic Model/Parameters | NZ | SZ | ||||
|---|---|---|---|---|---|---|
| co [mmol/L] qe [mmol/g] | 3.941 0.176 | 6.085 0.235 | 7.917 0.277 | 5.982 0.542 | 8.166 0.727 | 10.164 0.859 |
| Pseudo-first order model (PFO) qm [mmol/g] k1 [1/min] r2 RMSE χ2 × 103 | 0.157 0.018 0.899 0.019 2.205 | 0.213 0.018 0.952 0.019 2.353 | 0.248 0.016 0.945 0.025 3.414 | 0.504 0.020 0.977 0.033 2.888 | 0.658 0.020 0.950 0.060 7.326 | 0.773 0.017 0.946 0.075 9.522 |
| Pseudo-second order model (PSO) qm [mmol/g] k2 [g/(mmol∙min)] r2 RMSE χ2 × 104 | 0.171 0.138 0.961 0.013 5.428 | 0.234 0.097 0.985 0.010 3.060 | 0.273 0.073 0.981 0.014 6.697 | 0.547 0.048 0.992 0.018 1.604 | 0.718 0.035 0.985 0.032 1.182 | 0.849 0.025 0.982 0.043 1.637 |
| Elovich model αE [mmol/(g·min)] βE [g/mmol] r2 RMSE χ2 × 104 | 0.016 37.736 0.989 0.017 7.925 | 0.017 26.247 0.982 0.011 4.717 | 0.018 22.523 0.979 0.019 7.637 | 0.050 11.507 0.971 0.033 30.080 | 0.063 8.718 0.980 0.026 19.020 | 0.062 7.236 0.972 0.030 14.230 |
| Weber-Morris intra-particle diffusion model kWM1 [mmol/(g∙min1/2)] DWM1 × 107 [cm2/min] I RC [%] R2 kWM2 × 108 [mmol/(g∙min1/2)] DWM2 [cm2/min] R2 | 0.009 2.563 0.023 3.927 0.962 0.008 2.445 0.976 | 0.019 6.743 0.007 3.016 0.968 0.006 6.047 0.976 | 0.017 4.165 0.009 3.140 0.973 0.007 5.879 0.986 | 0.058 1.236 0.038 7.046 0.994 0.018 1.126 0.987 | 0.050 5.129 0.066 9.101 0.999 0.020 8.403 0.986 | 0.040 2.398 0.084 9.828 0.994 0.016 3.617 0.976 |
| Double exponential model qm [mmol/g] kB1 [1/min] B1 [mmol/L] kB2 × 103 [1/min] B2 [mmol/L] r1 × 103 [mmol/(g∙min)] r2 × 104 [mmol/(g∙min1/2)] r × 103 [mmol/(g∙min1/2)] RF [%] SF [%] r2 RMSE χ2∙103 | 0.175 0.039 0.552 3.447 1.181 2.153 4.071 2.560 31.852 68.148 0.997 0.660 3.684 | 0.236 0.048 1.074 3.648 1.249 5.155 4.556 5.611 46.233 53.767 0.995 0.717 7.666 | 0.277 0.059 1.006 3.738 1.683 5.935 6.291 6.564 37.412 62.588 0.994 0.194 2.757 | 0.514 3.454 0.854 6.259 4.286 294.972 26.830 297.655 16.615 83.385 0.991 0.018 1.542 | 0.693 3.694 1.622 8.421 5.309 599.167 44.710 603.638 23.402 76.598 0.982 0.033 1.711 | 0.830 3.671 1.935 8.446 6.360 710.339 53.720 715.711 23.327 76.673 0.985 0.036 1.003 |
| Vermeulen’s approximation qm [mmol/g] DV × 107 (cm2/min) r2 RMSE χ2 × 104 | 0.176 5.391 0.987 0.119 1.485 | 0.235 5.981 0.988 0.162 1.134 | 0.277 6.352 0.993 0.185 1.008 | 0.542 5.677 0.992 0.398 0.541 | 0.727 6.801 0.992 0.519 0.726 | 0.858 8.184 0.994 0.598 0.857 |
| co [mmol/L] | c1 [mmol/L] | c2 × 102 [mmol/L] | m1 [g] | m2 [g] | mtotal [g] | t1 [min] | t2 [min] | t1 + t2 [min] |
|---|---|---|---|---|---|---|---|---|
| 0.460 | 0.021 | 0.046 | 41.55 | 32.06 | 73.61 | 22.71 | 9.42 | 32.13 |
| 1.000 | 0.045 | 0.100 | 52.20 | 38.75 | 90.95 | 20.67 | 9.17 | 29.84 |
| 1.950 | 0.090 | 0.195 | 62.25 | 47.43 | 109.68 | 19.81 | 8.44 | 28.25 |
| 3.000 | 0.140 | 0.300 | 70.48 | 53.76 | 124.24 | 18.87 | 8.06 | 26.93 |
| 4.060 | 0.190 | 0.406 | 77.51 | 58.43 | 135.94 | 17.94 | 7.86 | 25.80 |
| 5.150 | 0.258 | 0.515 | 80.07 | 66.60 | 146.67 | 18.71 | 6.98 | 25.69 |
| 6.140 | 0.280 | 0.614 | 90.79 | 63.54 | 154.33 | 15.85 | 7.95 | 23.80 |
| 8.280 | 0.370 | 0.828 | 102.47 | 67.43 | 169.90 | 14.29 | 8.02 | 22.31 |
| 10.100 | 0.450 | 1.010 | 110.61 | 70.92 | 181.53 | 13.44 | 7.94 | 21.38 |
| 12.260 | 0.550 | 1.226 | 118.80 | 75.26 | 194.06 | 12.73 | 7.74 | 20.46 |
| co [mmol/L] | c1 [mmol/L] | c2 × 102 [mmol/L] | m1 [g] | m2 [g] | mtotal [g] | t1 [min] | t2 [min] | t1 + t2 [min] |
|---|---|---|---|---|---|---|---|---|
| 0.460 | 0.016 | 0.046 | 14.35 | 14.41 | 28.77 | 73.43 | 36.56 | 109.99 |
| 1.000 | 0.034 | 0.100 | 15.50 | 14.65 | 30.15 | 71.65 | 35.86 | 107.51 |
| 1.950 | 0.066 | 0.195 | 16.54 | 15.09 | 31.63 | 70.71 | 35.33 | 106.04 |
| 3.000 | 0.100 | 0.300 | 17.64 | 15.19 | 32.83 | 67.70 | 37.11 | 104.81 |
| 4.060 | 0.140 | 0.406 | 17.88 | 15.99 | 33.87 | 69.01 | 34.01 | 103.02 |
| 5.150 | 0.170 | 0.515 | 19.34 | 15.48 | 34.82 | 61.63 | 38.47 | 100.10 |
| 6.140 | 0.200 | 0.614 | 20.22 | 15.42 | 35.64 | 58.52 | 40.59 | 99.11 |
| 8.280 | 0.280 | 0.828 | 20.99 | 16.30 | 37.29 | 58.39 | 39.52 | 97.91 |
| 10.100 | 0.340 | 1.010 | 22.24 | 16.41 | 38.65 | 54.09 | 41.12 | 95.21 |
| 12.260 | 0.410 | 1.226 | 23.73 | 16.50 | 40.23 | 49.28 | 42.81 | 92.09 |
| co [mmol/L] | c1 [mmol/L] | c2 × 102 [mmol/L] | m1 = m2 [g] | 1st Stage t1 [min] | 2nd Stage t2 [min] | t1 + t2 [min] |
|---|---|---|---|---|---|---|
| 0.460 | 0.025 | 0.046 | 38.32 | 22.11 | 7.19 | 29.30 |
| 1.000 | 0.055 | 0.100 | 47.42 | 20.70 | 6.75 | 27.45 |
| 1.950 | 0.108 | 0.195 | 57.30 | 19.31 | 6.34 | 25.64 |
| 3.000 | 0.169 | 0.300 | 65.03 | 18.27 | 6.04 | 24.31 |
| 4.060 | 0.231 | 0.406 | 71.31 | 17.45 | 5.81 | 23.26 |
| 5.150 | 0.296 | 0.515 | 76.84 | 16.75 | 5.61 | 22.37 |
| 6.140 | 0.357 | 0.614 | 81.33 | 16.19 | 5.46 | 21.65 |
| 8.280 | 0.491 | 0.828 | 89.92 | 15.15 | 5.18 | 20.33 |
| 10.100 | 0.608 | 1.010 | 96.46 | 14.39 | 4.97 | 19.36 |
| 12.260 | 0.752 | 1.226 | 103.53 | 13.61 | 4.76 | 18.37 |
| co [mmol/L] | c1 [mmol/L] | c2 × 102 [mmol/L] | m1 = m2 [g] | 1st Stage t1 [min] | 2nd Stage t2 [min] | t1 + t2 [min] |
|---|---|---|---|---|---|---|
| 0.460 | 0.016 | 0.046 | 14.65 | 69.66 | 37.36 | 107.02 |
| 1.000 | 0.036 | 0.100 | 15.37 | 71.03 | 34.51 | 105.54 |
| 1.950 | 0.070 | 0.195 | 16.14 | 71.43 | 31.67 | 103.10 |
| 3.000 | 0.110 | 0.300 | 16.78 | 71.40 | 30.27 | 101.67 |
| 4.060 | 0.151 | 0.406 | 17.33 | 70.79 | 29.29 | 100.08 |
| 5.150 | 0.195 | 0.515 | 17.86 | 70.64 | 29.45 | 100.09 |
| 6.140 | 0.243 | 0.614 | 17.88 | 74.00 | 31.29 | 105.29 |
| 8.280 | 0.329 | 0.828 | 19.28 | 67.46 | 28.86 | 96.32 |
| 10.100 | 0.414 | 1.010 | 20.09 | 64.66 | 28.14 | 92.80 |
| 12.260 | 0.520 | 1.226 | 21.07 | 60.91 | 27.08 | 87.99 |
| co [mmol/L] | c1 [mmol/L] | c2 × 102 [mmol/L] | Total Removal [%] | Percentage Deviation [%] | |||
|---|---|---|---|---|---|---|---|
| Model | Exp. | Model | Exp. | Model | Exp. | ||
| 0.460 | 0.016 | 0.031 | 0.046 | 0.978 | 99.90 | 97.87 | 2.03 |
| 1.000 | 0.034 | 0.052 | 0.100 | 0.891 | 99.90 | 99.11 | 0.79 |
| 1.950 | 0.066 | 0.093 | 0.195 | 0.821 | 99.90 | 99.58 | 0.32 |
| 3.000 | 0.100 | 0.141 | 0.300 | 1.019 | 99.90 | 99.66 | 0.24 |
| 4.060 | 0.140 | 0.208 | 0.406 | 0.908 | 99.90 | 99.78 | 0.12 |
| 5.150 | 0.170 | 0.214 | 0.515 | 1.470 | 99.90 | 99.71 | 0.19 |
| 6.140 | 0.200 | 0.294 | 0.614 | 1.510 | 99.90 | 99.75 | 0.15 |
| 8.280 | 0.280 | 0.336 | 0.828 | 2.040 | 99.90 | 99.75 | 0.15 |
| 10.100 | 0.340 | 0.441 | 1.010 | 2.750 | 99.90 | 99.73 | 0.17 |
| 12.260 | 0.410 | 0.497 | 1.226 | 2.861 | 99.90 | 99.77 | 0.13 |
| co [mmol/L] | c1 [mmol/L] | c2 × 102 [mmol/L] | Total Removal [%] | Percentage Deviation [%] | |||
|---|---|---|---|---|---|---|---|
| Model | Exp. | Model | Exp. | Model | Exp. | ||
| 0.460 | 0.016 | 0.019 | 0.046 | 0.952 | 99.90 | 97.93 | 1.97 |
| 1.000 | 0.036 | 0.039 | 0.100 | 0.859 | 99.90 | 99.14 | 0.76 |
| 1.950 | 0.070 | 0.076 | 0.195 | 0.948 | 99.90 | 99.51 | 0.39 |
| 3.000 | 0.110 | 0.151 | 0.300 | 0.966 | 99.90 | 99.68 | 0.22 |
| 4.060 | 0.151 | 0.193 | 0.406 | 0.713 | 99.90 | 99.82 | 0.08 |
| 5.150 | 0.195 | 0.226 | 0.515 | 1.640 | 99.90 | 99.68 | 0.22 |
| 6.140 | 0.243 | 0.269 | 0.614 | 1.321 | 99.90 | 99.78 | 0.12 |
| 8.280 | 0.329 | 0.363 | 0.828 | 1.938 | 99.90 | 99.77 | 0.13 |
| 10.100 | 0.414 | 0.428 | 1.010 | 2.206 | 99.90 | 99.78 | 0.12 |
| 12.260 | 0.520 | 0.532 | 1.226 | 2.433 | 99.90 | 99.80 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugrina, M.; Jurić, A.; Nuić, I.; Trgo, M. Modeling, Simulation, Optimization, and Experimental Verification of Mercury Removal onto Natural and Sulfur-Impregnated Zeolite Clinoptilolite—Assessment of Feasibility for Remediation of Mercury-Contaminated Soil. Processes 2023, 11, 606. https://doi.org/10.3390/pr11020606
Ugrina M, Jurić A, Nuić I, Trgo M. Modeling, Simulation, Optimization, and Experimental Verification of Mercury Removal onto Natural and Sulfur-Impregnated Zeolite Clinoptilolite—Assessment of Feasibility for Remediation of Mercury-Contaminated Soil. Processes. 2023; 11(2):606. https://doi.org/10.3390/pr11020606
Chicago/Turabian StyleUgrina, Marin, Antonija Jurić, Ivona Nuić, and Marina Trgo. 2023. "Modeling, Simulation, Optimization, and Experimental Verification of Mercury Removal onto Natural and Sulfur-Impregnated Zeolite Clinoptilolite—Assessment of Feasibility for Remediation of Mercury-Contaminated Soil" Processes 11, no. 2: 606. https://doi.org/10.3390/pr11020606
APA StyleUgrina, M., Jurić, A., Nuić, I., & Trgo, M. (2023). Modeling, Simulation, Optimization, and Experimental Verification of Mercury Removal onto Natural and Sulfur-Impregnated Zeolite Clinoptilolite—Assessment of Feasibility for Remediation of Mercury-Contaminated Soil. Processes, 11(2), 606. https://doi.org/10.3390/pr11020606