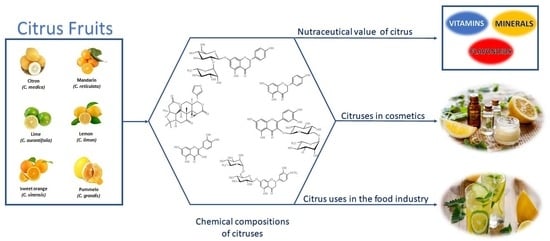
The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review
Abstract
1. Introduction
2. The Chemical Profiles and Compositions of Citruses
| Plant Part (Origin) Variety | Isolate/Isolation Method | Major Chemical Components | Reference |
|---|---|---|---|
| Lemon (C. limon L.) | |||
| Fruit (China) | EO, Hydrodistillation | (R)-(+)-Limonene (46%), Geranial (15.9%), Neral (10.6%), Citronellal (4.7%), α-Terpineol (4.0%), (−)-Isopulegol (3.9%), Linalool (2.3%). | [30] |
| Pulp (Indonesia) | Ethanolic, n-hexane, ethyl acetate extracts | Total phenolics (1.4–14.7 µg GAE/g), Flavonoids (8–30 µg QE/g), Luteolin-7-O-glucoside (0.024%), Rutin, Quercetin. | [31] |
| Peel (Tunisia) Beldi | Hydroethanolic extract | Total phenolics (105–204 mg GAE/g), Flavonoids (27–56 mg QE/g), Flavonols (9–26 mg RE/g), Condensed tannins (26–138 mg CE/g), Caffeoyl N-Tryptophan, Vicenin 2, Eriocitrin, Kaempferol-3-O-rutinoside, Quercetin-3-rutinoside, | [32] |
| Peel (Iraq) | Ethanolic, methanolic, ethyl acetate extracts | Coumarin, Ascorbic acid, Citric acid, Linoleic acid, Limonoid, Malic acid, D-Limonene, β-Carotene. | [29] |
| Peel (Indonesia) | Ethanolic, n-hexane, ethyl acetate extracts | Gallic acid (23.9 mg/L), 1,2-dihydroxybenzene (23.0 mg/L), Total phenolics (9–15.2 µg GAE/g), Flavonoids (25–29 µg QE/g). | [31] |
| Peel | EO, British Pharmacopoeia | D-Limonene (82.9%), β-Phellandrene (1.6%), β-Pinene (1.5%), γ-Terpinene (9.9%), β-Cymene (1.3%), α-Limonene diepoxide (1.2%). | [33] |
| Peel (Ethiopia) | EO, Clevenger-type apparatus | Limonene (49.7%), β-Pinene (17.1%), γ-Terpinene (7.5%), o-Cymene (2.2%), β-Bisabolene (2.4%), β-Caryophyllene (1.5%). | [34] |
| Peel (Nigeria) Osbeck | EO, Clevenger-type apparatus | Limonene (85.9%), Sabinene (3.9%), Myrcene (3.1%), Linalool (0.5%). | [35] |
| Peel (Iran) | EO, Clevenger-type apparatus | Limonene (61.4%), β-Pinene (13.1%), γ-Terpinene (11.3%), α-Pinene (2.4%), Sabinene (2.3%), Myrcene (1.6%), Geranial (1.5%), Neral (1.1%). | [36] |
| Peel (Algeria) | EO, Cold-pressing | Limonene (64.8%), γ-Terpinene (11.7%), β-Pinene (11.2%), α-Pinene (1.9%), β-Myrcene (1.7%), Geranial (1.7%), β-Bisabolen (1.0%). | [37] |
| Peel (Algeria) Eureka | EO, Clevenger-type apparatus | Limonene (61.3%), β-Pinene (9.7%), α-Citral (4.2%), γ-Terpinene (3.8%), cis-Citral (2.4%), β-Elemene (2.2%). | [38] |
| Peel (India) Burf | EO, Clevenger-type apparatus | Limonene (55.4%), Neral (10.4%), trans-Verbenol (6.4%), Decanal (3.3%), Ethyl cinnamate (2.2%), Ethyl p-methoxycinnamate (2.2%), cis-α-Bergamotene (1.6%), Geraniol (1.5%), trans-Carveol (1.3%), Nonanal (1.2%), Linalool (1.2%), α-Terpineol (1.1%). | [39] |
| Root (Cameroon) | Methanolic extract | Clausarin, Xanthyletin, Suberosin, E-suberenol, E-Methoxysuberenol, Thamnosmonin, Angelitriol, Hopey-hopin, Formlylumbelliferone, Atalantaflavone, Limonin, 1-(10–19) abeo-7α-acetoxy-10 β-hydroxyisoobacunoic acid-3,10-lactone. | [40] |
| Root (Cameroon) | EO, Hydrodistillation | Hexadecanoic acid, methyl ester (39.3%), β-Bisabolene (10.1%), (E)-9-octadecenoic acid, methyl ester (9.3%), α-Santalene (8%), Elemol (6.2%), (E)-5-Octadecene (6.1%), 1-Octadecene (5.7%). | [40] |
| Flowers (Tunisia) Osbeck | EO, Clevenger-type apparatus | Limonene (39.7%), β-Pinene (25.4%), α-Terpineol (7.3%), Nerolidol (6.9%), Farnesol (4.3%), Linalyl acetate (3.0%), Geranyl acetate (3.0%), Linalool (2.2%), Neryl acetate (1.7%). | [41] |
| Leaf (Cameroon) | Methanolic extract | Bergapten, 5-Hydroxy-6,7,8,4′-tetramethoxy flavone, 5-Hydroxy-6,7,8,3′,4′-pentamethoxy flavone, 5,4′-Dihydroxy-6,7,3′-trimethoxy flavone, 5,4′-Dihydroxy-6,7,8,3′-tetramethoxy flavone, 5,6,7,8,4′-Pentamethoxy flavone, 5-Hydroxynoracronicine, Asperfenamate, Stigmasterol, Sitosterol, Sitosterol-3-O-β-D-glucoside. | [40] |
| Leaf (Cameroon) Osbeck | Ethanolic, acetone, water extract | Alkaloids (12.2%), Saponins (5.5%), Total phenolics (208–289 mg GAE/g), Total flavonoids (447–1053 mg QE/g). | [42] |
| Leaf (Nigeria) Osbeck | EO, Clevenger-type apparatus | Limonene (31.5%), Sabinene (15.9%), Linalool (4.6%), (E)-β-Ocimene (3.9%), Myrcene (2.9%), α-Pinene (1.2%). | [35] |
| Leaf (Iran) | EO, Clevenger-type apparatus | Linalool (30.6%), Geraniol (15.9%), α-Terpineol (14.5%), Linalyl acetate (13.8%), Geranyl acetate (6.7%), β-Pinene (4.5%), Neryl acetate (4.2%). | [43] |
| Leaf (China) | EO, Hydrodistillation | Citronellal (75.3%), (R)-(+)-limonene (11.4%), Citronellol (6.7%), Citronellyl acetate (1.7%). | [30] |
| Orange (C. sinensis L.) | |||
| Pulp (Spain) Osbeck | Juice, Mechanical squeezing, HS-SPME | 1-Octanol (49–151), α-Pinene (28–65), β-Mircene (693–1340), Limonene (4310–5210), α-Terpinolene (54–106), Linalool (116–166), Valencene (698–1200)- units are ion peak areas divided by 106. | [44] |
| Pulp (China) Newhall navel | Juice, Mechanical squeezing | Sucrose (53.4–67.5 g/L), Glucose (25.7–29.5 g/L), Fructose (23.1-25.3 g/L), Total phenolics (4.3–5.1 mmol GAE/L), Total flavonoids (1.9–2.3 mmol/L), Vitamin C (0.51–0.63 mg/g), Hesperidin (478–839 mg/L), Narirutin (249–295 mg/L), Limonin (3.4–14.0 mg/L). | [45] |
| Pulp (Italy) Osbeck | Juice, Mechanical squeezing | Lucenin-2 (4.5–7.2 mg/L), Vicenin-2 (32.2–36.2 mg/L), Stellarin-2 (0.8–6.5 mg/L), Narirutin 4′-O-glucoside (0.5–10.1 mg/L), Quercetin-3-O-hexoside (0.7–4.1 mg/L), Narirutin (14.4–61.3 mg/L), Hesperidin (106–426 mg/L). | [46] |
| Pulp (Florida) Valencia | Juice, Mechanical squeezing | Insoluble solids (14–18 mg/g), Soluble solids (101–136 mg/g), Pectine (0.04–0.56 mg/g), Titrable acids (5.7–10.7 mg/g), Citric acid (6–1060 mg/g), Malic acid (2 mg/g), Sucrose (48–66 mg/g), Glucose (10–40 mg/g), Fructose (19–37 mg/g), Limonin (0.5–5.6 µg/g), D-Limonene (58–336 mg/L), Valencene (2.5–2.7 mg/L), Linalool (1.7–3.2 mg/L), Myrcene (2.6 mg/L), Acetaldehyde (4.9–8.1 mg/L), Ethyl acetate (3.3–3.9 mg/L). | [47] |
| Pulp (China) Osbeck (Tarocco blood oranges) | Juice, Mechanical squeezing | Total anthocyanine content (55–109 µg/g) (Delphinidin 3-glucoside, Cyanidin 3- galactoside, Cyanidin 3-glucoside, Delphynidin 3-(6″-malonylglucoside), Cyanidin 3-(3″-O-β-glucopyranosyl-6″-O-malonyl-β-glucopyranoside), Cyanidin 3-(6″-malonylglucoside, Cyanidin 3-(6″-dioxalylglucoside), Delphynidin-3-rutinoside, Cyanidin malonyl-(dioxalyl)-hexoside); D-limonene, Aromandendrene, Linalool, β-myrcene, D-carvone, ethyl butanoate. | [48] |
| Pulp (Mexico) | Methanolic extracts | Total phenolics (6.46 mg/g); Ferulic acid-O-hexoside (157.6 μg/g), Sinapic acid (414.7 μg/g), Isosakuranetin-7-O-rutinoside (428.8 μg/g), Naringenin-7-O-rutinoside (647.8 μg/g), Naringen-7-O-neohesperidoside (1428.8 μg/g), Naringenin-7-O-rutinoside-4′-O-glucoside (41.9 μg/g), Hesperetin-7-O-rutinoside (4434.9 μg/g), Apigenin-6,8-di-C-glucoside (216.8 μg/g), Vitexin-2″-O-xiloside (199.4 μg/g). | [49] |
| Peel (Egypt) | Water and ethanolic extracts | Narirutin (29 μg/g), Naringin (27 μg/g), Hesperetin (17 μg/g), Hesperetin-7-O-rutinoside naringenin (15 μg/g), Quinic acid (13 μg/g), Datiscetin-3-O-rutinoside (11 μg/g), Sakuranetin (9 μg/g). | [50] |
| Peel (Nigeria) Navel | Decoct | Quercitrin (22.6 mg/g), Rutin (17.9 mg/g), Quercetin (14.0 mg/g), Catechin (12.5 mg/g), Epicatechin (6.1 mg/g), Luteolin (5.9 mg/g), Naringin (5.7 mg/g), Kaempferol (3.8 mg/g), Caffeic acid (3.6 mg/g). | [51] |
| Peel (China) Osbeck Newhall | Ethanolic and ethyl acetate extracts | Sinensetin (67.3 μg/mg), Narirutin (55.6 μg/mg), Nobiletn (37.0 μg/mg), Hesperidin (31.5 μg/mg), 4′,5,6,7-Tetramethoxyflavone (22.5 μg/mg), 3,3′,4′,5,6,7-Hexamethoxyflavone (17.7 μg/mg), Didymin (12.5 μg/mg), 3,3′,4′,5,6,7,8-Heptamethoxyflavone (12.2 μg/mg). | [45] |
| Peel (China) | Methanolic extract, UAE | Isosinensetin (21.6–63.9 μg/g), Sinensetin (0.33–0.89 mg/g), 5,6,7,4′-Tetramethoxyflavone (8.6–21.7 μg/g), Nobiletin (0.42–1.01 mg/g), 5,7,8,4′-Tetramethoxyflavone (0.08–0.25 mg/g), 3,5,6,7,8,3′4′-Heptamethoxyflavone (0.15–0.35 mg/g), Tangeritin (0.07–0.15 mg/g), 5-hydroxy-6,7,8,3′,4′-Pentamethoxyflavone (11.3–46.7 μg/g), Gardenin A (2.9–19.0 μg/g). | [52] |
| Peel (USA) | Methanolic extract | p-Coumaric acid (18 µg/g), Ferulic acid (19 µg/g), Narirutin (1.34 mg/g), Hesperidin (16.3 mg/g). | [53] |
| Peel (China) Osbeck Brocade | Methanolic, DMSO, water extracts | Phenolic acids (Ferulic, p-Coumaric, Sinapic, Caffeic acid, Syringic, Vanillic, p-Hydroxybenzoic, Benzoic), Flavanones (Hesperetin, Hesperidin, Neohesperidin, Naringenin, Naringin, Didymin), Flavonols (Quercetin, Rutin), Flavones (Rhoifolin, Apiin, Luteolin), Polymethoxyflavones (Sinensetin, Tangeretin, Nobiletin). | [54] |
| Peel (Mexico) Valencia | Ionic Liquid-based MAE | Limonene (84.6–95.7%), 1-r-α-Pinene (0.2–1.9%), Linalol (0.9–1.3%), Eugenol methyl ether (0.2–1.5%), Eugenol (2.0%), Linalyl formate (1.5%), 1,2-Benzenedicarboxilic acid Mono(2-Ethylhexyl) ester (5.4%). | [55] |
| Peel (Ethiopia) | EO, Clevenger-type apparatus | Limonene (95.2%), N-methyl-1,3-propanediamine (2.9%), β-Myrcene (1.1%), 3-Carene (0.8%). | [34] |
| Peel (Pakistan) Mussami | EO, Clevenger-type apparatus | Limonene (48.9%), Geraniol (10.0%), Citraniol (10.1%), Eugenol (7.5%). | [56] |
| Peel (Pakistan) Red blood orange | EO, Clevenger-type apparatus | Limonene (46.3%), Geraniol (24%), Eugenol (12.9%), Citraniol (10.4%). | [56] |
| Peel (Iran) | EO, Clevenger-type apparatus | Limonene (90.5%), trans-Carveol (1.1%), Carvone (1.1%), cis-Linalool oxide (1.0%), β-Myrcene (0.9%). | [57] |
| Peel (Morocco) Navel | EO, MAHD | D-Limonene (89.9%), α-Sinensal (2.7%), β-Myrcene (2.3%), Capric aldehyde (1.9%), Linalool (1.2%), β-Pinene (0.7%), δ-Terpinene (0.6%). | [58] |
| Peel (Morocco) Navel | EO, Clevenger-type apparatus | D-Limonene (92.7%), α-Bergamotene (2.7%), β-Myrcene (2.2%), Sabinen (0.8%), (+)-Carene (0.8%), Capric aldehyde (0.7%), δ-Terpinene (0.6%), α-Pinene (0.6%), Linalool (0.2%). | [58] |
| Peel (Nigeria) Osbeck | EO, Clevenger-type apparatus | Limonene (92.1%), β-Myrcene (2.7%). | [59] |
| Seed (Nigeria) | Oil, Soxhlet Extraction | Total lipids (34.5%), Total saturate acids (28.5%), Total unsaturated acids (71.5%), Monounsaturated acid (29.7%), Polyunsaturated acids (41.8%); Linoleic acid (36.2%), Oleic acid (27.4%), Palmitic acid (21.1%), Stearic acid (4.8%), α-Linolenic acid (3.5%). | [60] |
| Leaf (Vietnam) Osbeck | EO, Clevenger-type apparatus | β-Pinene (16.9%), Limonene (13.8%), β-Ocimene (7.5%), Terpinen-4-ol (5.7%), Linalool (5.2%), β-Cubebene, (4.9%), Sabinene (4.7%), Nerol (3.8%), Geraniol (2.7%). | [61] |
| Leaf (Egypt) Navel cultivars | EO, Clevenger-type apparatus | Sabinene (8.3–28.8%), 2-Carene (11.3–16.7%), cis-β-Ocimene (10.2–13.9%), D-Limonene (6.5–12.0%), γ-Terpinene (2.0–4.5%), β-Citronellal (0.3–7.7%), Terpinen-4-ol (3.0–6.6%), β-Myrcene (3.4–5.6%), Linalool (0.2–5.3%), β-Elemene (2.6–14.2%). | [62] |
| Lime (C. aurantiifolia) | |||
| Pulp (Nigeria) | Juice | Flavonoids (7.1 mg/g), Tannins (5.3 mg/g), Phenolics (0.7 mg/g), Terpenes (0.6 mg/g). | [63] |
| Pulp (Iran) | EO, Clevenger-type apparatus, Extract, Static headspace | Limonene (49.3–71.7%), β-Pinene (8.5–21.7%), γ-Terpinene (7.3–9.0%), Myrcene (1.8%), α-Pinene (1.7–6.8%%), trans-Ferulic acid (2.3–2.8 mg/g), Hesperidin (0.3–2.1 mg/g), Ellagic acid (0.2–1.8 mg/g), Quercetin (0.03–0.8 mg/g), Rosmarinic acid, Hesperetin, Gallic acid, Catechin, Chlorogenic acid, p-Coumaric acid, Vanillin. | [64] |
| Peel (India) | EO, Clevenger-type apparatus | Palatinol-1C (13.3%), Limonene (12.9%), Carvon (9.1%), 2-Isopropenyl-5-methyl-4- hexanal (5.5%), cis-Cavacrol (5.3%). | [65] |
| Peel (Vietnam) | EO, Clevenger-type apparatus | Limonene (62.2%), γ-Terpinene (12.4%), β-Pinene(11.7%), β-Cymene (2.8%), 1R-α-Pinene (2.2%), Sabinene (1.5%). | [66] |
| Peel (Iran) | EO, Clevenger-type apparatus | Limonene (40.3%), β-Pinene (9.5%), α-Terpineol (10.9%), γ-Terpinolene (8.9%). | [57] |
| Peel (Italy) | Cold pressing | α-Phellandrene (48.5%), p-Cymene (16.5%), α-Pinene (12.6%), (E,E)-α-Farnesene (12.6%). | [67] |
| Peel (Vietnam) | EO, Clevenger-type apparatus, MAHD | Limonene (65.9 and 71.9%), α-Pinene (1.9 and 0.8%), β-Pinene (11.3 and 5.2%), β-Cymene (1.5 and 13.8%), α-Bergamotene (1.2 and 1.3%), Sabinene (1.5 and 1.6%). | [68] |
| Peel, Leaf (Brazil) | EO, Clevenger-type apparatus | Limonene (32.7–77.5%), Linalool (3.5–20.1%), Citronellal (3.2–14.5%), Citronellol (2.0–14.2%). | [69] |
| Leaf (India) | EO, Clevenger-type apparatus | Citral (13.5%), Limonene (11.6%), 1,2- Cyclohexanediol, 1-methyl-4-(1-methylethenyl) (11.3%), Geraniol (10.6%), Decanol (6.2%). | [65] |
| Leaf (Vietnam) | EO, Clevenger-type apparatus | Limonene (30.1%), β-Pinene (19.3%), Citronellol (3.9%), β-Caryophyllene (3.6%), β-Ocimene (3.5%), α-Terpineol (3.1%). | [61] |
| Leaf (Oman) | EO, Clevenger-type apparatus | D-Limonene (63.4%), 3,7-Dimethyl-2,6-octadien-1-ol (7.1%), Geraniol (6.2%), E-Citral (4.4%), Z-Citral (3.3%), β-Ocimene (2.3%). | [70] |
| Leaf (Nigeria) | EO, Clevenger-type apparatus | D-Limonene (57.8%), Neral (7.8%), Linalool (4.8%), Sulcatone (3.5%), Isogeraniol (3.5%). | [71] |
| Leaf (Benin) | Aqueous and ethanolic extracts | Phenolics (250–350 µg GAE/g), Flavonoids (6–24 µg RE/g). | [72] |
| Tangerine (C. reticulata) | |||
| Pulp (China) | Cyclodextrine-based liquid-phase pulsed discharge extraction | Total flavonoids (17.8 mg/g), Narirutin (4.2 mg/g), Hesperidin (15.7 mg/g), Nobiletin (0.6 mg/g), Tangeretin (0.5 mg/g). | [73] |
| Peel (China) Dahongpao | Methanolic extract | Tricin, Naringenin-7-O-glucoside (Prunin), Apigenin, Xanthohumol, Epicatechin gallate, Curcumin, Dihydromyricetin, Hesperetin 7-rutinoside (Hesperidin), Isoschaftoside, Astilbin, Vicenin-3, Eriocitrin, 6-Gingerol, Tectorigenin, Phloridzin, Naringenin 7-O-neohesperidoside (Naringin), Kaempferol 3-O-rutinoside (Nicotiflorin), Acacetin, Troxerutin (Trihydroxyethyl rutin), Quercetin 3-O-glucoside (Isotrifoliin), Biochanin A, Prunetin, Narirutin, Isoquercitroside, Theaflavin, Diosmin. | [74] |
| Peel (Portugal) Blanco | Aqueous and hydroethanolic extracts, SFE | Naringenin, Quercetin, Hesperidin, Naringin, Tangeretin, Rutin, Chlorogenic acid, Caffeic acid, Ferulic acid, Hesperitin. | [75] |
| Peel, Flesh, Seed (China) Blanco cv. Chachiensis (Chachi) | Acetone extracts | Peel: Naringin (555–581 µg/g), Hesperidin (3771–7491 µg/g), Nobiletin (1695–2011 µg/g), Tangeretin (597–646 µg/g), Chlorogenic acid (410–553 µg/g), Ferulic acid (270–356 µg/g). Flesh: Naringin (24.8–31.0 µg/g), Hesperidin (896–1076 µg/g), Neohesperidin (17.5–27.2 µg/g), Nobiletin (15.5–22.5 µg/g), Chlorogenic acid (79–99 µg/g), Caffeic acid (87–138 µg/g). Seed: Naringin (16.5–29.2 µg/g), Hesperidin (249–334 µg/g), Neohesperidin (20.2–56.3 µg/g), Ferulic acid (83.8–90.2 µg/g). | [76] |
| Peel (China) Blanco | EO, Hydrodistillation | D-Limonene (88.4%), γ-Terpinene (4.8%), Geranyl acetate, β-Elemen, δ-Elemen, Cyclohexane, 2,4-Diisopropenyl-1-methyl-1-vinyl, Gemacrene B, γ-Elemen, Neryl acetate, (-)-Spathulenol. | [77] |
| Peel (Brazil) Blanco | EO, Clevenger-type apparatus | Limonene (80.2%), Myrcene (6.7%), Linalool (3.7%), Sabinene (2.6%), α-Pinene (2.1%), ρ-Mentha-2,4(8) diene (1.5%), ρ-Mentha-1 (7),8-diene (0.7%), n-Decanal (0.5%), Terpinen-4-ol (0.3%), α-Terpineol (0.3%). | [78] |
| Peel (China) Chachi | EO, Hydrodistillation | D-Limonene (75.1%), γ-Terpinene (13.5%), Methyl methanthranilate, α-Sinensal, Champhene, Thymol, Citronellal, Perilla aldehyde, (R)-(+)-β-Citronellol. | [77] |
| Peel (Pakistan) Kinnow | EO, Clevenger-type apparatus | Limonene (54.6%), Citraniol (14%), Geraniol (12.7%), Eugenol (8.9%). | [56] |
| Peel | EO, British Pharmacopoeia. | D-Limonen (84.9%), δ-3-Carene (3.1%), β-Cymene (2.1%), β-Pinene (1.0%). | [33] |
| Peel (China) Ponkan | EO, Clevenger-type apparatus | Limonene (72.5%), γ-Terpinene (11.2%), β-Myrcene (3.0%), α-Pinene (1.3%), Linalool (1.9%), Octanal (0.6%), β-Pinene (0.6%), α-Terpinlene (0.5%), Sabinene (0.3%), β-Decanal (0.2%). | [79] |
| Grapefruit (C. paradisi) | |||
| Fruit, Juice-processing residues (Spain) | Extract, Steam explosion | D-limonene (87.1–93.7%), β-Myrcene (1.4–2.4%), Carvone (0.02–1.6%), (E)-Caryophyllene (0.4–1.5%), Pectic hydrocolloids (11–27 mg/g), Naringin (12–67 µg/g), Narirutin (4.1–7.9 µg/g), Naringin-4′-O-glucoside, Hesperidin glucoside. | [27] |
| Fruit (Iran) | Volatiles, Headspace single-drop microextraction | D-Limonene, β-Myrcene, α–Pinene, β-Pinene. | [80] |
| Pulp (Italy) Marsh, Star Ruby | Juice, Mechanical squeezing | Total Soluble Solids (10.8–13.4%),Total Acidity (0.6–1.0 mg citric acid/100 mL), Total phenolics (153–167 mg GAE/L), Total flavonoids (310–390 mg QE/L), Naringin (198–288 mg/L), Ascorbic acid (455–680 mg/L), Narirutin (37–39 mg/L), Poncirin (14–17 mg/L), Flavanones (narirutin, naringin, hesperidin, neohesperidin, and poncirin), flavones (rutin) and aglycones (quercetin, naringenin and hesperetin). | [81] |
| Pulp (Egypt) | Juice, Mechanical squeezing | Total soluble solids (11.6–12%),Total acidity (11.3–19.2 mg citric acid/L), Minerals (mg/L): P (890–930), Mg (150–340), Ca (210–480), K (1–6), Na (580–720), Fe (200–390), Cu (0.3–0.9), Zn (2–4), Mn (150–340), Total carbohydrates (8.1–85 g/L), Total fiber (44–66 mg/g), Total phenolics (7760–14080 mg GAE/L), Total flavonoids (195–251 mg CE/L), Total carotenoids (0.01–0.48 mg/L), Ascorbic acid (388–392 mg/L), Thiamine (5–6.5 mg/L), Riboflavin (0.4–0.5 mg/L). | [82] |
| Pulp (Spain) Star Ruby | Juice, Extract liquidization, Spray-drying, Oxalic acid and methanolic extracts | Total phenolics (12.7–12.9 mg GAE/g), Total flavonoids (43.1–65.9 mg QE/g), Delphinidin-3-glucoside, Hesperitin-7-O-glucoside, Hesperidin, Neohesperidin. | [83] |
| Pulp (Spain) Star Ruby | Juice, Extract, Freeze-drying, Spray-drying | α-Tocopherol (6–10 µg/g), Ascorbic acid (3.2–3.8 mg/g), Total phenolics (4.99–10.04 mg/g), Total phenolic acids (0.07–0.15 mg/g), Total flavonoids (4.9–9.9 mg/g), Narirutin (0.74–1.42 mg/g), Naringin (3.31–6.81 mg/g), Poncirin (0.28–0.48 mg/g). | [84] |
| Pulp (India) | Juice, Mechanical squeezing | Total soluble solids (10.3–12.4 °Brix), Acidity (1.2–2.0 g citric acid/kg), 1-(3-Ethyloxiranyl)-ethanone (up to 29%), 3-Hexen-2-one (9.9–11.6%), Limonene (0.7–15.4%). | [85] |
| Pulp (Pakistan) Shamber Tarnab | Juice | Total soluble solids (7.9 °Brix), Titrible acidity (1.4%), Ascorbic acid (~0.45 mg/g). | [28] |
| Peel (Sudan) | - | Ash (1.5–1.6%), Protein (1.1–1.2%), Oil (0.2–0.4%), Fiber (1.7–1.8%), Alcohol insoluble solids (9.5–10.5%), Titrable acidity (0.16–0.22%), Ascorbic acid (0.15–0.16%), Reducing sugars (10.2–10.4%), Total sugars (18.9–19.8%), Calcium (6.9–7.1 µg/g), Magnesium (1.7 µg/g), Total pectin (25%). | [86] |
| Peel (Spain) | Extract, ASE | Total phenolics (28–85 mg GAE/g), Naringin (43.5–160.1 mg/g), Naringenin (2.4–8.5 mg/g), Isonaringin (3.6–13.4 mg/g). | [87] |
| Peel (Egypt) | - | Proteins (64 µg/g), Fats (38 µg/g), Fibers (28 µg/g), Ash (82 µg/100 g), Carbohydrates (0.79 mg/g), Lycopene (0.43 mg/g), Ascorbic acid (0.52 mg/g), Total phenolics (10.78 mg GAE/g), Flavonoids (1.74 mg CE/g). | [26] |
| Peel (Argentina) | EO, Cold-pressing, Steam distillation | Limonene (87.9–88.5%), Myrcene (2.8–3.5%), β-pinene (1.2%), γ-Terpinene (1.1%). | [88] |
| Peel (Algeria) | EO, MAHD, Hydrodistillation | Limonene (85.5–87.5%), β-Myrcene (3.0–3.2%), Nootkatone (1.8%). | [89] |
| Peel (South Africa) | EO, Clevenger-type apparatus | D-limonene (87–90%), β-Myrcene (2–4%), γ-Terpinene (0.05–2%). | [90] |
| Peel (India) | EO, Clevenger-type apparatus | 1-Methyl-4-(1-methylethenyl)-cyclohexene (up to 84.3%), Myrcene (4.0–6.2%), 2,6,6-Trimethyl-bicyclo [3.1.1] hept-2-ene (1.0–1.6%) | [85] |
| Peel (Pakistan) | EO, Clevenger-type apparatus | Total phenolics (121 mg GAE/L), Flavonoids (76 mg CE/L), Triacetin (53.5%), Di-n-octyl-phthalate (17.3%), Octanal (9.2%), D-Limonene (9.2%), Alkaloids, Saponins. | [91] |
| Peel | EO, British Pharmacopoeia | D-Limonene (91.8%), δ-3-Carene (2.07%), β-Pinene (1.1%). | [33] |
| Peel (China) | EO, Molecular distillation | Limonene (93.3%), β-Myrcene (2.2%), α-Pinene (0.8%), Sabinene (0.6%), cis-Limonene oxide (0.4%), Carvone (0.4%), Octanal (0.4%), trans-Limonene oxide (0.3%). | [92] |
| Leaf (South Africa) | EO, Clevenger-type apparatus | β-Phellandrene (90–91%), Furanoid (0.6–2%), Caryophyllene (0.08–2%). | [90] |
| Pomelo (C. grandis) | |||
| Pulp (India) | Juice, Mechanical squeezing | Total phenolics (1834 mg GAE/L), Total flavonoids (529 mg QE/L), Fructose (12 g/L), Glucose (11 g/L), Sucrose (50 g/L), Citric acid (12 g/L), Malic acid (1.5 g/L), Tartaric acid (0.13 g/L), Succinic acid (0.22 g/L), Ascorbic acid (0.32 g/L), (R)-Limonene (1.67 mg/g), Octanal (13 µg/g), Linalool (21 µg/g), Ethyl butanoate (107 µg/g), Terpineol (13 µg/g), Citral (16 µg/g), α-Pinene (21 µg/g), Ethyl butyrate (1.02 mg/g), 2-Phenylethanol (1736 µg/g). | [93] |
| Pulp (China) Shatianyu, Lingpingyu Guangximiyu-R, Guangximiyu-W, Yuhuanyu | Acetone extracts | Total phenolics (0.92–1.71 mg GAE/g), Total flavonoids (0.13–1.93 mg CE/g); Cigranoside A (0.17–11.45 μg/g), Cigranoside B (0.19–17.73 μg/g), Cigranoside C (1.88–33.75 μg/g), Cigranoside D (0.69–38.0 μg/g), Cigranoside E (0.22–20.3 μg/g), Bergamjuicin (0.1–51.5 μg/g), Neoeriocitrin (0.8–14.7 μg/g), Melitidin (19.4–233.4 μg/g), Rhoifolin (1.64–4.14 μg/g), Naringin (24.5–301 μg/g), Hesperidin (0.004–0.028 μg/g), Isoquercitrin (0.1–1.09 μg/g). | [92] |
| Peel (Korea) Osbeck | Ethanolic extract (dichloromethane fraction) | Naringin (0.3 mg/g), Narirutin (0.3 mg/g), Neohesperidin (2.0 mg/g), Hesperidin (0.4 mg/g), Rutin (0.2 mg/g), Apigenin (1.0 mg/g), Hesperetin (0.8 mg/g), Isorhamnetin (1.4 mg/g), Kaempferol (1.4 mg/g), Luteolin (0.5 mg/g), Myricetin (1.3 mg/g), Naringenin (0.2 mg/g), Quercetin (0.6 mg/g), Rhaemnetin (1.9 mg/g), Taxifolin (7.0 mg/g), Nobiletin (70.5 mg/g), Sinensetin (76.2 mg/g), Tangeretin (14.1 mg/g). | [94] |
| Flavedo, albedo, juice sacs (China) Baishi, Cuixiangtian, Guanxi | Ethanolic extract, UAE | Limonin, Nomilin, Limonin glucoside. | [95] |
| Peel (Vietnam) | EO, Clevenger-type apparatus, Co-extraction, using citric acid | Limonene (87.9%), β-Pinene (2.7%), α-Phellandrene (1.3%), γ-Terpinene (0.5%), Linalool (0.26%), trans-β-ocimene (0.24%), trans-linalool oxide (0.18%), α-Terpinene (0.16%), cis-linalool oxide (0.12%), β-Citronellol (0.09%), trans-p-mentha-2,8-dien-1-ol (0.08%). Pectines (24%). | [96] |
| Peel (Vietnam) | EO, Hydrodistillation | Limonene (97.4%), β-Myrcene (1.2%), α-Phellandrene (0.7%), α-Pinene (0.5%), Sabinene (0.13%), β-Pinene (0.07%). | [97] |
| Peel (China) | EO, Steam distillation | D-Limonene (53.6%), Ocimene (4.4%), γ-Terpinene (1.6%), Myrcene (1.4%), α-Pinene (1.0%), β-Pinene (0.5%), Linalool (0.2%). | [98] |
| Leaf (Vietnam) Osbek | EO, Clevenger-type apparatus | Limonene (21.9%), Geraniol (10.7%), Nerol (10.4%), β-Caryophyllene (6.8%), β-Ocimene (6.4%), α-Phellandrene (4.0%), Citronellol (3.2%). | [61] |
3. Nutraceutical Value of Citrus
3.1. Dietary Fibers
3.2. Citric Acid
3.3. Polyphenolics: Flavonoids
3.4. Terpenoids: Carotenoids
3.5. Vitamins
3.6. Minerals
3.7. Essential Oils
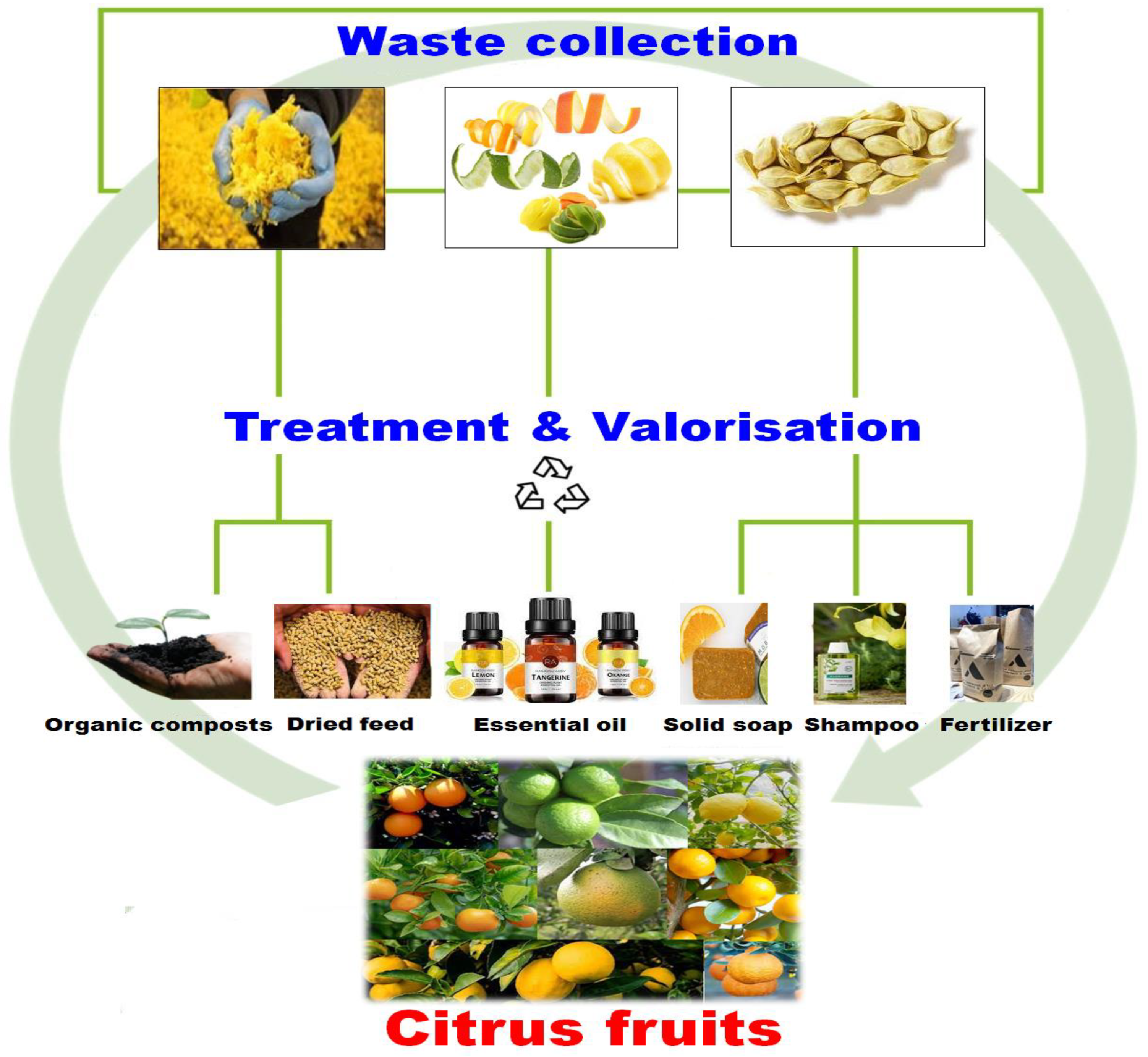
4. Citrus Uses in the Food Industry
4.1. Extraction of Bioactive Compounds for Food Applications
4.2. Food Industrial Applications
4.2.1. Functional Food Ingredient
4.2.2. Food Additive
4.2.3. Food Colorant
4.2.4. Flavoring Agent
4.2.5. Thickening Agent
4.3. Limitations of Applying Citrus Wastes in Food Industry
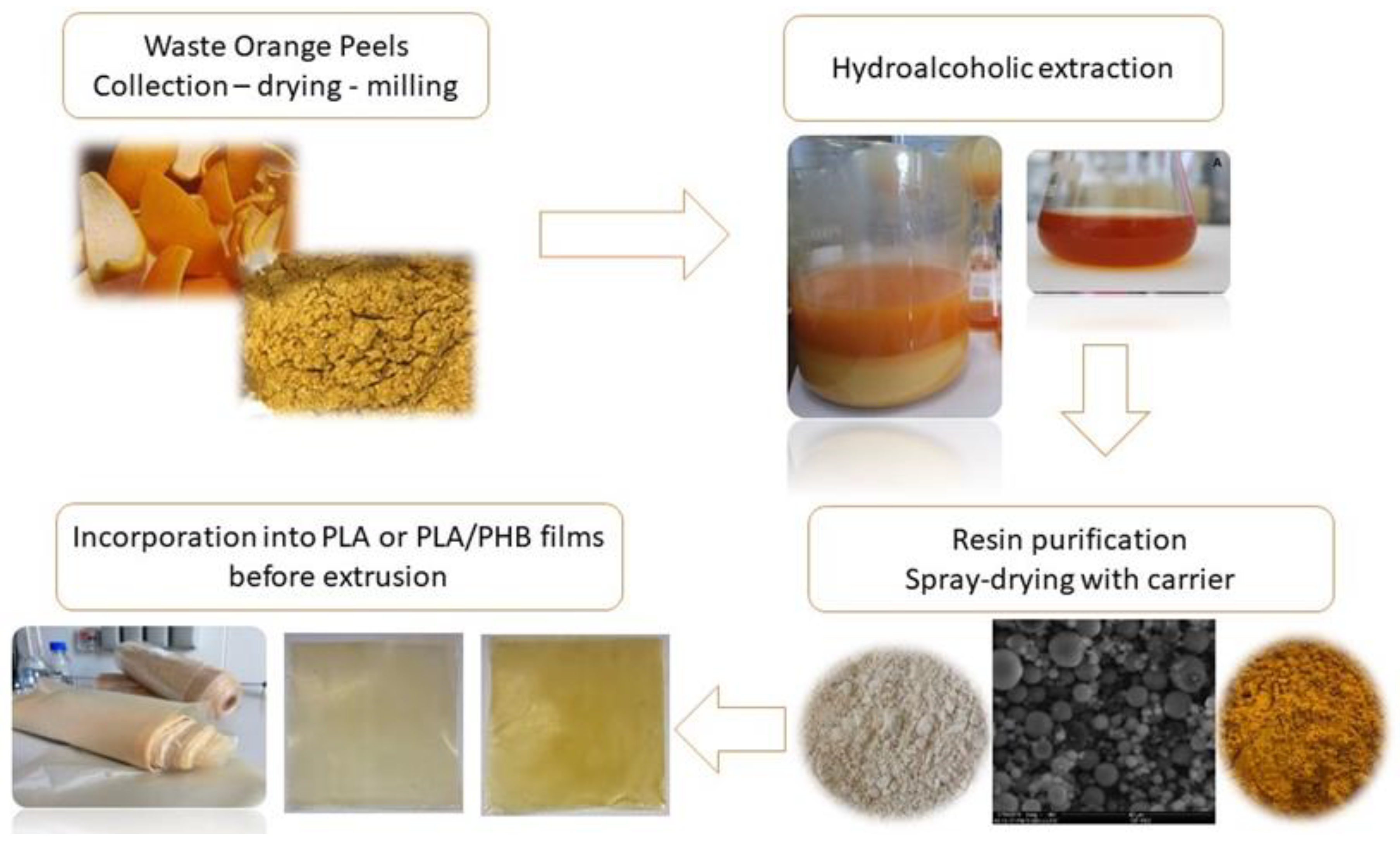
4.4. Application in Food Packaging
4.4.1. Natural Extracts Requirements for Incorporation into Packaging Material
4.4.2. Need for Encapsulation
4.4.3. Literature Examples of Citrus Extracts Use to Develop Antimicrobial/Antioxidant Packaging
5. The Use of Citruses in Cosmetics
5.1. The Extraction of Cosmeceuticals from Citrus Biowaste
5.2. The Use of Citrus Biowaste Extracts in Cosmetics
5.3. The Use of Citrus Essential Oils in Cosmetics
5.4. Cosmetic Formulations with Components Isolated from Citruses
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhong, G.; Nicolosi, E. Citrus origin, diffusion, and economic importance. In Compendium of Plant Genomes; Springer: Berlin/Heidelberg, Germany, 2020; pp. 5–21. [Google Scholar]
- de Araújo, E.F.; de Queiroz, L.P.; Machado, M.A. What is Citrus? Taxonomic implications from a study of cp-DNA evolution in the tribe Citreae (Rutaceae subfamily Aurantioideae). Org. Divers. Evol. 2003, 3, 55–62. [Google Scholar] [CrossRef]
- Berk, Z. Miscellaneous citrus products. In Citrus Fruit Processing; Academic Press: Cambridge, MA, USA, 2016; pp. 235–249. [Google Scholar]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- USDA. Citrus: World Markets and Trade. 2022; pp. 1–13. Available online: https://www.fas.usda.gov/data/citrus-world-markets-and-trade (accessed on 10 February 2023).
- Liu, Y.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Li, H. Citrus tree abiotic and biotic stress and implication of simulation and modeling tools in tree management. Tree For. Sci. Biotech 2009, 3, 66–78. [Google Scholar]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Colletti, A. Polyphenols Effect on Circulating Lipids and Lipoproteins: From Biochemistry to Clinical Evidence. Curr. Pharm. Des. 2018, 24, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lo, C.Y.; Ho, C.T. Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (Citrus sinensis) peel. J. Agric. Food Chem. 2006, 54, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Marín, F.R.; Soler-Rivas, C.; Benavente-García, O.; Castillo, J.; Pérez-Alvarez, J.A. By-products from different citrus processes as a source of customized functional fibres. Food Chem. 2007, 100, 736–741. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabro, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Colucci, G.; Apone, F. New Trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Robinson, P.H. Citrus by-products as ruminant feeds: A review. Anim. Feed Sci. Technol. 2006, 128, 175–217. [Google Scholar] [CrossRef]
- Mamma, D.; Christakopoulos, P. Biotransformation of Citrus By-Products into Value Added Products. Waste Biomass Valorization 2014, 5, 529–549. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Sinha, M.; Cho, M.H. Citrus waste derived nutra-/pharmaceuticals for health benefits: Current trends and future perspectives. J. Funct. Foods 2018, 40, 307–316. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef]
- Suri, S.; Singh, A.; Nema, P.K. Current applications of citrus fruit processing waste: A scientific outlook. Appl. Food Res. 2022, 2, 100050. [Google Scholar] [CrossRef]
- Braddock, R.; Cadwallader, K. Citrus by-products manufacture for food use. Food Technol. 1992, 46, 105–110. [Google Scholar]
- Idamokoro, E.M.; Hosu, Y.S. Out-Look on Worldwide Trends of Related Studies on Citrus waste as feed for livestock production: A scientometric analysis. Front. Res. Metr. Anal. 2022, 7, 869974. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Panesar, P.S.; Chopra, H.K. Citrus processing by-products: An overlooked repository of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2023, 63, 67–86. [Google Scholar] [CrossRef]
- Jiang, Z.; Mu, S.; Ma, C.; Liu, Y.; Ma, Y.; Zhang, M.; Li, H.; Liu, X.; Hou, J.; Tian, B. Consequences of ball milling combined with high-pressure homogenization on structure, physicochemical and rheological properties of citrus fiber. Food Hydrocoll. 2022, 127, 107515. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef]
- Hassan, M.A.; Hussein, S.M. Chemical and technological studies on pink grapefruit (Citrus paradise L.) peels. 1-effect of storage conditions on gross chemical composition, phytochemical components and oil stability of pink grapefruit peels. World J. Dairy Food Sci. 2017, 12, 115–123. [Google Scholar]
- Dorado, C.; Cameron, R.G.; Manthey, J.A.; Ferguson, K.L. Bench scale batch steam explosion of Florida red and white grapefruit juice processing residues. Future Foods 2021, 3, 100020. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ullah, I.; Sajid, M.; Basit, A.; Shehata, W.F.; Shah, S.T.; Alturki, S.M.; Ullah, A.; Aziz, I.; Ali, F. Influence of maturity stages on postharvest physico-chemical properties of grapefruit (Citrus paradisi var. ‘Shamber Tarnab’) under different storage durations. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12620. [Google Scholar] [CrossRef]
- Aziz, D.; Rasool, A.; Peshawa, H.; Mahmood, H. Characterization of Antioxidant Property and Chemical Composition of Lemon (Citrus lemon L.) Peel Extracts. J. Raparin Univ. 2016, 3, 1. [Google Scholar]
- Wu, K.; Jin, R.; Bao, X.; Yu, G.; Yi, F. Potential roles of essential oils from the flower, fruit and leaf of Citrus medica L. var. sarcodactylis in preventing spoilage of Chinese steamed bread. Food Biosci. 2021, 43, 101271. [Google Scholar] [CrossRef]
- Rizaldy, D.; Insanu, M.; Sabila, N.; Haniffadli, A.; Zahra, A.A.; Pratiwi, S.N.E.; Mudrika, S.N.; Hartati, R.; Fidrianny, I. Lemon (Citrus limon L.): Antioxidative Activity and Its Marker Compound. Biointerface Res. Appl. Chem. 2023, 13, 21. [Google Scholar]
- Makni, M.; Jemai, R.; Kriaa, W.; Chtourou, Y.; Fetoui, H. Citrus limon from Tunisia: Phytochemical and Physicochemical Properties and Biological Activities. Biomed. Res. Int. 2018, 2018, 6251546. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.; Teneva, D.; Tomova, T.; Goranov, B.; Denkova, Z.; Shopska, V.; Slavchev, A.; Hristova-Ivanova, Y. Chemical composition, antioxidant and antimicrobial activity of essential oils from tangerine (Citrus reticulata L.), grapefruit (Citrus paradisi L.), lemon (Citrus lemon L.) and cinnamon (Cinnamomum zeylanicum Blume). Z. Naturforsch. C J. Biosci. 2021, 76, 175–185. [Google Scholar] [CrossRef]
- Zeleke, Z. Extraction of essential oil from lemon and orange peel by Clevenger apparatus:Comparative GC_MS analysis of chemical composition, from Debre Berehan Market town Amahara Region Ethiopia. Ann. Biotechnol. 2022, 5, 1026. [Google Scholar] [CrossRef]
- Owolabi, M.S.; Avoseh, O.N.; Ogunwande, I.A.; Setzer, W.N.; Ogungbo, R.; Ogundajo, A.L.; Lawal, O.A.; Flamini, G. Chemical composition of Citrus limon (L.) Osbeck growing in Southwestern Nigeria: Essential oil chemotypes of both peel and leaf of lemon. Am. J. Essent. Oils Nat. Prod. 2018, 6, 36–40. [Google Scholar]
- Ghoorchibeigi, M.O.N.A.; Larijani, K.; Azar, P.A.; Zare, K.; Mehregan, I. Chemical composition and radical scavenging activity of Citrus limon peel essential oil. Orient. J. Chem. 2017, 33, 458–461. [Google Scholar] [CrossRef]
- Benoudjit, F.; Maameri, L.; Ouared, K. Evaluation of the quality and composition of lemon (Citrus limon) peel essential oil from an Algerian fruit juice industry. Alger. J. Environ. Sci. Technol. 2020, 6, 1575–1581. [Google Scholar]
- Himed, L.; Merniz, S.; Barkat, M. Chemical composition of Citrus limon (Eureka variety) essential oil and evaluation of its antioxidant and antibacterial activities. Afr. J. Biotechnol. 2018, 17, 356–361. [Google Scholar] [CrossRef]
- Paw, M.; Begum, T.; Gogoi, R.; Pandey, S.K.; Lal, M. Chemical composition of Citrus limon L. Burmf peel essential oil from North East India. J. Essent. Oil Bear. Plants 2020, 23, 337–344. [Google Scholar] [CrossRef]
- Nsangou, M.F.; Happi, E.N.; Fannang, S.V.; Atangana, A.F.; Waffo, A.F.K.; Wansi, J.D.; Isyaka, S.M.; Sadgrove, N.; Sewald, N.; Langat, M.K. Chemical composition and synergistic antimicrobial effects of a vegetatively propagated cameroonian lemon, citrus x limon (l.) osbeck. ACS Food Sci. Technol. 2021, 1, 354–361. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Halima, N.; Smaoui, S.; Hamdi, N. Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis. 2017, 16, 146. [Google Scholar] [CrossRef]
- Ehiobu, J.; Idamokoro, M.; Afolayan, A. Phytochemical content and antioxidant potential of leaf extracts of Citrus limon (L.) Osbeck collected in the Eastern Cape province, South Africa. S. Afr. J. Bot. 2021, 141, 480–486. [Google Scholar] [CrossRef]
- Hojjati, M.; Barzegar, H. Chemical composition and biological activities of lemon (Citrus limon) leaf essential oil. Nutr. Food Sci. Res. 2017, 4, 15–24. [Google Scholar] [CrossRef]
- Cuevas, F.J.; Moreno-Rojas, J.M.; Ruiz-Moreno, M.J. Assessing a traceability technique in fresh oranges (Citrus sinensis L. Osbeck) with an HS-SPME-GC-MS method. Towards a volatile characterisation of organic oranges. Food Chem. 2017, 221, 1930–1938. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Kaliaperumal, K.; Zhong, B. Variations of the chemical composition of Citrus sinensis Osbeck cv. Newhall fruit in relation to the symptom severity of Huanglongbing. J. Food Compos. Anal. 2022, 105, 104269. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Lagana, G.; Leuzzi, U.; Bellocco, E. C- and O-glycosyl flavonoids in Sanguinello and Tarocco blood orange (Citrus sinensis (L.) Osbeck) juice: Identification and influence on antioxidant properties and acetylcholinesterase activity. Food Chem. 2016, 196, 619–627. [Google Scholar] [CrossRef]
- Bai, J.; Baldwin, E.A.; McCollum, G.; Plotto, A.; Manthey, J.A.; Widmer, W.W.; Luzio, G.; Cameron, R. Changes in volatile and non-volatile flavor chemicals of “Valencia” orange juice over the harvest seasons. Foods 2016, 5, 4. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Ismail, B.B.; Wang, W.; Xu, E.; Pan, H.; Ye, X.; Liu, D.; Cheng, H. Effects of ethephon and low-temperature treatments on blood oranges (Citrus sinensis L. Osbeck): Anthocyanin accumulation and volatile profile changes during storage. Food Chem. 2022, 393, 133381. [Google Scholar] [CrossRef]
- Peña-Vázquez, G.I.; Dominguez-Fernández, M.T.; Camacho-Zamora, B.D.; Hernandez-Salazar, M.; Urías-Orona, V.; De Peña, M.P.; de la Garza, A.L. In vitro simulated gastrointestinal digestion impacts bioaccessibility and bioactivity of Sweet orange (Citrus sinensis) phenolic compounds. J. Funct. Foods 2022, 88, 104891. [Google Scholar] [CrossRef]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Omoba, O.S.; Obafaye, R.O.; Salawu, S.O.; Boligon, A.A.; Athayde, M.L. HPLC-DAD Phenolic characterization and antioxidant activities of ripe and unripe sweet orange peels. Antioxidants 2015, 4, 498–512. [Google Scholar] [CrossRef]
- Xing, T.T.; Zhao, X.J.; Zhang, Y.D.; Li, Y.F. Fast separation and sensitive quantitation of polymethoxylated flavonoids in the peels of Citrus using UPLC-Q-TOF-MS. J. Agric. Food Chem. 2017, 65, 2615–2627. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, D.I.; Kim, W.J.; Moon, S.K. Naringin inhibits matrix metalloproteinase-9 expression and AKT phosphorylation in tumor necrosis factor-alpha-induced vascular smooth muscle cells. Mol. Nutr. Food Res. 2009, 53, 1582–1591. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, B.; Chen, X.; Huang, P.; Chen, K.; Ma, Y.; Agarry, I.E.; Kan, J. Optimization and comparison of nonconventional extraction techniques for soluble phenolic compounds from brocade orange (Citrus sinensis) peels. J. Food Sci. 2022, 87, 4917–4929. [Google Scholar] [CrossRef]
- Franco-Vega, A.; López-Malo, A.; Palou, E.; Ramírez-Corona, N. Effect of imidazolium ionic liquids as microwave absorption media for the intensification of microwave-assisted extraction of Citrus sinensis peel essential oils. Chem. Eng. Process.-Process Intensif. 2021, 160, 108277. [Google Scholar] [CrossRef]
- Qadir, R.; Farooq Anwar, T.r.M.; Shahid, M.; Zahoor, S. 34. Variations in chemical composition, antimicrobial and haemolytic activities of peel essential oils from three local Citrus cultivars. Pure Appl. Biol. (PAB) 2018, 7, 282–291. [Google Scholar] [CrossRef]
- Ghazian, F.; Sadati, S.N.; Khanavi, M.; Kashani, L.M.-T. Chemical composition, radical scavenging and β-carotene bleaching assay of essential oils from Citrus aurantifolia, Citrus sinensis peel, and Zataria multiflora aerial parts. Tradit. Integr. Med. 2016, 1, 59–65. [Google Scholar]
- Brahmi, F.; Mokhtari, O.; Legssyer, B.; Hamdani, I.; Asehraou, A.; Hasnaoui, I.; Rokni, Y.; Diass, K.; Oualdi, I.; Tahani, A. Chemical and biological characterization of essential oils extracted from citrus fruits peels. Mater. Today Proc. 2021, 45, 7794–7799. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Olumuyiwa, T.A.; Olasehinde, T.A.; Ademiluyi, A.O.; Adeyemo, A.C. Insecticidal activity of essential oil from orange peels (Citrus sinensis) against Tribolium confusum, Callosobruchus maculatus and Sitophilus oryzae and its inhibitory effects on acetylcholinesterase and Na+/K+-ATPase activities. Phytoparasitica 2017, 45, 501–508. [Google Scholar] [CrossRef]
- Atolani, O.; Adamu, N.; Oguntoye, O.S.; Zubair, M.F.; Fabiyi, O.A.; Oyegoke, R.A.; Adeyemi, O.S.; Areh, E.T.; Tarigha, D.E.; Kambizi, L.; et al. Chemical characterization, antioxidant, cytotoxicity, Anti-Toxoplasma gondii and antimicrobial potentials of the Citrus sinensis seed oil for sustainable cosmeceutical production. Heliyon 2020, 6, e03399. [Google Scholar] [CrossRef]
- Chi, P.T.L.; Van Hung, P.; Le Thanh, H.; Phi, N.T.L. Valorization of Citrus Leaves: Chemical composition, antioxidant and antibacterial activities of essential oils. Waste Biomass Valorization 2020, 11, 4849–4857. [Google Scholar] [CrossRef]
- Kammoun, A.K.; Altyar, A.E.; Gad, H.A. Comparative metabolic study of Citrus sinensis leaves cultivars based on GC–MS and their cytotoxic activity. J. Pharm. Biomed. Anal. 2021, 198, 113991. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.C.; Akinsete, T.O.; Odeniyi, O.A. Phytochemical composition and comparative evaluation of antimicrobial activities of the juice extract of Citrus aurantifolia and its silver nanoparticles. Niger. J. Pharm. Res. 2016, 12, 59–64. [Google Scholar]
- Tavallali, H.; Bahmanzadegan, A.; Rowshan, V.; Tavallali, V. Essential oil composition, antioxidant activity, phenolic compounds, total phenolic and flavonoid contents from pomace of Citrus aurantifolia. J. Med. Plants By-Prod. 2021, 10, 103–116. [Google Scholar]
- Sarma, R.; Adhikari, K.; Mahanta, S.; Khanikor, B. Insecticidal activities of Citrus aurantifolia essential oil against Aedes aegypti (Diptera: Culicidae). Toxicol. Rep. 2019, 6, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Phong, H.X.; Cang, M.; Bach, L.G.; Van Muoi, N. Kinetic modeling of essential oil hydro-distillation from peels of Pomelo (Citrus grandis L.) fruit grown in Southern Vietnam. Sains Malays. 2021, 50, 3251–3261. [Google Scholar]
- Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Hanus, P.; Kowalczewski, P.; Bakay, L.; Kačániová, M. The potential use of Citrus aurantifolia L. essential oils for decay control, quality preservation of agricultural products, and anti-insect activity. Agronomy 2022, 12, 735. [Google Scholar] [CrossRef]
- Ngo, T.; Tran, T.; Dao, T.; Tran, T.; Ngo, H.; Huynh, X.; Tran, T.; Mai, H. Yield and composition analysis of Vietnamese lemon (Citrus aurantifolia) essential oils obtained from hydrodistillation and microwave-assisted hydrodistillation. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Chennai, India, 16–17 September 2020; p. 012123. [Google Scholar]
- Lemes, R.S.; Alves, C.C.F.; Estevam, E.B.B.; Santiago, M.B.; Martins, C.H.G.; Santos, T.; Crotti, A.E.M.; Miranda, M.L.D. Chemical composition and antibacterial activity of essential oils from Citrus aurantifolia leaves and fruit peel against oral pathogenic bacteria. Acad. Bras. Cienc. 2018, 90, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Al-Aamri, M.S.; Al-Abousi, N.M.; Al-Jabri, S.S.; Alam, T.; Khan, S.A. Chemical composition and in-vitro antioxidant and antimicrobial activity of the essential oil of Citrus aurantifolia L. leaves grown in Eastern Oman. J. Taibah Univ. Med. Sci. 2018, 13, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.A.; Usman, L.A.; Akolade, J.O.; Idowu, O.A.; Abdulazeez, A.T.; Amuzat, A.O. Antidiabetic potentials of Citrus aurantifolia leaf essential oil. Drug Res. 2019, 69, 201–206. [Google Scholar] [CrossRef]
- Dougnon, V.T.; Klotoé, J.; Sènou, M.; Roko, G.; Dougnon, G.; Fabiyi, K.; Amadou, A.; Aniambossou, A.; Assogba, P.; Bankolé, H. Chemical composition, cytotoxicity and antibacterial activity of selected extracts of Euphorbia hirta, Citrus aurantifolia and Heterotis rotundifolia on enteropathogenic bacteria. EC Microbiol. 2017, 12, 180–195. [Google Scholar]
- Zhu, K.; Chen, H.; Zhang, Y.; Liu, Y.; Zheng, X.; Xu, J.; Ye, J.; Deng, X. Carotenoid extraction, detection, and analysis in citrus. Methods Enzym. 2022, 670, 179–212. [Google Scholar]
- Wang, F.; Chen, L.; Chen, H.; Chen, S.; Liu, Y. Analysis of flavonoid metabolites in citrus peels (Citrus reticulata “Dahongpao”) using UPLC-ESI-MS/MS. Molecules 2019, 24, 2680. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Citrus reticulata Blanco peels as a source of antioxidant and anti-proliferative phenolic compounds. Ind. Crops Prod. 2018, 111, 141–148. [Google Scholar] [CrossRef]
- Wang, L.-F.; Rhim, J.-W. Grapefruit seed extract incorporated antimicrobial LDPE and PLA films: Effect of type of polymer matrix. LWT 2016, 74, 338–345. [Google Scholar] [CrossRef]
- Duan, L.; Guo, L.; Dou, L.-L.; Zhou, C.-L.; Xu, F.-G.; Zheng, G.-D.; Li, P.; Liu, E.-H. Discrimination of Citrus reticulata Blanco and Citrus reticulata ‘Chachi’by gas chromatograph-mass spectrometry based metabolomics approach. Food Chem. 2016, 212, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.A.; da Camara, C.A. Chemical composition and bioactivity of peel oils from Citrus aurantiifolia and Citrus reticulata and enantiomers of their major constituent against Sitophilus zeamais (Coleoptera: Curculionidae). J. Stored Prod. Res. 2017, 73, 30–36. [Google Scholar] [CrossRef]
- He, Q.; Xiao, K. The effects of tangerine peel (Citri reticulatae pericarpium) essential oils as glazing layer on freshness preservation of bream (Megalobrama amblycephala) during superchilling storage. Food Control 2016, 69, 339–345. [Google Scholar] [CrossRef]
- Sadeghian, F.; Ebrahimi, P.; Shakeri, A.; Jamali, M. Extraction of Citrus paradisi volatile components by headspace single-drop microextraction and statistical modeling. J. Chromatogr. Sci. 2016, 54, 1263–1269. [Google Scholar] [CrossRef]
- Sicari, V.; Pellicanò, T.; Giuffrè, A.; Zappia, C.; Capocasale, M.; Poiana, M. Physical chemical properties and antioxidant capacities of grapefruit juice (Citrus paradisi) extracted from two different varieties. Int. Food Res. J. 2018, 25, 1978–1984. [Google Scholar]
- Seleim, M.; Hassan, M.A.; Saleh, A. Physico-chemical evaluation of white and pink grapefruit (Citrus paradisi) juice. Assiut J. Agric. Sci. 2019, 50, 112–122. [Google Scholar]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Agudelo, C.; Barros, L.; Santos-Buelga, C.; Martínez-Navarrete, N.; Ferreira, I. Phytochemical content and antioxidant activity of grapefruit (Star Ruby): A comparison between fresh freeze-dried fruits and different powder formulations. LWT 2017, 80, 106–112. [Google Scholar] [CrossRef]
- Ahmed, S.; Rattanpal, H.; Gul, K.; Dar, R.A.; Sharma, A. Chemical composition, antioxidant activity and GC-MS analysis of juice and peel oil of grapefruit varieties cultivated in India. J. Integr. Agric. 2019, 18, 1634–1642. [Google Scholar] [CrossRef]
- Mohamed, H. Extraction and Characterization of Pectin from Grapefruit Peels. MOJ Food Process. Technol. 2016, 2, 31–38. [Google Scholar] [CrossRef]
- Castro-Vazquez, L.; Alanon, M.E.; Rodriguez-Robledo, V.; Perez-Coello, M.S.; Hermosin-Gutierrez, I.; Diaz-Maroto, M.C.; Jordan, J.; Galindo, M.F.; Arroyo-Jimenez Mdel, M. Bioactive flavonoids, antioxidant behaviour, and cytoprotective effects of dried grapefruit peels (Citrus paradisi Macf.). Oxid. Med. Cell Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri, K.; Raofie, F.; Qomi, M.; Davoodi, A. Response Surface Methodology for Optimization of Supercritical Fluid Extraction of Orange Peel Essential Oil. Pharm. Biomed. Res. 2020, 6, 303–312. [Google Scholar] [CrossRef]
- El Houda, A.K.N.; Boudina, A.; Ahmed, A.; Oukil, S.; Foudil-Cherif, Y. Chemical composition, antimicrobial and insecticidal activities of Citrus paradisi peel essential oil from Algeria. J. Microbiol. Biotechnol. Food Sci. 2021, 9, 1093–1098. [Google Scholar] [CrossRef]
- Miya, G.; Nyalambisa, M.; Oyedeji, O.; Gondwe, M.; Oyedeji, A. Chemical Profiling, Toxicity and Anti-Inflammatory Activities of Essential Oils from Three Grapefruit Cultivars from KwaZulu-Natal in South Africa. Molecules 2021, 26, 3387. [Google Scholar] [CrossRef]
- Amjad, Y.; Anwar, M.; Aamir, M. Phytochemical investigation and in-vitro evaluation of antibacterial and antioxidant activities of grapefruit (Citrus paradisi) peel essential oils. J. Toxicol. Pharm. Sci. 2019, 3, 21–27. [Google Scholar]
- Deng, M.; Dong, L.; Jia, X.; Huang, F.; Chi, J.; Muhammad, Z.; Ma, Q.; Zhao, D.; Zhang, M.; Zhang, R. The flavonoid profiles in the pulp of different pomelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars and their in vitro bioactivity. Food Chem. X 2022, 15, 100368. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Eom, T.; Choi, J.H.; Kim, J.; Kim, J.; Unno, T. Dichloromethane fraction of Citrus grandis induces apoptosis in a human colorectal cancer cell lines via apoptotic signaling pathway. J. Funct. Foods 2022, 88, 104903. [Google Scholar] [CrossRef]
- Huang, X.; Muneer, M.A.; Li, J.; Hou, W.; Ma, C.; Jiao, J.; Cai, Y.; Chen, X.; Wu, L.; Zheng, C. Integrated nutrient management significantly improves pomelo (Citrus grandis) root growth and nutrients uptake under acidic soil of Southern China. Agronomy 2021, 11, 1231. [Google Scholar] [CrossRef]
- Tuan, N.T.; Dang, L.; Huong, B.; Danh, L. One step extraction of essential oils and pectin from pomelo (Citrus grandis) peels. Chem. Eng. Process.-Process Intensif. 2019, 142, 107550. [Google Scholar] [CrossRef]
- Dao, P.T.; Tran, N.Y.T.; Tran, Q.N.; Bach, G.L.; Lam, T.V. Kinetics of pilot-scale essential oil extraction from pomelo (Citrus maxima) peels: Comparison between linear and nonlinear models. Alex. Eng. J. 2022, 61, 2564–2572. [Google Scholar] [CrossRef]
- Guo, J.; Lai, X.P.; Li, J.X.; Yue, J.Q.; Zhang, S.Y.; Li, Y.Y.; Gao, J.Y.; Wang, Z.R.; Duan, H.F.; Yang, J.D. First Report on Citrus Chlorotic Dwarf Associated Virus on Lemon in Dehong Prefecture, Yunnan, China. Plant Dis. 2015, 99, 1287. [Google Scholar] [CrossRef]
- Rafiq, S.; Kaul, R.; Sofi, S.A.; Bashir, N.; Nazir, F.; Ahmad Nayik, G. Citrus peel as a source of functional ingredient: A review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Drzikova, B.; Dongowski, G.; Gebhardt, E.; Habel, A. The composition of dietary fibre-rich extrudates from oat affects bile acid binding and fermentation in vitro. Food Chem. 2005, 90, 181–192. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Veronese, F.M.; Pasut, G. PEGylation: Posttranslational bioengineering of protein biotherapeutics. Drug Discov. Today Technol. 2008, 5, e57–e64. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Xu, F.; An, H.; Zhang, J.; Xu, Z.; Jiang, F. Effects of Fruit Load on Sugar/Acid Quality and Puffiness of Delayed-Harvest Citrus. Horticulturae 2021, 7, 189. [Google Scholar] [CrossRef]
- Moufida, S.; Marzouk, B. Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry 2003, 62, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Anastassiadis, S.; Morgunov, I.G.; Kamzolova, S.V.; Finogenova, T.V. Citric acid production patent review. Recent Pat. Biotechnol. 2008, 2, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.G.; Malik, M.; Connell, E.; Muller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Tomás-Navarro, M.; Vallejo, F.; Tomás-Barberán, F.A. Bioavailability and metabolism of citrus fruit beverage flavanones in humans. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Reshef, N.; Hayari, Y.; Goren, C.; Boaz, M.; Madar, Z.; Knobler, H. Antihypertensive effect of sweetie fruit in patients with stage I hypertension. Am. J. Hypertens. 2005, 18, 1360–1363. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.A.; Gonzalez-Sarrias, A.; Vallejo, F.; Espin, J.C.; Tomas-Barberan, F.A. Hesperetin and its sulfate and glucuronide metabolites inhibit TNF-alpha induced human aortic endothelial cell migration and decrease plasminogen activator inhibitor-1 (PAI-1) levels. Food Funct. 2016, 7, 118–126. [Google Scholar] [CrossRef]
- Raso, G.M.; Meli, R.; Di Carlo, G.; Pacilio, M.; Di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Tsai, S.H.; Lin-Shiau, S.Y.; Lin, J.K. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br. J. Pharm. 1999, 126, 673–680. [Google Scholar] [CrossRef]
- Testai, L.; Calderone, V. Nutraceutical value of Citrus flavanones and their implications in cardiovascular disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruit and vegetable consumption and risk of stroke: A meta-analysis of cohort studies. Neurology 2005, 65, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Guirro, M.; Gual-Grau, A.; Gibert-Ramos, A.; Alcaide-Hidalgo, J.M.; Canela, N.; Arola, L.; Mayneris-Perxachs, J. Metabolomics Elucidates Dose-Dependent Molecular Beneficial Effects of Hesperidin Supplementation in Rats Fed an Obesogenic Diet. Antioxidants 2020, 9, 79. [Google Scholar] [CrossRef]
- Fidélix, M.; Milenkovic, D.; Sivieri, K. Microbiota modulation and effects on metabolic biomarkers by orange juice: A controlled clinical trial. Food Funct. 2020, 11, 1599. [Google Scholar] [CrossRef]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Siddiqui, R.A.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodríguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Silveira, J.Q.; Cesar, T.B.; Manthey, J.A.; Baldwin, E.A.; Bai, J.; Raithore, S. Pharmacokinetics of flavanone glycosides after ingestion of single doses of fresh-squeezed orange juice versus commercially processed orange juice in healthy humans. J. Agric. Food Chem. 2014, 62, 12576–12584. [Google Scholar] [CrossRef]
- von Lintig, J.; Hessel, S.; Isken, A.; Kiefer, C.; Lampert, J.M.; Voolstra, O.; Vogt, K. Towards a better understanding of carotenoid metabolism in animals. Biochim. Biophys. Acta 2005, 1740, 122–131. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barrandon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-carotene supplementation and cancer risk: A systematic review and metaanalysis of randomized controlled trials. Int. J. Cancer 2010, 127, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Mavrommatis, A.; Zografaki, M.E.; Marka, S.; Myrtsi, E.D.; Giamouri, E.; Christodoulou, C.; Evergetis, E.; Iliopoulos, V.; Koulocheri, S.D.; Moschopoulou, G.; et al. Effect of a Carotenoid Extract from Citrus reticulata By-Products on the Immune-Oxidative Status of Broilers. Antioxidants 2022, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Rampersaud, G.C.; Valim, M.F. 100% citrus juice: Nutritional contribution, dietary benefits, and association with anthropometric measures. Crit. Rev. Food Sci. Nutr. 2017, 57, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, E.; Armando Laudicina, V.; Antonietta Germanà, M. Current and potential use of Citrus essential oils. Curr. Org. Chem. 2013, 17, 3042–3049. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Khan, G.S.; Mushtaq, Z.; Zubair, M. Essential Oils. In Essential Oil Research; Malik, S., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Tocmo, R.; Pena-Fronteras, J.; Calumba, K.F.; Mendoza, M.; Johnson, J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1969–2012. [Google Scholar] [CrossRef] [PubMed]
- Wedamulla, N.E.; Fan, M.; Choi, Y.-J.; Kim, E.-K. Citrus peel as a renewable bioresource: Transforming waste to food additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Kaur, H.; Pancham, P.; Kaur, R.; Agarwal, S.; Singh, M. Synthesis and characterization of Citrus limonum essential oil based nanoemulsion and its enhanced antioxidant activity with stability for transdermal application. J. Biomater. Nanobiotechnol. 2020, 11, 215–236. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K. Phenols in Citrus Peel Byproducts. Concentrations of hydroxycinnamates and polymethoxylated flavones in Citrus peel molasses. J. Agric. Food Chem. 2001, 49, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Cadwallader, K.R.; Braddock, R.J. Enzymatic Hydration of (4R)-(+)-Limonene to (4R)-(+)-α-Terpineol. Dev. Food Sci. 1992, 29, 571–584. [Google Scholar]
- Tripodo, M.M.; Lanuzza, F.; Micali, G.; Coppolino, R.; Nucita, F. Citrus waste recovery: A new environmentally friendly procedure to obtain animal feed. Bioresour. Technol. 2004, 91, 111–115. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K.; Cameron, R.G. Hydrolysis of grapefruit peel waste with cellulase and pectinase enzymes. Bioresour. Technol. 2007, 98, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Bekatorou, A.; Koutinas, A.A.; Soupioni, M.; Banat, I.M.; Marchant, R. Use of Saccharomyces cerevisiae cells immobilized on orange peel as biocatalyst for alcoholic fermentation. Bioresour. Technol. 2007, 98, 860–865. [Google Scholar] [CrossRef]
- Schiewer, S.; Patil, S.B. Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Bioresour. Technol. 2008, 99, 1896–1903. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Peschel, W.; Sánchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzía, I.; Jiménez, D.; Lamuela-Raventós, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, M.; Choudary, J.A.; Ahmad, S.; Siddiqui, H.L. Synthesis of potential biologically active 1,2-Benzothiazin-3-yl-quinazolin-4(3H)-ones. Chem. Pharm. Bull. 2006, 54, 1175–1178. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Chu, Y.-L.; Sridhar, K.; Tsai, P.-J. Effect of ultrasound, high-pressure processing, and enzymatic hydrolysis on carbohydrate hydrolyzing enzymes and antioxidant activity of lemon (Citrus limon) flavedo. LWT 2021, 138, 110511. [Google Scholar] [CrossRef]
- Šafranko, S.; Ćorković, I.; Jerković, I.; Jakovljević, M.; Aladić, K.; Šubarić, D.; Jokić, S. Green extraction techniques for obtaining bioactive compounds from Mandarin peel (Citrus unshiu var. Kuno): Phytochemical analysis and process optimization. Foods 2021, 10, 1043. [Google Scholar] [CrossRef]
- Ciriminna, R.; Forest, B.; Meneguzzo, F.; Pagliaro, M.; Hamann, M.T. Technical and Economic feasibility of a stable yellow natural colorant production from wastelemon peel. Appl. Sci. 2020, 10, 6812. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Esparza-Estrada, J.; Vanegas-Mahecha, P. Ultrasound-assisted extraction of total carotenoids from mandarin epicarp and application as natural colorant in bakery products. LWT 2021, 139, 110598. [Google Scholar] [CrossRef]
- Singhal, S.; Swami Hulle, N.R. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Zayed, A.; Badawy, M.T.; Farag, M.A. Valorization and extraction optimization of Citrus seeds for food and functional food applications. Food Chem. 2021, 355, 129609. [Google Scholar] [CrossRef] [PubMed]
- Dar, N.G.; Hussain, A.; Paracha, G.M.; Akhter, S. Evaluation of Different Techniques for Extraction of Antioxidants as Bioactive Compounds from Citrus Peels (Industrial by Products). Am.-Eurasian J. Agric. Environ. Sci 2015, 15, 676–682. [Google Scholar]
- Al-Juhaimi, F.Y. Citrus fruits by-products as sources of bioactive compounds with antioxidant potential. Pak. J. Bot 2014, 46, 1459–1462. [Google Scholar]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.E.; Madrid, Y. Citrus peels waste as a source of value-added compounds: Extraction and quantification of bioactive polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Montero-Calderon, A.; Cortes, C.; Zulueta, A.; Frigola, A.; Esteve, M.J. Green solvents and ultrasound-assisted extraction of bioactive orange (Citrus sinensis) peel compounds. Sci. Rep. 2019, 9, 16120. [Google Scholar] [CrossRef]
- Benassi, L.; Alessandri, I.; Vassalini, I. Assessing green methods for pectin extraction from waste orange peels. Molecules 2021, 26, 1766. [Google Scholar] [CrossRef]
- Caggia, C.; Palmeri, R.; Russo, N.; Timpone, R.; Randazzo, C.L.; Todaro, A.; Barbagallo, S. Employ of Citrus by-product as fat replacer ingredient for bakery confectionery products. Front. Nutr. 2020, 7, 46. [Google Scholar] [CrossRef]
- Iftikhar, M.; Wahab, S.; Haq, N.u.; Malik, S.N.; Amber, S.; Taran, N.U.; Rehman, S.U. Utilization of citrus plant waste (peel) for the development of food product. Pure Appl. Biol. (PAB) 2019, 8, 1991–1998. [Google Scholar] [CrossRef]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Bianchi, A.; Sgherri, C.; Quartacci, M.F.; De Leo, M.; Pistelli, L.; Palla, F.; et al. Bread Fortified with Cooked Purple Potato Flour and Citrus Albedo: An evaluation of its compositional and sensorial properties. Foods 2021, 10, 942. [Google Scholar] [CrossRef] [PubMed]
- Nishad, J.; Koley, T.K.; Varghese, E.; Kaur, C. Synergistic effects of nutmeg and citrus peel extracts in imparting oxidative stability in meat balls. Food Res. Int. 2018, 106, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Garza, G.; Antonyan, N.; Loera-Hernández, I. Orange peel dehydration and creation of new edible products. Int. J. Food Eng. 2018, 4, 327–331. [Google Scholar] [CrossRef]
- Romero-Lopez, M.R.; Osorio-Diaz, P.; Bello-Perez, L.A.; Tovar, J.; Bernardino-Nicanor, A. Fiber concentrate from orange (Citrus sinensis L.) bagase: Characterization and application as bakery product ingredient. Int. J. Mol. Sci. 2011, 12, 2174–2186. [Google Scholar] [CrossRef]
- Ojha, P.; Thapa, S. Quality evaluation of biscuit incorporated with mandarin peel powder. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2017, 18, 19. [Google Scholar]
- Younis, K.; Ahmad, S.; Malik, M.A. Mosambi peel powder incorporation in meat products: Effect on physicochemical properties and shelf life stability. Appl. Food Res. 2021, 1, 100015. [Google Scholar] [CrossRef]
- Barman, K.; Chowdhury, D.; Baruah, P.K. Development of β-carotene loaded nanoemulsion using the industrial waste of orange (Citrus reticulate) peel to improve in vitro bioaccessibility of carotenoids and use as natural food colorant. J. Food Process. Preserv. 2020, 44, e14429. [Google Scholar] [CrossRef]
- Bora, H.; Kamle, M.; Mahato, D.K.; Tiwari, P.; Kumar, P. Citrus essential oils (CEOs) and their applications in food: An overview. Plants 2020, 9, 357. [Google Scholar] [CrossRef]
- Tekgül, Y.; Baysal, T. Comparative evaluation of quality properties and volatile profiles of lemon peels subjected to different drying techniques. J. Food Process Eng. 2018, 41, e12902. [Google Scholar] [CrossRef]
- Matsuo, Y.; Akita, K.; Taguchi, H.; Fujii, S.; Yoshie-Stark, Y.; Araki, T. Utilization and evaluation of Citrus natsudaidai peel waste as a source of natural food additives. Food Chem. 2022, 373, 131464. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Q.; Teoh, W.H.; Yusoff, R.; Ngoh, G.C. Comparisons of process intensifying methods in the extraction of pectin from pomelo peel. Chem. Eng. Process. Process Intensif. 2019, 143, 107586. [Google Scholar] [CrossRef]
- Mann, S.; Aggarwal, K.S.M.P. Development of Phytochemical Rich Ice Cream Incorporating Kinnow Peel. Glob. J. Sci. Front. Res. 2013, 13, 1–13. [Google Scholar]
- Khan, A.; Butt, M.S.; Randhawa, M.; Karim, R.; Sultan, M.; Ahmed, W. Extraction and characterization of pectin from grapefruit (Duncan cultivar) and its utilization as gelling agent. Int. Food Res. J. 2014, 21, 2195–2199. [Google Scholar]
- Lavelli, V. Circular food supply chains—Impact on value addition and safety. Trends Food Sci. Technol. 2021, 114, 323–332. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological activities and safety of Citrus spp. Essential oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food Packaging: A Comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Miltz, J.; Hoojjat, P.; Han, J.K.; Giacin, J.R.; Harte, B.R.; Gray, I.J. Loss of antioxidants from high-density polyethylene. ACS Symp. Ser. 1988, 365, 83–93. [Google Scholar]
- Ha, J.-U.; Kim, Y.-M.; Lee, D.-S. Multilayered antimicrobial polyethylene films applied to the packaging of ground beef. Packag. Technol. Sci. 2001, 14, 55–62. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Barba, F.J.; Gomez, B.; Putnik, P.; Bursac Kovacevic, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.W. Antimicrobial wrapping paper coated with a ternary blend of carbohydrates (alginate, carboxymethyl cellulose, carrageenan) and grapefruit seed extract. Carbohydr. Polym. 2018, 196, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Antioxidant packaging with encapsulated green tea for fresh minced meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef]
- Zanetti, M.; Carniel, T.K.; Dalcanton, F.; dos Anjos, R.S.; Gracher Riella, H.; de Araújo, P.H.H.; de Oliveira, D.; Antônio Fiori, M. Use of encapsulated natural compounds as antimicrobial additives in food packaging: A brief review. Trends Food Sci. Technol. 2018, 81, 51–60. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 374–395. [Google Scholar] [CrossRef]
- Ghabraie, M.; Vu, K.D.; Tata, L.; Salmieri, S.; Lacroix, M. Antimicrobial effect of essential oils in combinations against five bacteria and their effect on sensorial quality of ground meat. LWT Food Sci. Technol. 2016, 66, 332–339. [Google Scholar] [CrossRef]
- Song, H.Y.; Shin, Y.J.; Song, K.B. Preparation of a barley bran protein–gelatin composite film containing grapefruit seed extract and its application in salmon packaging. J. Food Eng. 2012, 113, 541–547. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.W. Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef]
- Jridi, M.; Boughriba, S.; Abdelhedi, O.; Nciri, H.; Nasri, R.; Kchaou, H.; Kaya, M.; Sebai, H.; Zouari, N.; Nasri, M. Investigation of physicochemical and antioxidant properties of gelatin edible film mixed with blood orange (Citrus sinensis) peel extract. Food Packag. Shelf Life 2019, 21, 100342. [Google Scholar] [CrossRef]
- Bassani, A.; Rossi, F.; Fiorentini, C.; Garrido, G.D.; Reklaitis, G.V.R.; Bonadies, I.; Spigno, G. Model of spray-drying for encapsulation of natural extracts. Comput. Aided Chem. Eng. 2020, 48, 355–360. [Google Scholar]
- Fiorentini, C.; Duserm Garrido, G.; Bassani, A.; Cortimiglia, C.; Zaccone, M.; Montalbano, L.; Martinez-Nogues, V.; Cocconcelli, P.S.; Spigno, G. Citrus peel extracts for industrial-scale production of bio-based active food packaging. Foods 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Colon, M.; Nerin, C. Role of catechins in the antioxidant capacity of an active film containing green tea, green coffee, and grapefruit extracts. J. Agric. Food Chem. 2012, 60, 9842–9849. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.D.L.L.; Silva, A.M.; Delerue-Matos, C.; Rodrigues, F. Cosmetics—Food waste recovery. In Food Waste Recovery; Academic Press: Cambridge, MA, USA, 2021; pp. 503–528. [Google Scholar]
- Murador, D.C.; Braga, A.R.C.; Martins, P.L.G.; Mercadante, A.Z.; de Rosso, V.V. Ionic liquid associated with ultrasonic-assisted extraction: A new approach to obtain carotenoids from orange peel. Food Res. Int. 2019, 126, 108653. [Google Scholar] [CrossRef] [PubMed]
- Boukroufa, M.; Boutekedjiret, C.; Chemat, F. Development of a green procedure of citrus fruits waste processing to recover carotenoids. Resour. Effic. Technol. 2017, 3, 252–262. [Google Scholar] [CrossRef]
- Tsitsagi, M.; Ebralidze, K.; Chkhaidze, M.; Rubashvili, I.; Tsitsishvili, V. Sequential extraction of bioactive compounds from tangerine (Citrus Unshiu) peel. Ann. Agrar. Sci. 2018, 16, 236–241. [Google Scholar] [CrossRef]
- Buniowska, M.; Carbonell-Capella, J.; Zulueta, A.; Frigola, A.; Esteve, M.J. Bioaccessibility of bioactive compounds and antioxidant capacity from orange peel after pulsed electric fields and high voltage electrical discharges. MOJ Food Process. Technol. 2015, 1, 17. [Google Scholar]
- Ferhat, M.A.; Meklati, B.Y.; Smadja, J.; Chemat, F. An improved microwave Clevenger apparatus for distillation of essential oils from orange peel. J. Chromatogr. A 2006, 1112, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Estimation of mass transfer coefficients of the extraction process of essential oil from orange peel using microwave assisted extraction. J. Food Eng. 2016, 170, 136–143. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; de Cindio, B.; Loizzo, M.R.; Conforti, F.; Statti, G.A.; Menabeni, R.; Bettini, R.; Menichini, F. Chemical composition and bioactivity of Citrus medica L. cv. Diamante essential oil obtained by hydrodistillation, cold-pressing and supercritical carbon dioxide extraction. Nat. Prod Res. 2011, 25, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Garrido, G.; Chou, W.H.; Vega, C.; Goïty, L.; Valdés, M. Influence of extraction methods on fatty acid composition, total phenolic content and antioxidant capacity of Citrus seed oils from the Atacama Desert, Chile. J. Pharm. Pharmacogn. Res. 2019, 7, 389–407. [Google Scholar]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green extraction of polyphenols from grapefruit peels using high voltage electrical discharges, deep eutectic solvents and aqueous glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Casquete, R.; Castro, S.M.; Martín, A.; Ruiz-Moyano, S.; Saraiva, J.A.; Córdoba, M.G.; Teixeira, P. Evaluation of the effect of high pressure on total phenolic content, antioxidant and antimicrobial activity of citrus peels. Innov. Food Sci. Emerg. Technol. 2015, 31, 37–44. [Google Scholar] [CrossRef]
- Inoue, T.; Tsubaki, S.; Ogawa, K.; Onishi, K.; Azuma, J.-I. Isolation of hesperidin from peels of thinned Citrus unshiu fruits by microwave-assisted extraction. Food Chem. 2010, 123, 542–547. [Google Scholar] [CrossRef]
- Omar, J.; Alonso, I.; Garaikoetxea, A.; Etxebarria, N. Optimization of focused ultrasound extraction (FUSE) and supercritical fluid extraction (SFE) of Citrus peel volatile oils and antioxidants. Food Anal. Methods 2013, 6, 1244–1252. [Google Scholar] [CrossRef]
- Kim, S.-S.; Lee, J.-A.; Kim, J.-Y.; Lee, N.-H.; Hyun, C.-G. Citrus peel wastes as functional materials for cosmeceuticals. Appl. Biol. Chem. 2008, 51, 7–12. [Google Scholar] [CrossRef]
- Apraj, V.D.; Pandita, N.S. Evaluation of Skin Anti-aging potential of Citrus reticulata Blanco peel. Pharmacogn. Res 2016, 8, 160–168. [Google Scholar] [CrossRef]
- Nakagawa, M.; Kawai, K.; Kawai, K. Contact allergy to kojic acid in skin care products. Contact Dermat. 1995, 32, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef] [PubMed]
- Kamalambigeswari, R.; Sharmila, S.; Kowsalya, E.; Rebecca, L.J. Formulation of face cream using methanolic extract of Citrus limon. Drug Invent. Today 2019, 11, 11. [Google Scholar]
- Riski, R.; Nur, S.; Pabontong, E. Activity test and formulation of antioxidant cream from ethanol extract combination of several Citrus fruit peels (Citrus sp.). J. Pharm. Med. 2021, 6, 23–30. [Google Scholar]
- Nareswari, N.; Kuncoro, A. Preparation of essential oil ointment of lime leaves (Citrus amblycarpa) and stability test on base type used. Biofarmasi J. Nat. Prod. Biochem. 2017, 14, 63–68. [Google Scholar] [CrossRef]
- Jayarathna, W.U.; Hettihewa, S.K.; Karunanayaka, K.D.S.V.; Samarasingha, S.; Dissanayake, G. Formulation of herbal cream using essential oils of Cymbopogon citrus (Lemon grass) and evaluation of mosquito repellent activity against Aedes aegypti, Anopheles stephensi, Culex quinquefasciatus. In Proceedings of the 1st Research Symposium of Faculty of Allied Health Sciences; University of Ruhuna: Galle, Sri Lanka, 2018; ISSN 2659-2029. [Google Scholar]
- Abelan, U.S.; de Oliveira, A.C.; Cacoci, E.S.P.; Martins, T.E.A.; Giacon, V.M.; Velasco, M.V.R.; Lima, C. Potential use of essential oils in cosmetic and dermatological hair products: A review. J. Cosmet. Dermatol. 2022, 21, 1407–1418. [Google Scholar] [CrossRef] [PubMed]
- Wuttisin, N.; Nararatwanchai, T.; Sarikaphuti, A. Matrix metalloproteinase-2 inhibition activity of Plukenetia volubilis L. leaves extract for anti-aging application. Food Res. 2021, 5, 120–126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Hsouna, A.; Sadaka, C.; Generalić Mekinić, I.; Garzoli, S.; Švarc-Gajić, J.; Rodrigues, F.; Morais, S.; Moreira, M.M.; Ferreira, E.; Spigno, G.; et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants 2023, 12, 481. https://doi.org/10.3390/antiox12020481
Ben Hsouna A, Sadaka C, Generalić Mekinić I, Garzoli S, Švarc-Gajić J, Rodrigues F, Morais S, Moreira MM, Ferreira E, Spigno G, et al. The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants. 2023; 12(2):481. https://doi.org/10.3390/antiox12020481
Chicago/Turabian StyleBen Hsouna, Anis, Carmen Sadaka, Ivana Generalić Mekinić, Stefania Garzoli, Jaroslava Švarc-Gajić, Francisca Rodrigues, Simone Morais, Manuela M. Moreira, Eduarda Ferreira, Giorgia Spigno, and et al. 2023. "The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review" Antioxidants 12, no. 2: 481. https://doi.org/10.3390/antiox12020481
APA StyleBen Hsouna, A., Sadaka, C., Generalić Mekinić, I., Garzoli, S., Švarc-Gajić, J., Rodrigues, F., Morais, S., Moreira, M. M., Ferreira, E., Spigno, G., Brezo-Borjan, T., Akacha, B. B., Saad, R. B., Delerue-Matos, C., & Mnif, W. (2023). The Chemical Variability, Nutraceutical Value, and Food-Industry and Cosmetic Applications of Citrus Plants: A Critical Review. Antioxidants, 12(2), 481. https://doi.org/10.3390/antiox12020481