Citric Acid and Magnolol Ameliorate Clostridium perfringens Challenge in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. C. perfringens Preparation
2.2. CA and MA Preparation
2.3. Analysis of Effects of CA and MA on C. perfringens
2.4. Investigation of the Protective Effect of CA and MA on Chickens against C. perfringens
2.5. Sampling
2.6. Pathological Sectioning
2.7. ELISA for Measuring Bioactive Proteins and Peptides
2.8. Real-Time qPCR
2.9. 16S rRNA Sequencing of Cecal Contents
2.10. Statistical Analysis
3. Results
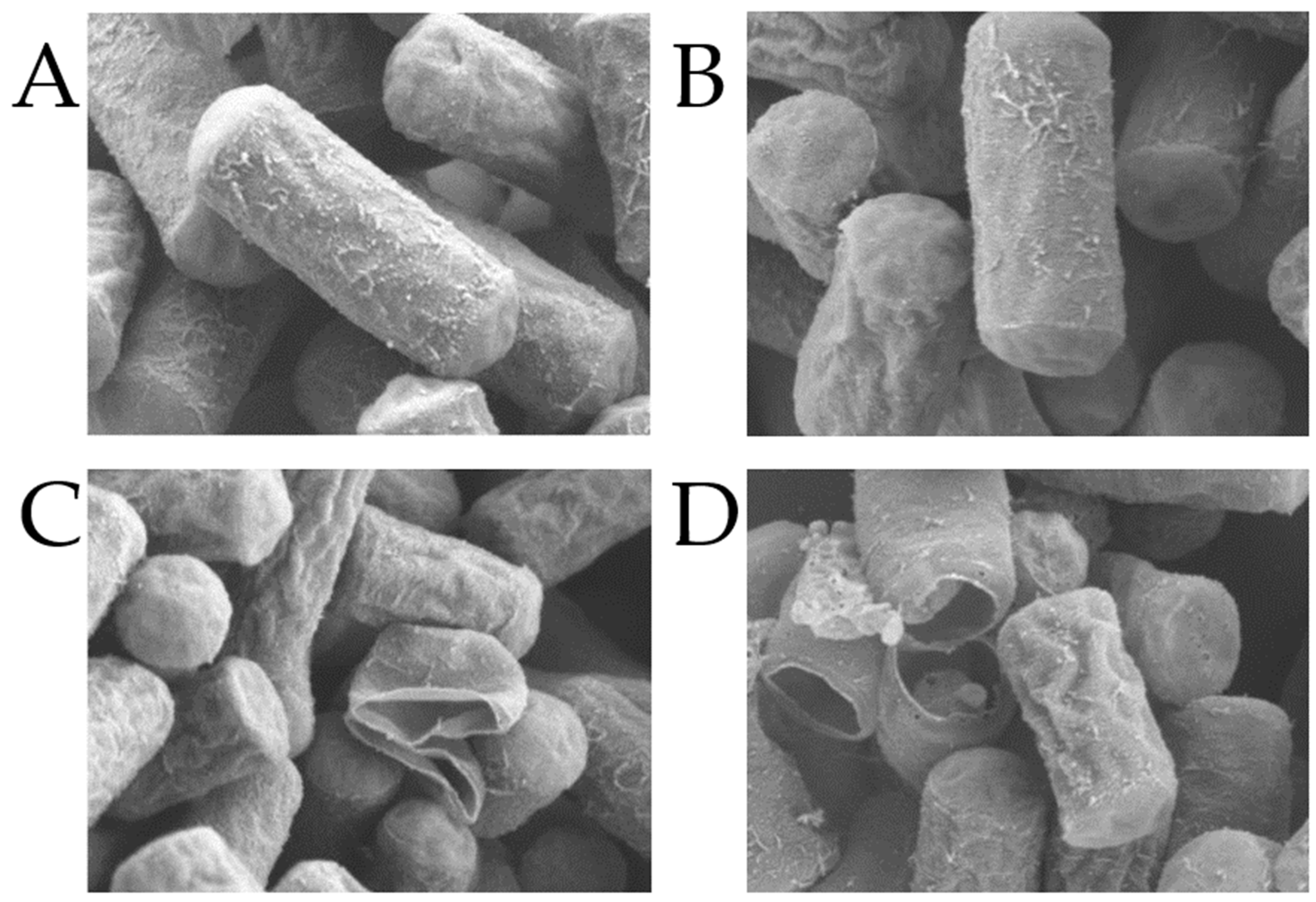
3.1. Analysis of Effects of CA and MA on C. perfringens
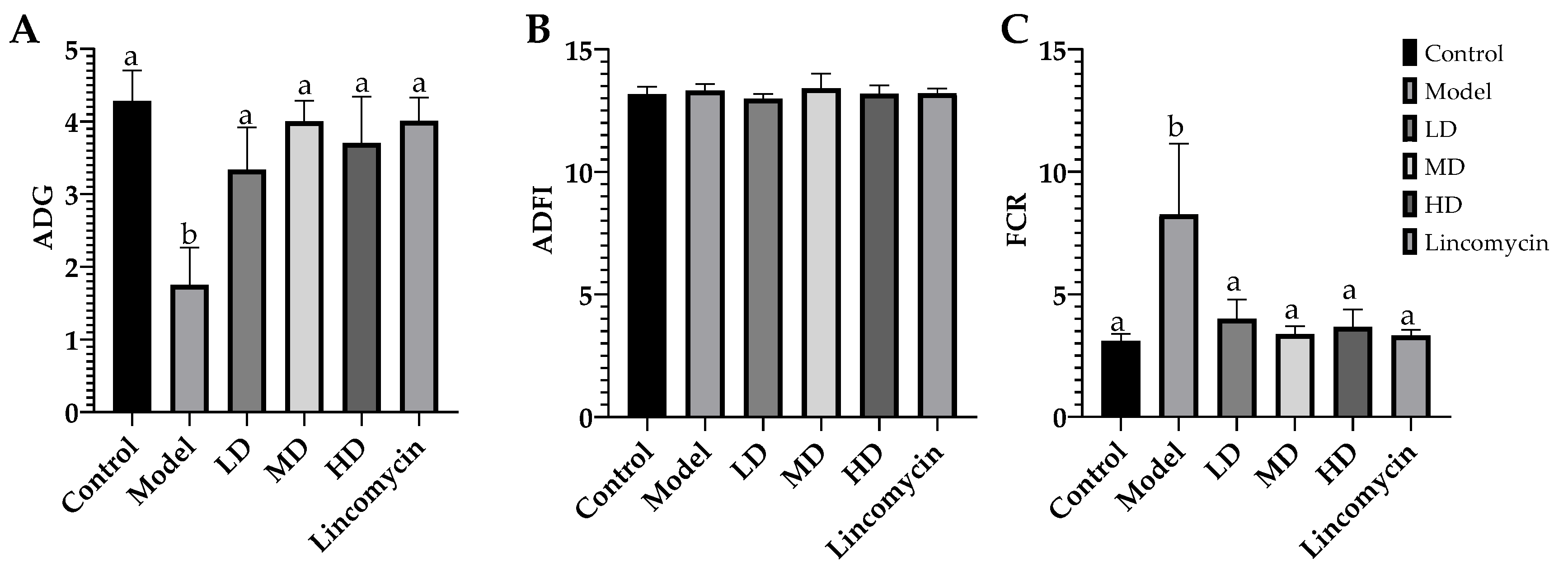
3.2. Growth Performance of Chickens
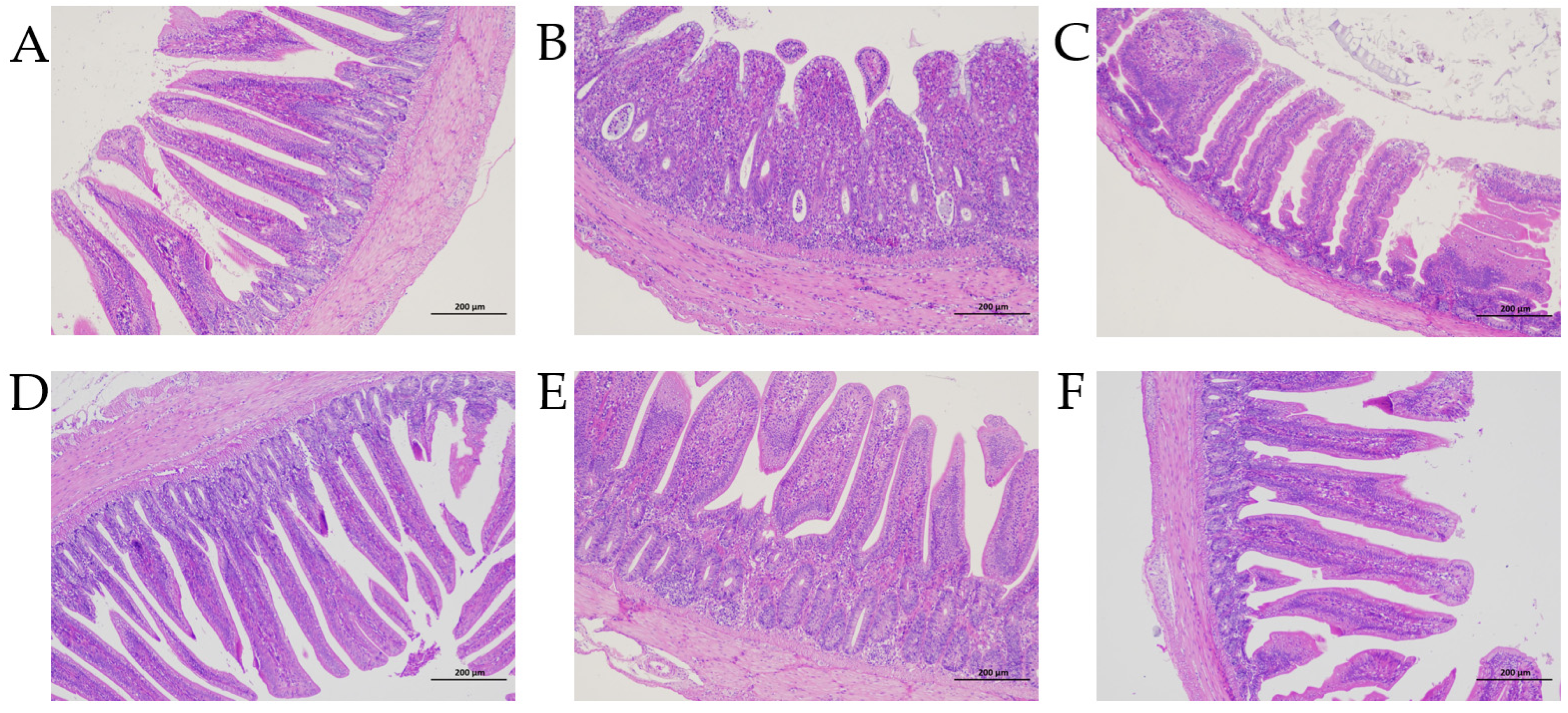
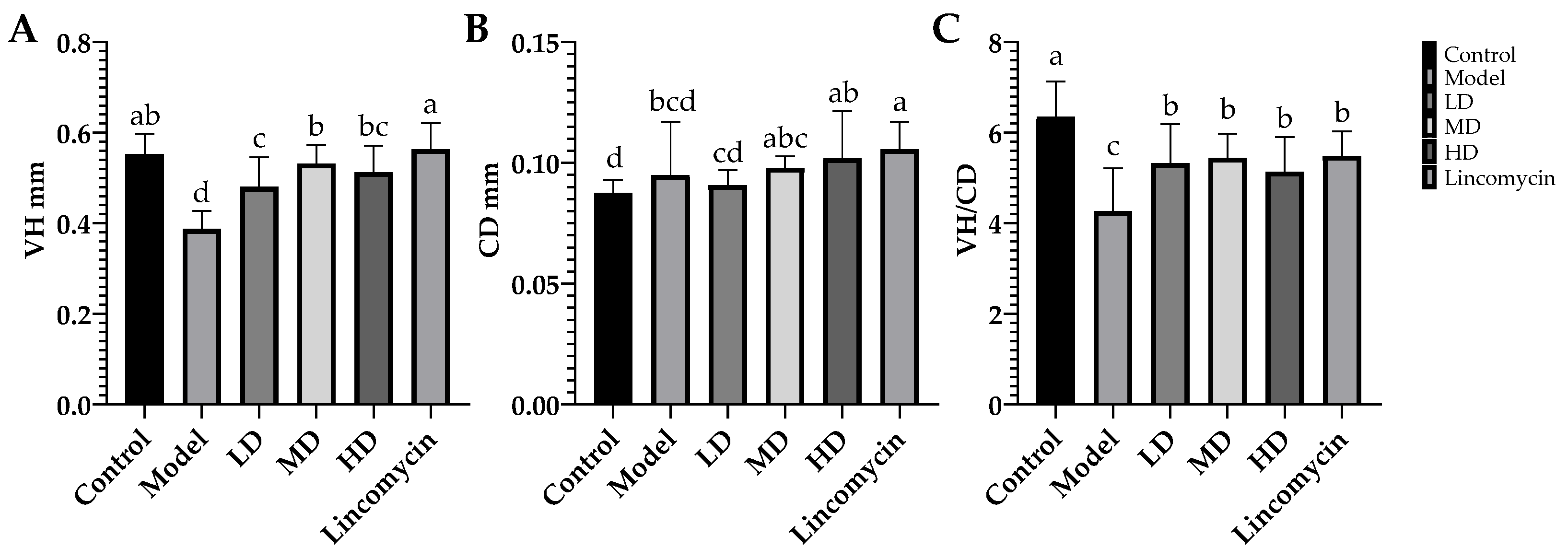
3.3. Enteropathy
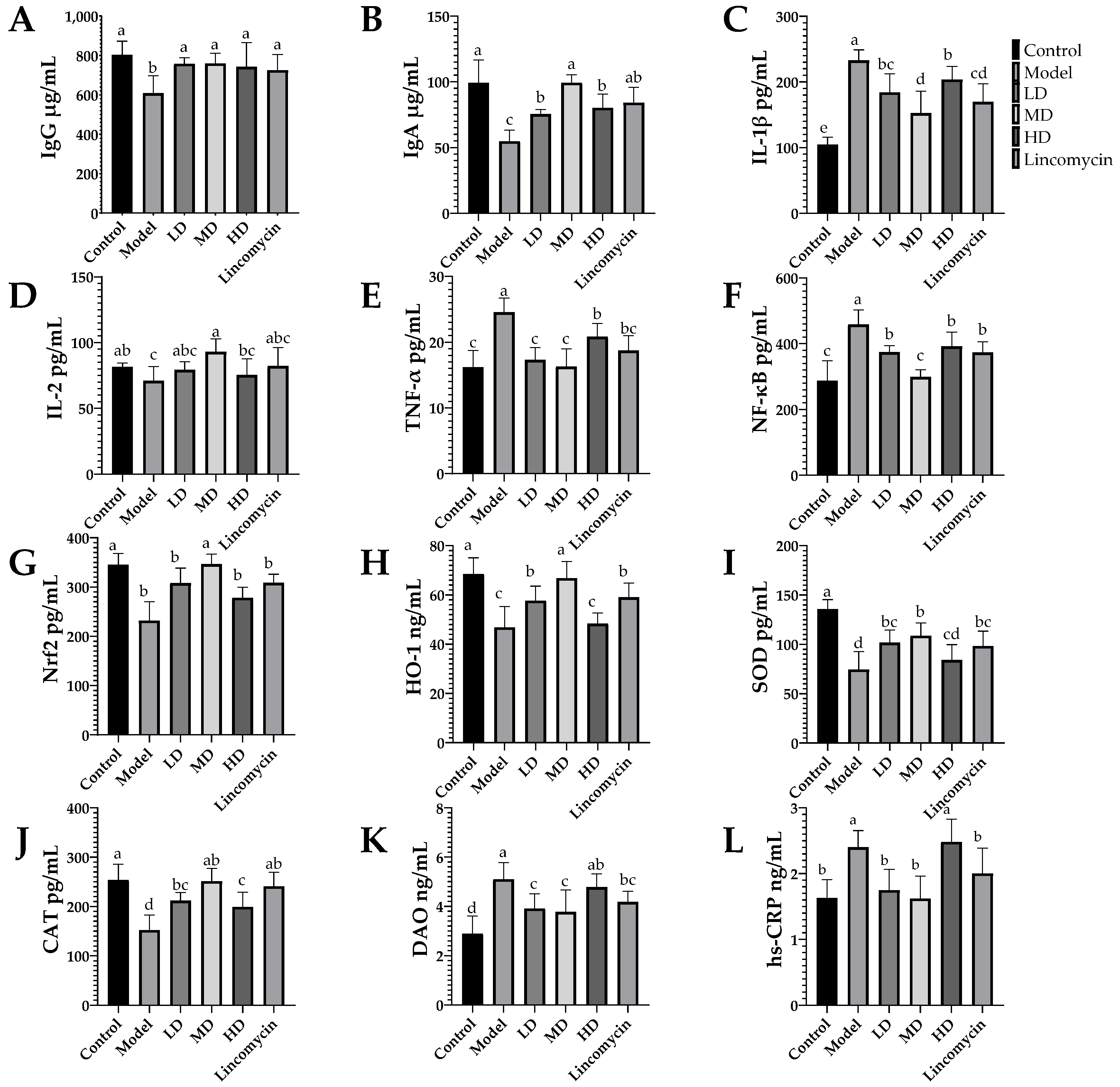
3.4. ELISA Results
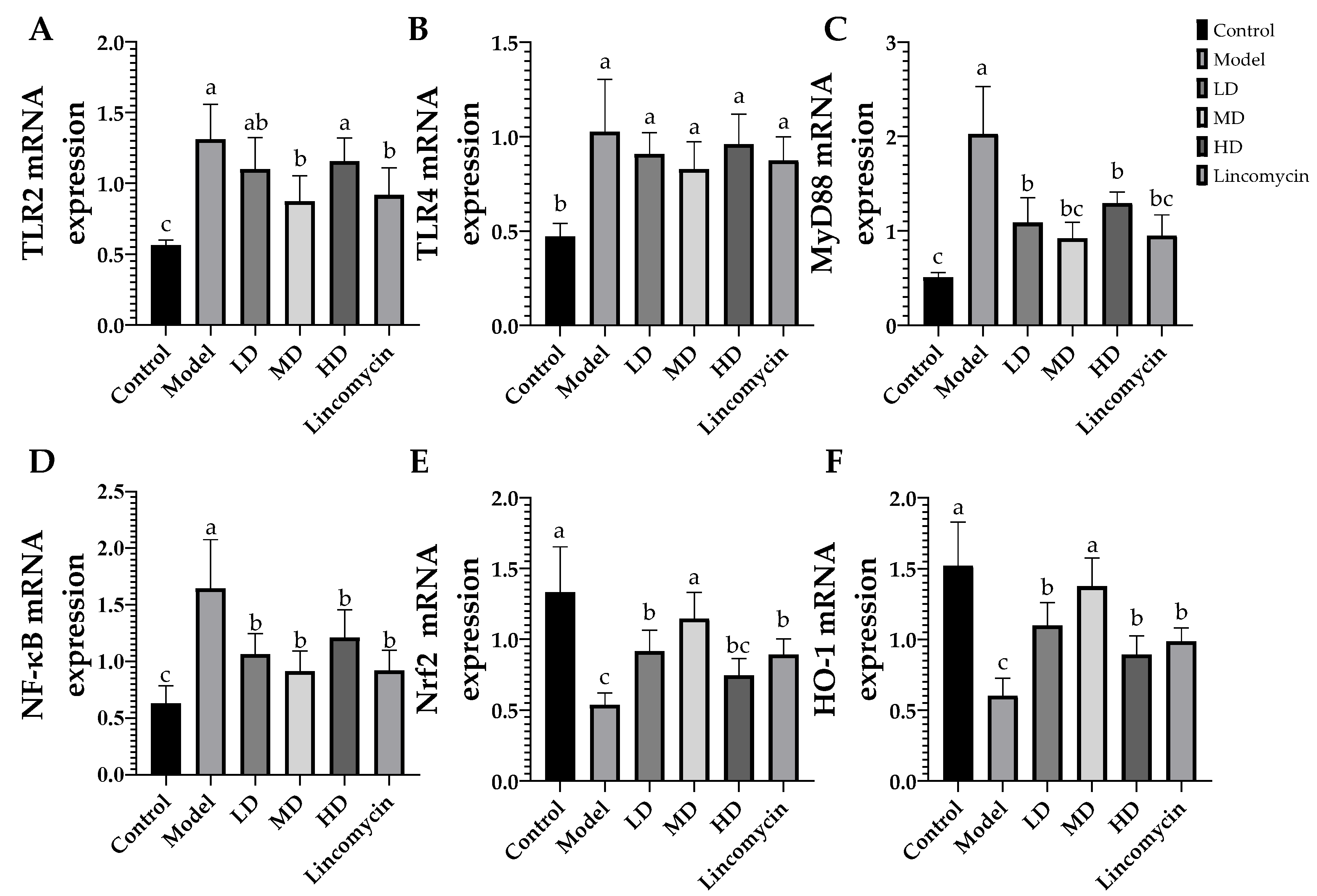
3.5. RT-qPCR Results
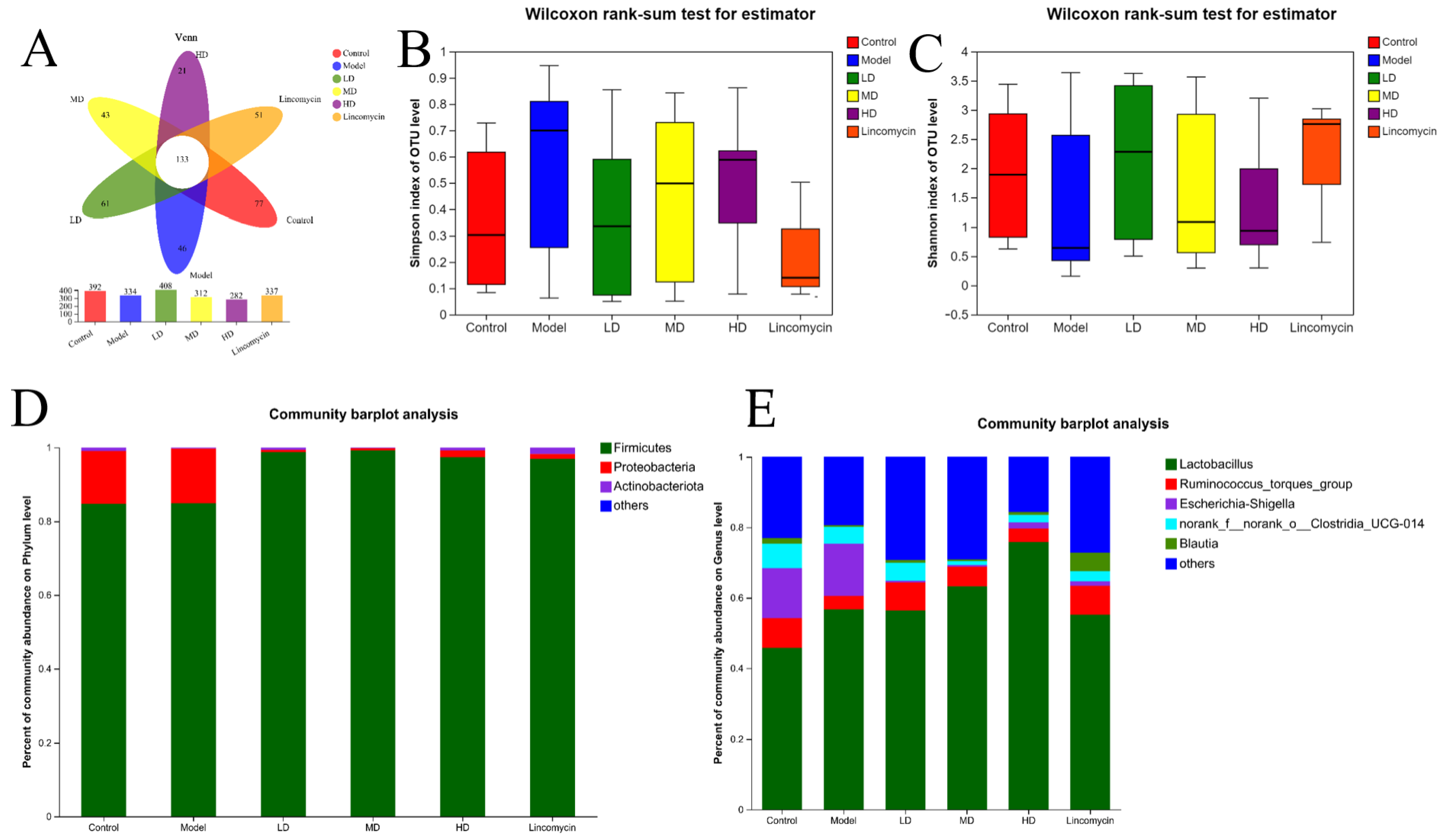
3.6. 16S rRNA Sequencing Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, K.K.; Songer, J.G. Necrotic enteritis in chickens: A paradigm of enteric infection by Clostridium perfringens type A. Anaerobe 2009, 15, 55–60. [Google Scholar] [CrossRef]
- Park, I.; Oh, S.; Nam, H.; Celi, P.; Lillehoj, H.S. Antimicrobial activity of sophorolipids against Eimeria maxima and Clostridium perfringens, and their effect on growth performance and gut health in necrotic enteritis. Poult Sci. 2022, 101, 101731. [Google Scholar] [CrossRef]
- Van Immerseel, F.; De Buck, J.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium perfringens in poultry: An emerging threat for animal and public health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Kulkarni, R.R.; Gaghan, C.; Gorrell, K.; Sharif, S.; Taha-Abdelaziz, K. Probiotics as Alternatives to Antibiotics for the Prevention and Control of Necrotic Enteritis in Chickens. Pathogens 2022, 11, 692. [Google Scholar] [CrossRef] [PubMed]
- Gochez, D.; Raicek, M.; Pinto Ferreira, J.; Jeannin, M.; Moulin, G.; Erlacher-Vindel, E. OIE Annual Report on Antimicrobial Agents Intended for Use in Animals: Methods Used. Front. Vet. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
- Karaffa, L.; Kubicek, C.P. Citric acid and itaconic acid accumulation: Variations of the same story? Appl. Microbiol. Biotechnol. 2019, 103, 2889–2902. [Google Scholar] [CrossRef]
- Tanpong, S.; Cherdthong, A.; Tengjaroenkul, B.; Tengjaroenkul, U.; Wongtangtintharn, S. Evaluation of physical and chemical properties of citric acid industrial waste. Trop. Anim. Health Prod. 2019, 51, 2167–2174. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, B.; Zhang, H. Butyric and Citric Acids and Their Salts in Poultry Nutrition: Effects on Gut Health and Intestinal Microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, Toxicity, Bioavailability, and Formulation of Magnolol: An Update. Front. Pharmacol. 2021, 12, 632767. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.L.; Nam, Y.R.; Kim, H.J.; Woo, J.H.; Kung, W.N.; Nam, J.H.; Kim, W.K. In-vitro and in-vivo anti-allergic effects of magnolol on allergic rhinitis via inhibition of ORAI1 and ANO1 channels. J. Ethnopharmacol. 2022, 289, 115061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Huang, X.; Shi, W.; Zhang, R.; Chen, M.; Huang, H.; Wu, L. Insights on the Multifunctional Activities of Magnolol. Biomed. Res Int. 2019, 2019, 1847130. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Ding, H.; Liu, Y.; Yang, Q.; Xu, N.; Yang, Y.; Ai, X. Magnolol protects channel catfish from Aeromonas hydrophila infection via inhibiting the expression of aerolysin. Vet. Microbiol. 2017, 211, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Xie, K.; Yi, J.; Song, Z.; Zhang, H.; He, X. The effects of magnolol supplementation on growth performance, meat quality, oxidative capacity, and intestinal microbiota in broilers. Poult. Sci. 2022, 101, 101722. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.M. Testing BWPoS. BSAC standardized disc susceptibility testing method (version 8). J. Antimicrob. Chemother. 2009, 64, 454–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, S.; Guo, Y. Xylanase supplementation to a wheat-based diet alleviated the intestinal mucosal barrier impairment of broiler chickens challenged by Clostridium perfringens. Avian Pathol. 2012, 41, 291–298. [Google Scholar] [CrossRef]
- Zhang, B.; Gan, L.; Shahid, M.S.; Lv, Z.; Fan, H.; Liu, D.; Guo, Y. In vivo and in vitro protective effect of arginine against intestinal inflammatory response induced by Clostridium perfringens in broiler chickens. J. Anim. Sci. Biotechnol. 2019, 10, 73. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, B.; Robinson, K.; Belem, T.; Lyu, W.; Deng, Z.; Ramanathan, R.; Zhang, G. Butyrate in combination with forskolin alleviates necrotic enteritis, increases feed efficiency, and improves carcass composition of broilers. J. Anim. Sci. Biotechnol. 2022, 13, 3. [Google Scholar] [CrossRef]
- Abdelli, N.; Pérez, J.F.; Vilarrasa, E.; Luna, I.C.; Melo-Duran, D.; D’Angelo, M.; Solà-Oriol, D. Targeted-Release Organic Acids and Essential Oils Improve Performance and Digestive Function in Broilers Under a Necrotic Enteritis Challenge. Animals 2020, 10, 259. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Q.; Diarra, M.S.; Yu, H.; Hua, Y.; Gong, J. Functional assessment of encapsulated citral for controlling necrotic enteritis in broiler chickens. Poult. Sci. 2016, 95, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, X.; Wang, Y.; Guo, Y.; Zhu, P.; Li, G.; Zhang, J.; Ma, Q.; Zhao, L. Correction: Dietary ellagic acid ameliorated Clostridium perfringens-induced subclinical necrotic enteritis in broilers via regulating inflammation and cecal microbiota. J. Anim. Sci. Biotechnol. 2022, 13, 66. [Google Scholar] [CrossRef]
- Mot, D.; Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Progress and problems in vaccination against necrotic enteritis in broiler chickens. Avian Pathol. 2014, 43, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, W.; Liu, D.; Guo, Y. Effects of Lactobacillus acidophilus on the growth performance and intestinal health of broilers challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Pekel, A.Y.; Alp, M.; Kocabağlı, N. Effects of dietary supplementation of citric acid, copper, and microbial phytase on growth performance and mineral retention in broiler chickens fed a low available phosphorus diet. J. Appl. Poult. Res. 2012, 21, 335–347. [Google Scholar] [CrossRef]
- Khosravinia, H.; Nourmohammadi, R.; Afzali, N. Productive performance, gut morphometry, and nutrient digestibility of broiler chicken in response to low and high dietary levels of citric acid. J. Appl. Poult. Res. 2015, 24, 470–480. [Google Scholar] [CrossRef]
- Ho, K.Y.; Tsai, C.C.; Chen, C.P.; Huang, J.S.; Lin, C.C. Antimicrobial activity of honokiol and magnolol isolated from Magnolia officinalis. Phytother. Res. 2001, 15, 139–141. [Google Scholar] [CrossRef]
- Lin, M.H.; Chen, M.C.; Chen, T.H.; Chang, H.Y.; Chou, T.C. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-gamma-dependent inhibition of NF-kB activation. Int. Immunopharmacol. 2015, 28, 270–278. [Google Scholar] [CrossRef]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant Activity of Magnolol and Honokiol: Kinetic and Mechanistic Investigations of Their Reaction with Peroxyl Radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, H.; Zhao, N.; Du, E.; Jin, F.; Fan, Q.; Guo, W.; Huang, S.; Wei, J. Effects of magnolol and honokiol blend on performance, egg quality, hepatic lipid metabolism, and intestinal morphology of hens at late laying cycle. Animal 2022, 16, 100532. [Google Scholar] [CrossRef]
- Pasare, C.; Medzhitov, R. Toll-like receptors: Linking innate and adaptive immunity. Microbes Infect. 2004, 6, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Ertel, W.; Morrison, M.H.; Wang, P.; Ba, Z.F.; Ayala, A.; Chaudry, I.H. The complex pattern of cytokines in sepsis. Association between prostaglandins, cachectin, and interleukins. Ann. Surg. 1991, 214, 141–148. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.M.; Baird, A.W. Cytokine regulation of epithelial permeability and ion transport. Gut 1999, 44, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, D.; Tong, Y.; Li, Q.; Yang, W.; Wang, T.; Yang, Z. Trans-Anethole Alleviates Subclinical Necro-Haemorrhagic Enteritis-Induced Intestinal Barrier Dysfunction and Intestinal Inflammation in Broilers. Front. Microbiol. 2022, 13, 831882. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Li, P.; Yan, S.; Liu, Y.; Gao, M.; Lv, H.; Lv, Z.; Guo, Y. Effects of Dietary Astragalus Polysaccharide Supplementation on the Th17/Treg Balance and the Gut Microbiota of Broiler Chickens Challenged With Necrotic Enteritis. Front. Immunol. 2022, 13, 781934. [Google Scholar] [CrossRef]
- Shojadoost, B.; Alizadeh, M.; Boodhoo, N.; Astill, J.; Karimi, S.H.; Doost, J.S.; Taha-Abdelaziz, K.; Kulkarni, R.; Sharif, S. Effects of Treatment with Lactobacilli on Necrotic Enteritis in Broiler Chickens. Probiotics Antimicrob Proteins 2022, 14, 1110–1129. [Google Scholar] [CrossRef]
- Park, S.S.; Lillehoj, H.S.; Allen, P.C.; Park, D.W.; FitzCoy, S.; Bautista, D.A.; Lillehoj, E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Rochat, T.; Bermúdez-Humarán, L.; Gratadoux, J.-J.; Fourage, C.; Hoebler, C.; Corthier, G.; Langella, P. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb. Cell Fact. 2007, 6, 22. [Google Scholar] [CrossRef]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into antioxidant activities and anti-skin-aging potential of callus extract from Centella asiatica (L.). Sci. Rep. 2021, 11, 13459. [Google Scholar] [CrossRef]
- Li, Z.; Feng, H.; Han, L.; Ding, L.; Shen, B.; Tian, Y.; Zhao, L.; Jin, M.; Wang, Q.; Qin, H.; et al. Chicoric acid ameliorate inflammation and oxidative stress in Lipopolysaccharide and d-galactosamine induced acute liver injury. J. Cell. Mol. Med. 2020, 24, 3022–3033. [Google Scholar] [CrossRef]
- Zhao, Y.; Zeng, D.; Wang, H.; Qing, X.; Sun, N.; Xin, J.; Luo, M.; Khalique, A.; Pan, K.; Shu, G.; et al. Dietary Probiotic Bacillus licheniformis H2 Enhanced Growth Performance, Morphology of Small Intestine and Liver, and Antioxidant Capacity of Broiler Chickens Against Clostridium perfringens-Induced Subclinical Necrotic Enteritis. Probiotics Antimicrob. Proteins 2020, 12, 883–895. [Google Scholar] [CrossRef]
- Salim, H.M.; Kang, H.K.; Akter, N.; Kim, D.W.; Kim, J.H.; Kim, M.J.; Na, J.C.; Jong, H.B.; Choi, H.C.; Suh, O.S.; et al. Supplementation of direct-fed microbials as an alternative to antibiotic on growth performance, immune response, cecal microbial population, and ileal morphology of broiler chickens. Poult. Sci. 2013, 92, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Wad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, G.; Shahid, M.S.; Gan, L.; Fan, H.; Lv, Z.; Yan, S.; Guo, Y. Dietary l-arginine supplementation ameliorates inflammatory response and alters gut microbiota composition in broiler chickens infected with Salmonella enterica serovar Typhimurium. Poult. Sci. 2020, 99, 1862–1874. [Google Scholar] [CrossRef]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Barbosa, E.V.; Cardoso, C.V.; Silva, R.d.C.F.; Cerqueira, A.d.M.F.; Liberal, M.H.T.; Castro, H.C. An Update Review about An Emerging Poultry Pathogen. Vet. Sci. 2019, 7, 3. [Google Scholar] [CrossRef]
- Latorre, J.D.; Adhikari, B.; Park, S.H.; Teague, K.D.; Graham, L.E.; Mahaffey, B.D.; Baxter, M.F.A.; Hernandez-Velasco, X.; Kwon, Y.M.; Ricke, S.C.; et al. Evaluation of the Epithelial Barrier Function and Ileal Microbiome in an Established Necrotic Enteritis Challenge Model in Broiler Chickens. Front. Vet. Sci. 2018, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xueqin, N.; Zeng, D.; Ni, X.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; et al. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS ONE 2017, 12, e0182426. [Google Scholar] [CrossRef]
- Gu, X.; Li, Z.; Wang, J.; Chen, J.; Jiang, Q.; Liu, N.; Liu, X.; Zhang, F.; Tan, B.; Li, H.; et al. Fermented Cottonseed Meal as a Partial Replacement for Soybean Meal Could Improve the Growth Performance, Immunity and Antioxidant Properties, and Nutrient Digestibility by Altering the Gut Microbiota Profile of Weaned Piglets. Front. Microbiol. 2021, 12, 734389. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Wang, X.; Robinson, K.; Whitmore, M.A.; Stewart, S.N.; Zhao, J.; Zhang, G. Identification of an Intestinal Microbiota Signature Associated With the Severity of Necrotic Enteritis. Front. Microbiol. 2021, 12, 70369. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Primer Sequence (5′→3′) |
|---|---|
| TLR2 | F:GGGGCTCACAGGCAAAATC |
| R:AGCAGGGTTCTCAGGTTCACA | |
| TLR4 | F:AGTCTGAAATTGCTGAGCTCAAAT |
| R:GCGACGTTAAGCCATGGAAG | |
| MyD88 | F:GAAGTTGGGCCACGACTACCT |
| R:TTGCACTTGACCGGAATCAGC | |
| NF-κB | F:TGACCGCCAATAGCTTGTCC |
| R:ACAGCTAAATGCAATGCCGTTC | |
| Nrf-2 | F:GGGCAAGGCGTGAAGTTTTT |
| R:GGCTTTCTCCCGCTCTTTCT | |
| HO-1 | F:AGCTTCGCACAAGGAGTGTT |
| R:GGAGAGGTGGTCAGCATGTC |
| Medicine | C. perfringens |
|---|---|
| MIC | |
| MA | 40 μg/mL |
| CA | 2000 μg/mL |
| Different Multiples of MIC | C. perfringens | ||
|---|---|---|---|
| MIC of MA | MIC of CA | FICI | |
| 4 × MA − 1 × CA | 20 μg/mL | 250 μg/mL | 0.625 |
| 3 × MA − 1 × CA | 15 μg/mL | 250 μg/mL | 0.5 |
| 2 × MA − 1 × CA | 20 μg/mL | 500 μg/mL | 0.75 |
| 1 × MA − 1 × CA | 20 μg/mL | 1000 μg/mL | 1 |
| 1 × MA − 2 × CA | 10 μg/mL | 1000 μg/mL | 0.75 |
| 1 × MA − 3 × CA | 10 μg/mL | 1500 μg/mL | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Zhong, X.; Yang, Y.; Zhang, G.; Si, H. Citric Acid and Magnolol Ameliorate Clostridium perfringens Challenge in Broiler Chickens. Animals 2023, 13, 577. https://doi.org/10.3390/ani13040577
Ding X, Zhong X, Yang Y, Zhang G, Si H. Citric Acid and Magnolol Ameliorate Clostridium perfringens Challenge in Broiler Chickens. Animals. 2023; 13(4):577. https://doi.org/10.3390/ani13040577
Chicago/Turabian StyleDing, Xieying, Xin Zhong, Yunqiao Yang, Geyin Zhang, and Hongbin Si. 2023. "Citric Acid and Magnolol Ameliorate Clostridium perfringens Challenge in Broiler Chickens" Animals 13, no. 4: 577. https://doi.org/10.3390/ani13040577
APA StyleDing, X., Zhong, X., Yang, Y., Zhang, G., & Si, H. (2023). Citric Acid and Magnolol Ameliorate Clostridium perfringens Challenge in Broiler Chickens. Animals, 13(4), 577. https://doi.org/10.3390/ani13040577





