Recent Progress in Solid Electrolytes for All-Solid-State Metal(Li/Na)–Sulfur Batteries
Abstract
:1. Introduction
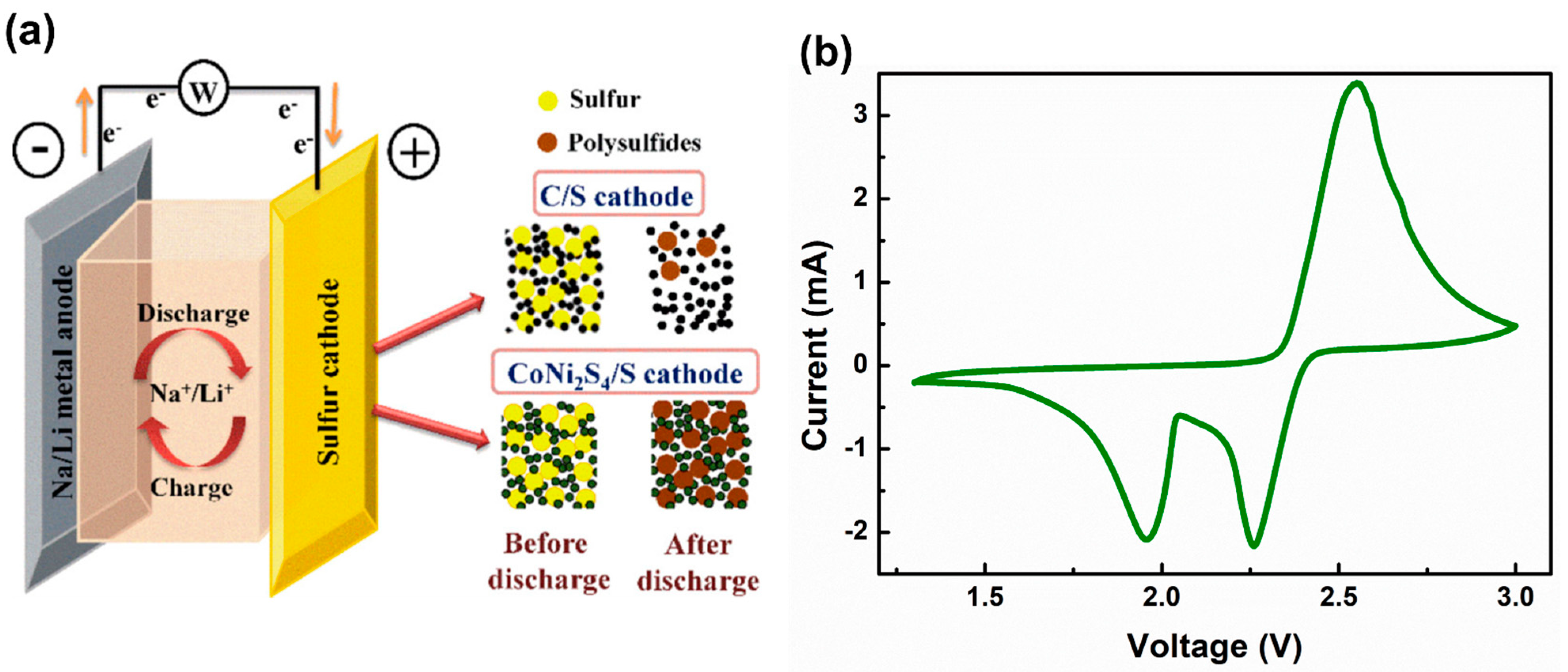
1.1. Li-S Battery
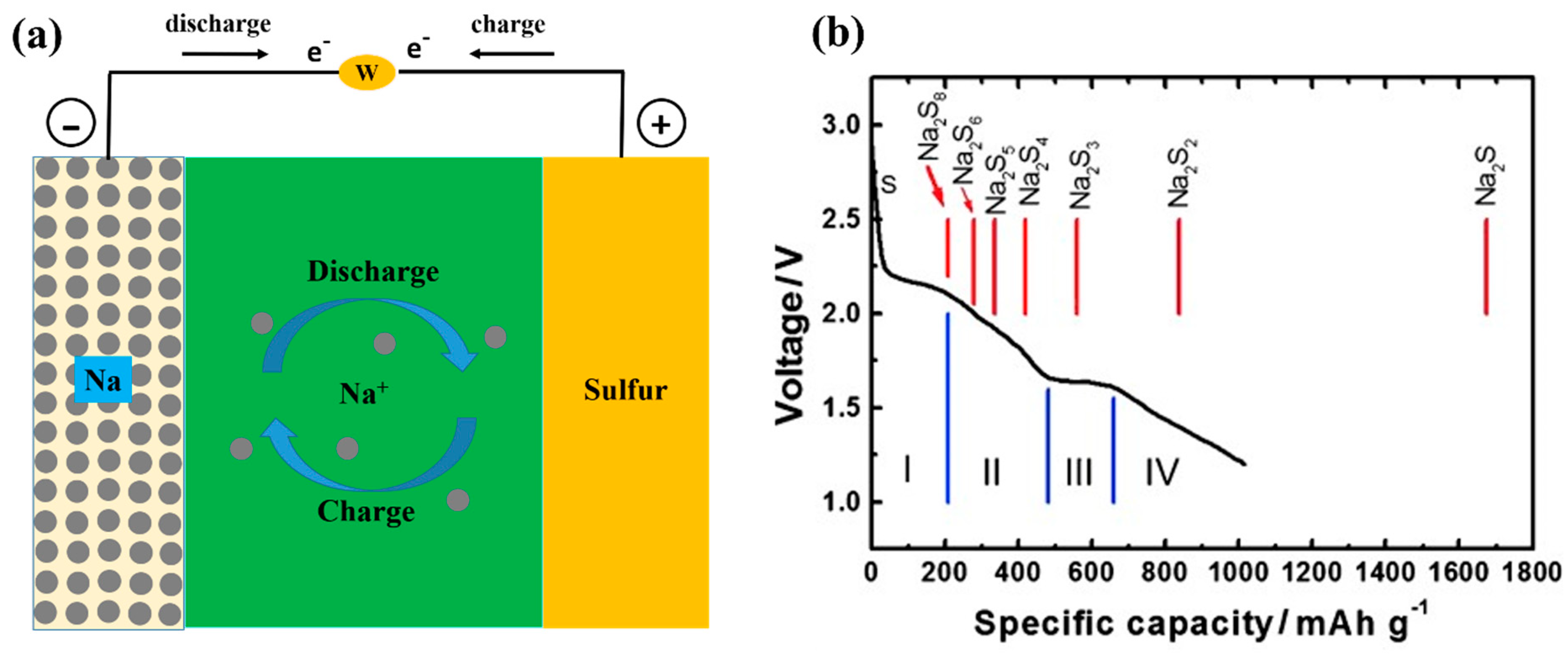
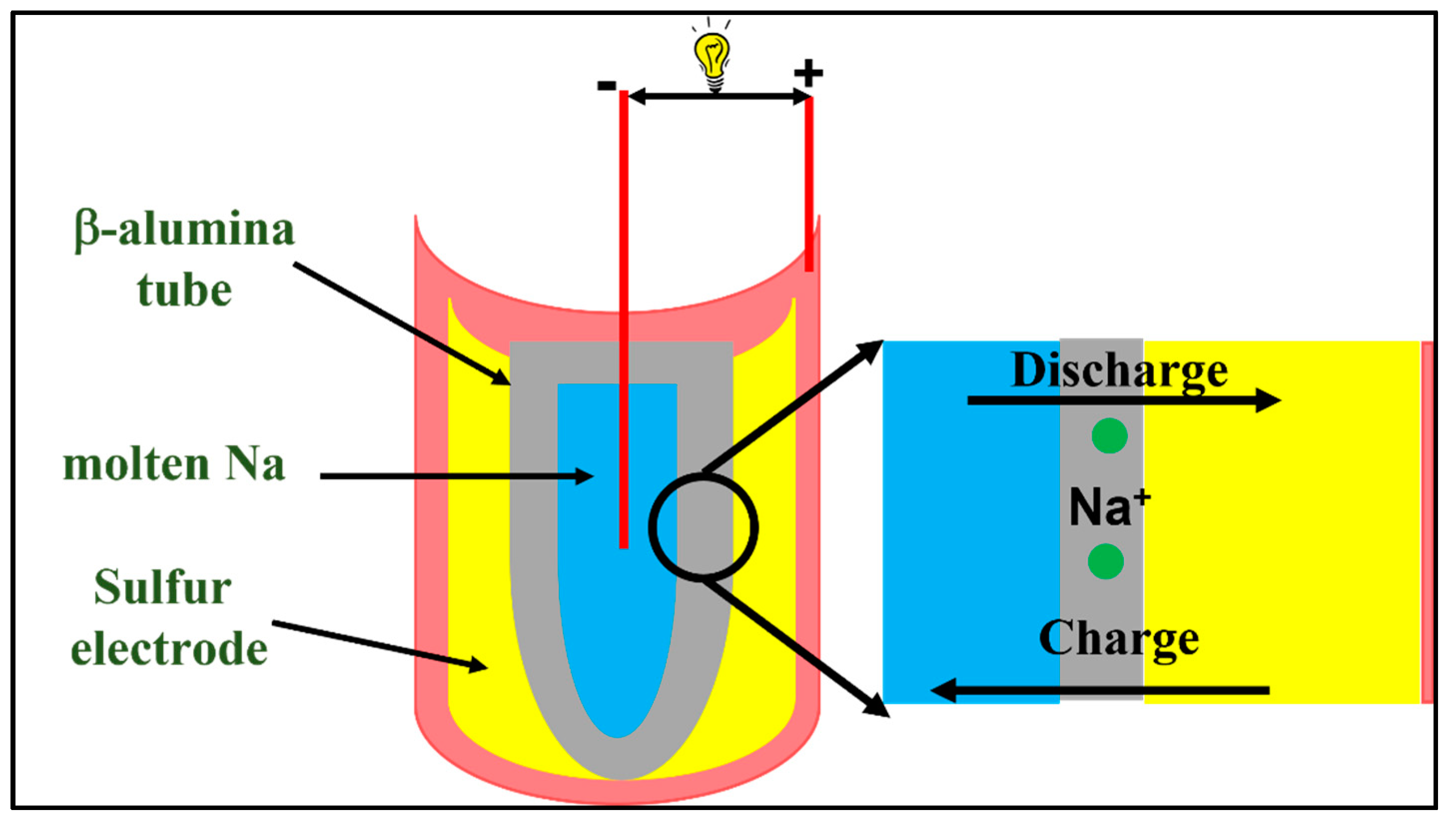
1.2. Sodium Sulfur (Na-S) Battery
2. Replacement of Liquid Organic Electrolyte with Solid Electrolytes (SEs)
3. All-solid-state Metal–sulfur Batteries
4. Fundamental Requirements of Solid Electrolyte to Meet the Practical Applications
5. Recent Progress in Solid Electrolyte for Li-S Batteries
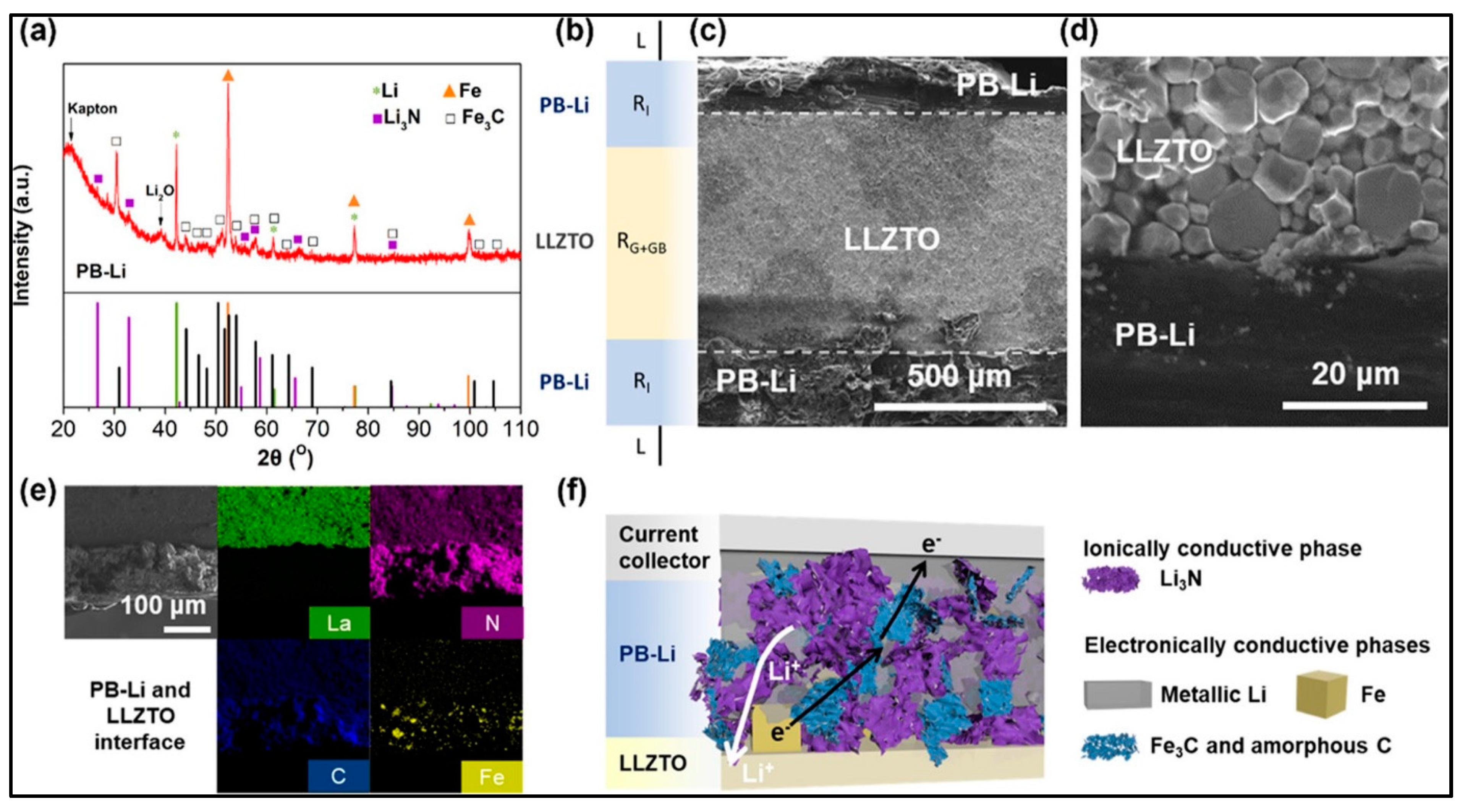
5.1. Inorganic Solid Electrolyte
5.2. Polymer Electrolyte
5.3. Composite Electrolytes
6. Recent Progress in Solid Electrolyte for Na-S Batteries
7. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J. For the Grid: A Battery of Choices. Science 2011, 334, 928. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J. Building Better Batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Grätzel, M. Themed Issue: Nanomaterials for Energy Conversion and Storage. J. Mater. Chem. 2012, 22, 46. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 4, 135. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the Development of Advanced Li-Ion Batteries: A Review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Evers, S.; Nazar, L.F. New Approaches for High Energy Density Lithium–Sulfur Battery Cathodes. Acc. Chem. Res. 2013, 46, 1135–1143. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A Highly Ordered Nanostructured Carbon–Sulphur Cathode for Lithium–Sulphur Batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Zhao, S.; Jiang, S.X.; Huang, C.; Wang, X.Y.; Yang, Z.H.; Xiang, K.X.; Zhang, Y. Suppressing the polysulfide shuttle effect by heteroatom-doping for high performance lithium sulfur batteries. ACS Sustain. Chem. Eng. 2018, 6, 7545–7557. [Google Scholar] [CrossRef]
- Gope, S.; Singh, D.K.; Eswaramoorthy, M.; Bhattacharyya, A.J. An Extremely High Surface Area Mesoporous-Microporous-Networked Pillared Carbon for High Stability Li-S and Intermediate Temperature Na-S Batteries. ChemistrySelect 2017, 2, 9249–9255. [Google Scholar] [CrossRef]
- Song, Y.; Wang, H.; Ma, Q.L.; Li, D.; Yu, W.S.; Liu, G.X.; Wang, T.T.; Yang, Y.; Dong, X.T.; Wang, J.X. Dandelion derived nitrogen-doped hollow carbon host for encapsulating sulfur in lithium sulfur battery. ACS Sustain. Chem. Eng. 2019, 7, 3042–3051. [Google Scholar] [CrossRef]
- Zegeye, T.A.; Kuo, C.F.J.; Chen, H.M.; Tripathi, A.M.; Lin, M.H.; Cheng, J.H.; Duma, A.D.; Su, W.N.; Hwang, B.J. Dual-Confined Sulfur in Hybrid Nanostructured Materials for Enhancement of Lithium-Sulfur Battery Cathode Capacity Retention. ChemElectroChem 2017, 4, 636–647. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.-M. Li–O2 and Li–S Batteries with High Energy Storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef]
- Zegeye, T.A.; Tsai, M.C.; Cheng, J.H.; Lin, M.H.; Chen, H.M.; Rick, J.; Su, W.N.; Kuo, C.F.J.; Hwang, B.J. Controllable Embedding of Sulfur in High Surface Area Nitrogen Doped Three Dimensional Reduced Graphene Oxide by Solution Drop Impregnation Method for High Performance Lithium-Sulfur Batteries. J. Power Sources 2017, 353, 298–311. [Google Scholar] [CrossRef]
- Xin, S.; Gu, L.; Zhao, N.H.; Yin, Y.X.; Zhou, L.J.; Guo, Y.G.; Wan, L.J. Smaller Sulfur Molecules Promise Better Lithium’sulfur Batteries. J. Am. Chem. Soc. 2012, 134, 18510–18513. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Park, J.W.; Hasa, I.; Sun, Y.K.; Scrosati, B.; Hassoun, J. Alternative Materials for Sodium Ion-Sulphur Batteries. J. Mater. Chem. A 2013, 17, 5256–5261. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-Enhanced Redox Chemistry of Polysulphides on a Metallic and Polar Host for Lithium-Sulphur Batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Liu, B.; Zhang, R.; Seh, Z.W.; Zhuo, D.; Liu, Y.; Sun, J. Catalytic Oxidation of Li2S on the Surface of Metal Sulfides for Li–S Batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Lao, M.; Zhao, G.; Li, X.; Chen, Y.; Dou, S.X.; Sun, W. Homogeneous Sulfur-Cobalt Sulfide Nanocomposites as Lithium-Sulfur Battery Cathodes with Enhanced Reaction Kinetics. ACS Appl. Energy Mater. 2018, 1, 167–172. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Bhattacharyya, A.J. Efficient Magnesium Plating and Stripping in DOL/DME- Mg(HMDS) 2—Based Electrolytes and Application in Mg/S Batteries. ACS Appl. Energy Mater. 2021, 4, 14121–14128. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Gomes, R.; Bhattacharyya, A.J. Probing the Polysul Fi de Con Fi Nement in Two Di Ff Erent Sulfur Hosts for a Mg|S Battery Employing Operando Raman and Ex-Situ UV—Visible Spectroscopy. J. Phys. Chem. Lett. 2022, 13, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Peng, H.J.; Zhang, R.; Hou, T.Z.; Huang, J.Q.; Li, B.; Zhang, Q. An Analogous Periodic Law for Strong Anchoring of Polysulfides on Polar Hosts in Lithium Sulfur Batteries: S- or Li-Binding on First-Row Transition-Metal Sulfides? ACS Energy Lett. 2017, 2, 795–801. [Google Scholar] [CrossRef]
- Yu, M.; Ma, J.; Song, H.; Wang, A.; Tian, F.; Wang, Y.; Qiu, H.; Wang, R. Atomic Layer Deposited TiO2 on a Nitrogen-Doped Graphene/Sulfur Electrode for High Performance Lithium–Sulfur Batteries. Energy Environ. Sci. 2016, 9, 1495–1503. [Google Scholar] [CrossRef]
- Du, W.; Wang, Z.; Zhu, Z.; Hu, S.; Zhu, X.; Shi, Y.; Pang, H.; Qian, X. Facile Synthesis and Superior Electrochemical Performances of CoNi2S4/Graphene Nanocomposite Suitable for Supercapacitor Electrodes. J. Mater. Chem. A 2014, 2, 9613–9619. [Google Scholar] [CrossRef]
- Pan, H.; Hu, Y.-S.; Chen, L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013, 6, 2338–2360. [Google Scholar] [CrossRef]
- Park, C.-W.; Ahn, J.-H.; Ryu, H.-S.; Kim, K.-W.; Ahn, H.-J. Room-Temperature Solid-State Sodium/Sulfur Battery. Electrochem. Solid-State Lett. 2006, 9, A123. [Google Scholar] [CrossRef]
- Vineeth, S.K.; Tebyetekerwa, M.; Liu, H.; Soni, C.B.; Sungjemmenla, N.; Zhao, X.S.; Kumar, V. Progress in the Development of Solid-State Electrolytes for Reversible Room-Temperature Sodium-Sulfur Batteries. Mater. Adv. 2022, 2, 6415–6440. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Carbon Materials for Lithium Sulfur Batteries—Ten Critical Questions. Chem.—A Eur. J. 2016, 22, 7324–7351. [Google Scholar] [CrossRef]
- Mikhaylik, Y. Fundamental chemistry of Sion power Li/S battery. In Proceedings of the International Battery Association and Hawaii Battery Conference, Waikoloa, HI, USA, 9–12 January 2006. [Google Scholar]
- Feature, N. The Rechargeable Battery Revolution: A Better Battery. Nature 2014, 507, 26–28. [Google Scholar]
- ENDLiS ENERGY. 2017. Available online: http://www.endlisenergy.com/home.html (accessed on 1 August 2022).
- Ji, X.; Nazar, L.F. Advances in Li/S Batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Whittingham, M.S. Lithium Batteries and Cathode Materials. Chem. Rev. 2004, 104, 4271–4301. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Tsai, W.L.; Huang, M.H.; Lee, W.K.; Hsu, Y.J.; Pan, K.C.; Huang, Y.H.; Ting, H.C.; Sarma, M.; Ho, Y.Y.; Hu, H.C.; et al. A Versatile Thermally Activated Delayed Fluorescence Emitter for Both Highly Efficient Doped and Non-Doped Organic Light Emitting Devices. Chem. Commun. 2015, 51, 13662–13665. [Google Scholar] [CrossRef]
- Kumaresan, K.; Mikhaylik, Y.; White, R.E. A Mathematical Model for a Lithium–Sulfur Cell. J. Electrochem. Soc. 2008, 155, A576. [Google Scholar] [CrossRef]
- Moy, D.; Manivannan, A.; Narayanan, S.R. Direct Measurement of Polysulfide Shuttle Current: A Window into Understanding the Performance of Lithium-Sulfur Cells. J. Electrochem. Soc. 2015, 162, A1–A7. [Google Scholar] [CrossRef]
- Barchasz, C.; Molton, F.; Duboc, C.; Leprêtre, J.C.; Patoux, S.; Alloin, F. Lithium/Sulfur Cell Discharge Mechanism: An Original Approach for Intermediate Species Identification. Anal. Chem. 2012, 84, 3973–3980. [Google Scholar] [CrossRef]
- Kawase, A.; Shirai, S.; Yamoto, Y.; Arakawa, R.; Takata, T. Electrochemical Reactions of Lithium-Sulfur Batteries: An Analytical Study Using the Organic Conversion Technique. Phys. Chem. Chem. Phys. 2014, 16, 9344–9350. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium Sulfur Batteries, a Mechanistic Review. Energy Environ. Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Jayanthi, S.; Adarakatti, P.S.; Sood, A.K.; Bhattacharyya, A.J. Probing the Extent of Polysul Fi de Con Fi Nement Using a CoNi 2 S 4 Additive Inside a Sulfur Cathode of a Na/Li—Sulfur Rechargeable Battery. ACS Appl. Mater. Interfaces 2020, 12, 8120–28128. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Yang, S.; Cheng, F.; Liang, J.; Chen, J. A Quantum-Chemical Study on the Discharge Reaction Mechanism of Lithium-Sulfur Batteries. J. Energy Chem. 2013, 22, 72–77. [Google Scholar] [CrossRef]
- Busche, M.R.; Adelhelm, P.; Sommer, H.; Schneider, H.; Leitner, K.; Janek, J. Systematical Electrochemical Study on the Parasitic Shuttle-Effect in Lithium-Sulfur-Cells at Different Temperatures and Different Rates. J. Power Sources 2014, 259, 289–299. [Google Scholar] [CrossRef]
- Wujcik, K.H.; Velasco-Velez, J.; Wu, C.H.; Pascal, T.; Teran, A.A.; Marcus, M.A.; Cabana, J.; Guo, J.; Prendergast, D.; Salmeron, M.; et al. Fingerprinting Lithium-Sulfur Battery Reaction Products by X-Ray Absorption Spectroscopy. J. Electrochem. Soc. 2014, 161, A1100–A1106. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal Component Analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Park, M.S.; Yu, J.S.; Kim, K.J.; Jeong, G.; Kim, J.H.; Jo, Y.N.; Hwang, U.; Kang, S.; Woo, T.; Kim, Y.J. One-Step Synthesis of a Sulfur-Impregnated Graphene Cathode for Lithium-Sulfur Batteries. Phys. Chem. Chem. Phys. 2012, 14, 6796–6804. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, G.; Cui, Y. Nanostructured Sulfur Cathodes. Chem. Soc. Rev. 2013, 42, 3018–3032. [Google Scholar] [CrossRef] [PubMed]
- Raiß, C.; Peppler, K.; Janek, J.; Adelhelm, P. Pitfalls in the Characterization of Sulfur/Carbon Nanocomposite Materials for Lithium-Sulfur Batteries. Carbon N. Y. 2014, 79, 245–255. [Google Scholar] [CrossRef]
- Fujimori, T.; Morelos-Gómez, A.; Zhu, Z.; Muramatsu, H.; Futamura, R.; Urita, K.; Terrones, M.; Hayashi, T.; Endo, M.; Young Hong, S.; et al. Conducting Linear Chains of Sulphur inside Carbon Nanotubes. Nat. Commun. 2013, 4, 2162. [Google Scholar] [CrossRef]
- Ma, L.; Hendrickson, K.E.; Wei, S.; Archer, L.A. Nanomaterials: Science and Applications in the Lithium-Sulfur Battery. Nano Today 2015, 10, 315–338. [Google Scholar] [CrossRef]
- Song, X.; Gao, T.; Wang, S.; Bao, Y.; Chen, G.; Ding, L.X.; Wang, H. Free-Standing Sulfur Host Based on Titanium-Dioxide-Modified Porous-Carbon Nanofibers for Lithium-Sulfur Batteries. J. Power Sources 2017, 356, 172–180. [Google Scholar] [CrossRef]
- Liu, Y.; Li, G.; Chen, Z.; Peng, X. CNT-Threaded N-Doped Porous Carbon Film as Binder-Free Electrode for High-Capacity Supercapacitor and Li/S Battery. J. Mater. Chem. A 2017, 5, 9775–9784. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Guan, B.; Wang, D.; Liu, L.M.; Lou, X.W. A Sulfur Host Based on Titanium Monoxide@carbon Hollow Spheres for Advanced Lithium-Sulfur Batteries. Nat. Commun. 2016, 7, 13065. [Google Scholar] [CrossRef] [Green Version]
- Agostini, M.; Hassoun, J.; Liu, J.; Jeong, M.; Nara, H.; Momma, T.; Osaka, T.; Sun, Y.K.; Scrosati, B. A Lithium-Ion Sulfur Battery Based on a Carbon-Coated Lithium-Sulfide Cathode and an Electrodeposited Silicon-Based Anode. ACS Appl. Mater. Interfaces 2014, 6, 10924–10928. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; You, Y.; Yan, Y.; Zhang, D.; Cong, H.P.; Qin, H.; Zhang, C.; Cao, F.F.; Jiang, K.C.; Wang, Y.; et al. Conductive Carbon Network inside a Sulfur-Impregnated Carbon Sponge: A Bioinspired High-Performance Cathode for Li/S Battery. ACS Appl. Mater. Interfaces 2016, 8, 22261–22269. [Google Scholar] [CrossRef]
- Shen, J.; Feng, Y.; Wang, P.; Qiu, G.; Zhang, L.; Lu, L.; Wang, H.; Wang, R.; Linkov, V.; Ji, S. Conductive Sulfur-Rich Copolymer Composites as Lithium-Sulfur Battery Electrodes with Fast Kinetics and a High Cycle Stability. ACS Sustain. Chem. Eng. 2020, 8, 10389–10401. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, S.; Shen, Y.; Shao, G.; Zhang, F. “room-like” TiO2Array as a Sulfur Host for Lithium-Sulfur Batteries: Combining Advantages of Array and Closed Structures. ACS Sustain. Chem. Eng. 2020, 8, 7609–7616. [Google Scholar] [CrossRef]
- Yu, M.; Li, R.; Wu, M.; Shi, G. Graphene Materials for Lithium-Sulfur Batteries. Energy Storage Mater. 2015, 1, 51–73. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Lahan, H.; Sekkar, V.; John, B.; Bhattacharyya, A.J. High-Performance Li-Metal-Free Sulfur Battery Employing a Lithiated Anatase TiO2 Anode and a Freestanding Li2S—Carbon Aerogel Cathode. ACS Sustain. Chem. Eng. 2022, 10, 410–420. [Google Scholar] [CrossRef]
- Palomares, V.; Serras, P.; Villaluenga, I.; Hueso, K.B.; Carretero-González, J.; Rojo, T. Na-Ion Batteries, Recent Advances and Present Challenges to Become Low Cost Energy Storage Systems. Energy Environ. Sci. 2012, 5, 5884–5901. [Google Scholar] [CrossRef]
- Hassoun, J.; Scrosati, B. A High-Performance Polymer Tin Sulfur Lithium Ion Battery. Angew. Chem.—Int. Ed. 2010, 49, 2371–2374. [Google Scholar] [CrossRef]
- Hosaka, T.; Kubota, K.; Hameed, A.S.; Komaba, S. Research Development on K-Ion Batteries. Chem. Rev. 2020, 120, 6358–6466. [Google Scholar] [CrossRef]
- Lu, X.; Kirby, B.W.; Xu, W.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. Advanced Intermediate-Temperature Na/S Battery. Energy Environ. Sci. 2013, 6, 299–306. [Google Scholar] [CrossRef]
- Wei, S.; Xu, S.; Agrawral, A.; Choudhury, S.; Lu, Y.; Tu, Z.; Ma, L.; Archer, L.A. A Stable Room-Temperature Sodium-Sulfur Battery. Nat. Commun. 2016, 7, 11722. [Google Scholar] [CrossRef]
- Hueso, K.B.; Armand, M.; Rojo, T. High Temperature Sodium Batteries: Status, Challenges and Future Trends. Energy Environ. Sci. 2013, 6, 734–749. [Google Scholar] [CrossRef]
- Hueso, K.B.; Palomares, V.; Armand, M.; Rojo, T. Challenges and Perspectives on High and Intermediate-Temperature Sodium Batteries. Nano Res. 2017, 10, 4082–4114. [Google Scholar] [CrossRef]
- Ye, H.; Ma, L.; Zhou, Y.; Wang, L.; Han, N.; Zhao, F.; Deng, J.; Wu, T.; Li, Y.; Lu, J. Amorphous MoS3 as the Sulfur-Equivalent Cathode Material for Room-Temperature Li–S and Na–S Batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 13091–13096. [Google Scholar] [CrossRef]
- Carter, R.; Oakes, L.; Douglas, A.; Muralidharan, N.; Cohn, A.P.; Pint, C.L. A Sugar-Derived Room-Temperature Sodium Sulfur Battery with Long Term Cycling Stability. Nano Lett. 2017, 17, 1863–1869. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, B.; Lai, W.; Xu, Y.; Chou, S.L.; Liu, H.K.; Dou, S.X. Room-Temperature Sodium-Sulfur Batteries: A Comprehensive Review on Research Progress and Cell Chemistry. Adv. Energy Mater. 2017, 7, 1602829. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kajiyama, M.; Iwatate, J.; Nishikawa, H.; Hitomi, S.; Okuyama, R.; Usui, R.; Yamada, Y.; Komaba, S. P2-Type Nax [Fe1/2Mn1/2[O2 Made from Earth-Abundant Elements for Rechargeable Na Batteries. Nat. Mater. 2012, 11, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian Blue: A New Framework of Electrode Materials for Sodium Batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X. Ambient Temperature Sodium-Sulfur Batteries. Small 2015, 11, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Manthiram, A. Capacity Enhancement and Discharge Mechanisms of Room-Temperature Sodium-Sulfur Batteries. ChemElectroChem 2014, 1, 1275–1280. [Google Scholar] [CrossRef]
- Source of Detrimental Dendrite Growth in Lithium Batteries Discover. MSE Supplies Admin, 17 October 2019.
- Yang, X.; Li, X.; Adair, K.; Zhang, H.; Sun, X. Structural Design of Lithium–Sulfur Batteries: From Fundamental Research to Practical Application; Springer: Singapore, 2018; Volume 1. [Google Scholar]
- Zhang, C.; Wu, H.B.; Yuan, C.; Guo, Z.; Lou, X.W. Confining Sulfur in Double-Shelled Hollow Carbon Spheres for Lithium-Sulfur Batteries. Angew. Chem.—Int. Ed. 2012, 51, 9592–9595. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Ji, X.; Nazar, L. High “c” Rate Li-S Cathodes: Sulfur Imbibed Bimodal Porous Carbons. Energy Environ. Sci. 2011, 4, 2878–2883. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous Hollow Carbon@sulfur Composites for High-Power Lithium-Sulfur Batteries. Angew. Chem.—Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef]
- Cuisinier, M.; Cabelguen, P.E.; Adams, B.D.; Garsuch, A.; Balasubramanian, M.; Nazar, L.F. Unique Behaviour of Nonsolvents for Polysulphides in Lithium-Sulphur Batteries. Energy Environ. Sci. 2014, 7, 2697–2705. [Google Scholar] [CrossRef]
- Chen, J.; Wu, D.; Walter, E.; Engelhard, M.; Bhattacharya, P.; Pan, H.; Shao, Y.; Gao, F.; Xiao, J.; Liu, J. Molecular-Confinement of Polysulfides within Mesoscale Electrodes for the Practical Application of Lithium Sulfur Batteries. Nano Energy 2015, 13, 267–274. [Google Scholar] [CrossRef]
- Kumar, P.; Wu, F.Y.; Hu, L.H.; Ali Abbas, S.; Ming, J.; Lin, C.N.; Fang, J.; Chu, C.W.; Li, L.J. High-Performance Graphene/Sulphur Electrodes for Flexible Li-Ion Batteries Using the Low-Temperature Spraying Method. Nanoscale 2015, 7, 8093–8100. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, D.J.; Jung, H.G.; Sun, Y.K.; Hassoun, J.; Scrosati, B. An Advanced Lithium-Sulfur Battery. Adv. Funct. Mater. 2013, 23, 1076–1080. [Google Scholar] [CrossRef]
- Chang, C.H.; Chung, S.H.; Manthiram, A. Ultra-Lightweight PANiNF/MWCNT-Functionalized Separators with Synergistic Suppression of Polysulfide Migration for Li-S Batteries with Pure Sulfur Cathodes. J. Mater. Chem. A 2015, 3, 18829–18834. [Google Scholar] [CrossRef]
- Quartarone, E.; Mustarelli, P. Electrolytes for Solid-State Lithium Rechargeable Batteries: Recent Advances and Perspectives. Chem. Soc. Rev. 2011, 40, 2525–2540. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium Battery Chemistries Enabled by Solid-State Electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Knauth, P.; Mate, L.; Charles, C.S.; Cedex, C.M.; Tuller, H.L. Solid-State Ionics: Roots, Status, and Future Prospects. J. Am. Ceram. Soc. 2002, 80, 1654–1680. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent Progress of the Solid-State Electrolytes for High-Energy Metal-Based Batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Judez, X.; Martinez-Ibañez, M.; Santiago, A.; Armand, M.; Zhang, H.; Li, C. Quasi-Solid-State Electrolytes for Lithium Sulfur Batteries: Advances and Perspectives. J. Power Sources 2019, 438, 438–478. [Google Scholar] [CrossRef]
- Yao, W.; Zheng, W.; Xu, J.; Tian, C.; Han, K.; Sun, W.; Xiao, S. ZnS-SnS@NC Heterostructure as Robust Lithiophilicity and Sulfiphilicity Mediator toward High-Rate and Long-Life Lithium-Sulfur Batteries. ACS Nano 2021, 15, 7114–7130. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and Reality of Post-Lithium-Ion Batteries with High Energy Densities. Nat. Rev. Mater. 2016, 1, 256–262. [Google Scholar] [CrossRef]
- Xu, J.; Yu, F.; Hua, J.; Tang, W.; Yang, C.; Hu, S.; Zhao, S.; Zhang, X.; Xin, Z.; Niu, D. Donor Dominated Triazine-Based Microporous Polymer as a Polysulfide Immobilizer and Catalyst for High-Performance Lithium-Sulfur Batteries. Chem. Eng. J. 2020, 392, 123694. [Google Scholar] [CrossRef]
- Yang, L.; Li, Q.; Wang, Y.; Chen, Y.; Guo, X.; Wu, Z.; Chen, G.; Zhong, B.; Xiang, W.; Zhong, Y. A Review of Cathode Materials in Lithium-Sulfur Batteries. Ionics 2020, 26, 5299–5318. [Google Scholar] [CrossRef]
- Wang, P.; Xi, B.; Huang, M.; Chen, W.; Feng, J.; Xiong, S. Emerging Catalysts to Promote Kinetics of Lithium–Sulfur Batteries. Adv. Energy Mater. 2021, 11, 2002893. [Google Scholar] [CrossRef]
- Ding, B.; Wang, J.; Fan, Z.; Chen, S.; Lin, Q.; Lu, X.; Dou, H.; Kumar Nanjundan, A.; Yushin, G.; Zhang, X.; et al. Solid-State Lithium–Sulfur Batteries: Advances, Challenges and Perspectives. Mater. Today 2020, 40, 114–131. [Google Scholar] [CrossRef]
- Yuan, S.; Bao, J.L.; Li, C.; Xia, Y.; Truhlar, D.G.; Wang, Y. Dual Lithiophilic Structure for Uniform Li Deposition. ACS Appl. Mater. Interfaces 2019, 11, 10616–10623. [Google Scholar] [CrossRef]
- Ong, S.P.; Mo, Y.; Richards, W.D.; Miara, L.; Lee, H.S.; Ceder, G. Phase Stability, Electrochemical Stability and Ionic Conductivity of the Li10±1MP2X12 (M = Ge, Si, Sn, Al or P, and X = O, S or Se) Family of Superionic Conductors. Energy Environ. Sci. 2013, 6, 148–156. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Son, B.; Mukherjee, S.; Schuppert, N.; Bates, A.; Kwon, O.; Choi, M.J.; Chung, H.Y.; Park, S. A Review of Lithium and Non-Lithium Based Solid State Batteries. J. Power Sources 2015, 282, 299–322. [Google Scholar] [CrossRef]
- Yao, X.; Huang, B.; Yin, J.; Peng, G.; Huang, Z.; Gao, C.; Liu, D.; Xu, X. All-Solid-State Lithium Batteries with Inorganic Solid Electrolytes: Review of Fundamental Science. Chin. Phys. B 2015, 25, 18802. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, L.; Liu, S.; Ai, B.; Zhao, S.; Zhang, W.; Pan, K.; Zhang, L. Improvement of the Interface between the Lithium Anode and a Garnet-Type Solid Electrolyte of Lithium Batteries Using an Aluminum-Nitride Layer. Nanomaterials 2022, 12, 2023. [Google Scholar] [CrossRef]
- Cai, M.; Jin, J.; Xiu, T.; Song, Z.; Badding, M.E.; Wen, Z. In-Situ Constructed Lithium-Salt Lithiophilic Layer Inducing Bi-Functional Interphase for Stable LLZO/Li Interface. Energy Storage Mater. 2022, 47, 61–69. [Google Scholar] [CrossRef]
- Huang, B.; Hua, H.; Lai, P.; Shen, X.; Li, R.; He, Z.; Zhang, P.; Zhao, J. Constructing Ion-Selective Coating Layer with Lithium Ion Conductor LLZO and Binder Li-Nafion for Separator Used in Lithium-Sulfur Batteries. ChemElectroChem 2022, 9, e202200416. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Tang, Z.; Mei, Y.; Xiang, X.; Deng, J. Study on the Suppression of Lithium Dendrites Based on Solid Electrolyte Li7La3Zr2O12 with Annealing Treatment of Al Interlayer. J. Alloys Compd. 2022, 904, 163908. [Google Scholar] [CrossRef]
- He, X.; Yan, F.; Gao, M.; Shi, Y.; Ge, G.; Shen, B.; Zhai, J. Cu-Doped Alloy Layer Guiding Uniform Li Deposition on a Li-LLZO Interface under High Current Density. ACS Appl. Mater. Interfaces 2021, 13, 42212–42219. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-Power All-Solid-State Batteries Using Sulfide Superionic Conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; McShane, E.J.; McCloskey, B.D. Promising Routes to a High Li+ Transference Number Electrolyte for Lithium Ion Batteries. ACS Energy Lett. 2017, 2, 2563–2575. [Google Scholar] [CrossRef]
- Su, S.; Ma, J.; Zhao, L.; Lin, K.; Li, Q.; Lv, S.; Kang, F.; He, Y.B. Progress and perspective of the cathode/electrolyte interface construction in all-solid-state lithium batteries. Carbon Energy 2021, 3, 866–894. [Google Scholar] [CrossRef]
- Xiao, S.; Huang, L.; Lv, W.; He, Y.B. A Highly Efficient Ion and Electron Conductive Interlayer to Achieve Low Self-Discharge of Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2022, 14, 1783–1790. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Zheng, P.; Sun, J.; Liu, C.; Chai, J.; Li, X.; Zheng, Y.; Liu, Z. Recent Advances in Stability Issues of Inorganic Solid Electrolytes and Composite Solid Electrolytes for All-Solid-State Batteries. Chem. Rec. 2022, 22, e202200116. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, R.; Li, Y.; Liu, Y.; Xin, C.; Richter, F.H.; Nan, C.W. Interfacial Challenges for All-Solid-State Batteries Based on Sulfide Solid Electrolytes. J. Mater. 2021, 7, 209–218. [Google Scholar] [CrossRef]
- Li, G.; Yang, Z.; Jiang, Y.; Jin, C.; Huang, W.; Ding, X.; Huang, Y. Towards Polyvalent Ion Batteries: A Zinc-Ion Battery Based on NASICON Structured Na3V2(PO4)3. Nano Energy 2016, 25, 211–217. [Google Scholar] [CrossRef]
- Kwon, W.J.; Kim, H.; Jung, K.N.; Cho, W.; Kim, S.H.; Lee, J.W.; Park, M.S. Enhanced Li+ Conduction in Perovskite Li3XLa2/3-X□1/3-2xTiO3 Solid-Electrolytes via Microstructural Engineering. J. Mater. Chem. A 2017, 5, 6257–6262. [Google Scholar] [CrossRef]
- Deshmukh, A.; Thripuranthaka, M.; Chaturvedi, V.; Das, A.K.; Shelke, V.; Shelke, M.V. A Review on Recent Advancements in Solid State Lithium-Sulfur Batteries: Fundamentals, Challenges, and Perspectives. Prog. Energy 2022, 4, 042001. [Google Scholar] [CrossRef]
- Wang, H.C.; Cao, X.; Liu, W.; Sun, X. Research Progress of the Solid State Lithium-Sulfur Batteries. Front. Energy Res. 2019, 7, 1–20. [Google Scholar] [CrossRef]
- Lang, B.; Ziebarth, B.; Elsässer, C. Lithium Ion Conduction in LiTi2(PO4)3 and Related Compounds Based on the NASICON Structure: A First-Principles Study. Chem. Mater. 2015, 27, 5040–5048. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast Lithium Ion Conduction in Garnet-Type Li7La3Zr2O12. Angew. Chem.—Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.T.; Wang, C.A.; Xie, H.; Goodenough, J.B. Optimizing Li+ Conductivity in a Garnet Framework. J. Mater. Chem. 2012, 22, 15357–15361. [Google Scholar] [CrossRef]
- Zhong, Y.; Cao, C.; Tadé, M.O.; Shao, Z. Ionically and Electronically Conductive Phases in a Composite Anode for High-Rate and Stable Lithium Stripping and Plating for Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2022, 14, 38786–38794. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; Mangelis, P.; Maravelakis, E.; Mountakis, N.; Papadakis, V.; Neonaki, M.; Thomadaki, G. Thermomechanical Response of Polycarbonate/Aluminum Nitride Nanocomposites in Material Extrusion Additive Manufacturing. Materials 2022, 15, 8806. [Google Scholar] [CrossRef]
- Zhou, D.; Ren, G.X.; Zhang, N.; Yu, P.F.; Zhang, H.; Zheng, S.; Tian, Z.W.; Du, S.Y.; Chen, J.X.; Liu, X.S. Garnet Electrolytes with Ultralow Interfacial Resistance by SnS2Coating for Dendrite-Free All-Solid-State Batteries. ACS Appl. Energy Mater. 2021, 4, 2873–2880. [Google Scholar] [CrossRef]
- Zhu, P.; Yan, C.; Zhu, J.; Zang, J.; Li, Y.; Jia, H.; Dong, X.; Du, Z.; Zhang, C.; Wu, N.; et al. Flexible Electrolyte-Cathode Bilayer Framework with Stabilized Interface for Room-Temperature All-Solid-State Lithium-Sulfur Batteries. Energy Storage Mater. 2019, 17, 220–225. [Google Scholar] [CrossRef]
- Dai, X.; Zou, K.; Jing, W.; Xu, P.; Sun, J.; Guo, S.; Tan, Q.; Liu, Y.; Zhou, T.; Chen, Y. A Dual-Functional Interlayer for Li-S Batteries Using Carbon Fiber Film Cladded Electron-Deficient Li2B4O7. J. Mater. Chem. A 2022, 10, 16152–16162. [Google Scholar] [CrossRef]
- Yu, X.; Bi, Z.; Zhao, F.; Manthiram, A. Polysulfide-Shuttle Control in Lithium-Sulfur Batteries with a Chemically/Electrochemically Compatible NaSICON-Type Solid Electrolyte. Adv. Energy Mater. 2016, 6, 1601392. [Google Scholar] [CrossRef]
- Yao, X.; Huang, N.; Han, F.; Zhang, Q.; Wan, H.; Mwizerwa, J.P.; Wang, C.; Xu, X. High-Performance All-Solid-State Lithium–Sulfur Batteries Enabled by Amorphous Sulfur-Coated Reduced Graphene Oxide Cathodes. Adv. Energy Mater. 2017, 7, 1602923. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.H.; Shen, Y.; Li, L.; Nan, C.W. High-Conductivity Argyrodite Li6PS5Cl Solid Electrolytes Prepared via Optimized Sintering Processes for All-Solid-State Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 42279–42285. [Google Scholar] [CrossRef] [PubMed]
- Umeshbabu, E.; Zheng, B.; Zhu, J.; Wang, H.; Li, Y.; Yang, Y. Stable Cycling Lithium-Sulfur Solid Batteries with Enhanced Li/Li10GeP2S12 Solid Electrolyte Interface Stability. ACS Appl. Mater. Interfaces 2019, 11, 18436–18447. [Google Scholar] [CrossRef]
- Wan, H.; Liu, S.; Deng, T.; Xu, J.; Zhang, J.; He, X.; Ji, X.; Yao, X.; Wang, C. Bifunctional Interphase-Enabled Li10GeP2S12Electrolytes for Lithium-Sulfur Battery. ACS Energy Lett. 2021, 6, 862–868. [Google Scholar] [CrossRef]
- Xia, Y.; Li, J.; Zhang, J.; Zhou, X.; Huang, H.; He, X.; Gan, Y.; Xiao, Z.; Zhang, W. Yttrium Stabilized Argyrodite Solid Electrolyte with Enhanced Ionic Conductivity and Interfacial Stability for All-Solid-State Batteries. J. Power Sources 2022, 543, 231846. [Google Scholar] [CrossRef]
- Qian, J.; Jin, B.; Li, Y.; Zhan, X.; Hou, Y.; Zhang, Q. Research Progress on Gel Polymer Electrolytes for Lithium-Sulfur Batteries. J. Energy Chem. 2021, 56, 420–437. [Google Scholar] [CrossRef]
- Han, D.D.; Liu, S.; Liu, Y.T.; Zhang, Z.; Li, G.R.; Gao, X.P. Lithiophilic Gel Polymer Electrolyte to Stabilize the Lithium Anode for a Quasi-Solid-State Lithium-Sulfur Battery. J. Mater. Chem. A 2018, 6, 18627–18634. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, Q.; Sun, C.; Wang, J.; Zhong, X.; Chen, B.; Li, C.; Gao, R.; Han, Z.; Zhou, G. Crosslinked Nanofiber-Reinforced Solid-State Electrolytes with Polysulfide Fixation Effect Towards High Safety Flexible Lithium–Sulfur Batteries. Adv. Funct. Mater. 2022, 32, 2203272. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, H.R.; Ren, Y.X.; Zhou, D.; Kang, F.Y.; Zhao, T.S. In-Situ Fabrication of a Freestanding Acrylate-Based Hierarchical Electrolyte for Lithium-Sulfur Batteries. Electrochim. Acta 2016, 213, 871–878. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Liu, J.; Miller, J.D. Natural Halloysite Nano-Clay Electrolyte for Advanced All-Solid-State Lithium-Sulfur Batteries. Nano Energy 2017, 31, 478–485. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, N.; Qu, W.; Yang, C.; Li, L.; Wu, F.; Chen, R. Vertical Channels Design for Polymer Electrolyte to Enhance Mechanical Strength and Ion Conductivity. ACS Appl. Mater. Interfaces 2021, 13, 42957–42965. [Google Scholar] [CrossRef]
- Xie, P.; Yang, R.; Zhou, Y.; Zhang, B.; Tian, X. Rationally Designing Composite Gel Polymer Electrolyte Enables High Sulfur Utilization and Stable Lithium Anode. Chem. Eng. J. 2022, 450, 138195. [Google Scholar] [CrossRef]
- Ellis, B.L.; Nazar, L.F. Sodium and Sodium-Ion Energy Storage Batteries. Curr. Opin. Solid State Mater. Sci. 2012, 16, 168–177. [Google Scholar] [CrossRef]
- Delmas, C. Sodium and Sodium-Ion Batteries: 50 Years of Research. Adv. Energy Mater. 2018, 8, 1703137. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-Lithium-Ion Battery Cell Production and Its Compatibility with Lithium-Ion Cell Production Infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Sodium-Ion Batteries: Present and Future. Chem. Soc. Rev. 2017, 46, 3529–3614. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, C.H.; Choi, S.H.; Ahn, J.P.; Ahn, J.H.; Kim, K.W.; Cairns, E.J.; Ahn, H.J. A Singular Flexible Cathode for Room Temperature Sodium/Sulfur Battery. J. Power Sources 2016, 307, 31–37. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Busche, M.; Eufinger, C.; Janek, J. From Lithium to Sodium: Cell Chemistry of Room Temperature Sodium-Air and Sodium-Sulfur Batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef]
- Tanibata, N.; Deguchi, M.; Hayashi, A.; Tatsumisago, M. All-Solid-State Na/S Batteries with a Na3PS4 Electrolyte Operating at Room Temperature. Chem. Mater. 2017, 29, 5232–5238. [Google Scholar] [CrossRef]
- Fan, X.; Yue, J.; Han, F.; Chen, J.; Deng, T.; Zhou, X.; Hou, S.; Wang, C. High-Performance All-Solid-State Na-S Battery Enabled by Casting-Annealing Technology. ACS Nano 2018, 12, 3360–3368. [Google Scholar] [CrossRef]
- Ge, Z.; Li, J.; Liu, J. High Sodium Ion Mobility of PEO-NaTFSI-Na3Zr2Si2PO12 Composite Solid Electrolyte for All-Solid-State Na-S Battery. ChemistrySelect 2022, 7, e20220062. [Google Scholar] [CrossRef]
- Yue, J.; Han, F.; Fan, X.; Zhu, X.; Ma, Z.; Yang, J.; Wang, C. High-Performance All-Inorganic Solid-State Sodium-Sulfur Battery. ACS Nano 2017, 11, 4885–4891. [Google Scholar] [CrossRef] [PubMed]
- Jhang, L.-J.; Wang, D.; Silver, A.; Li, X.; Reed, D.; Wang, D. Stable All-Solid-State Sodium-Sulfur Batteries for Low-Temperature Operation Enabled by Sodium Alloy Anode and Confined Sulfur Cathode. Nano Energy 2023, 105, 107995. [Google Scholar] [CrossRef]


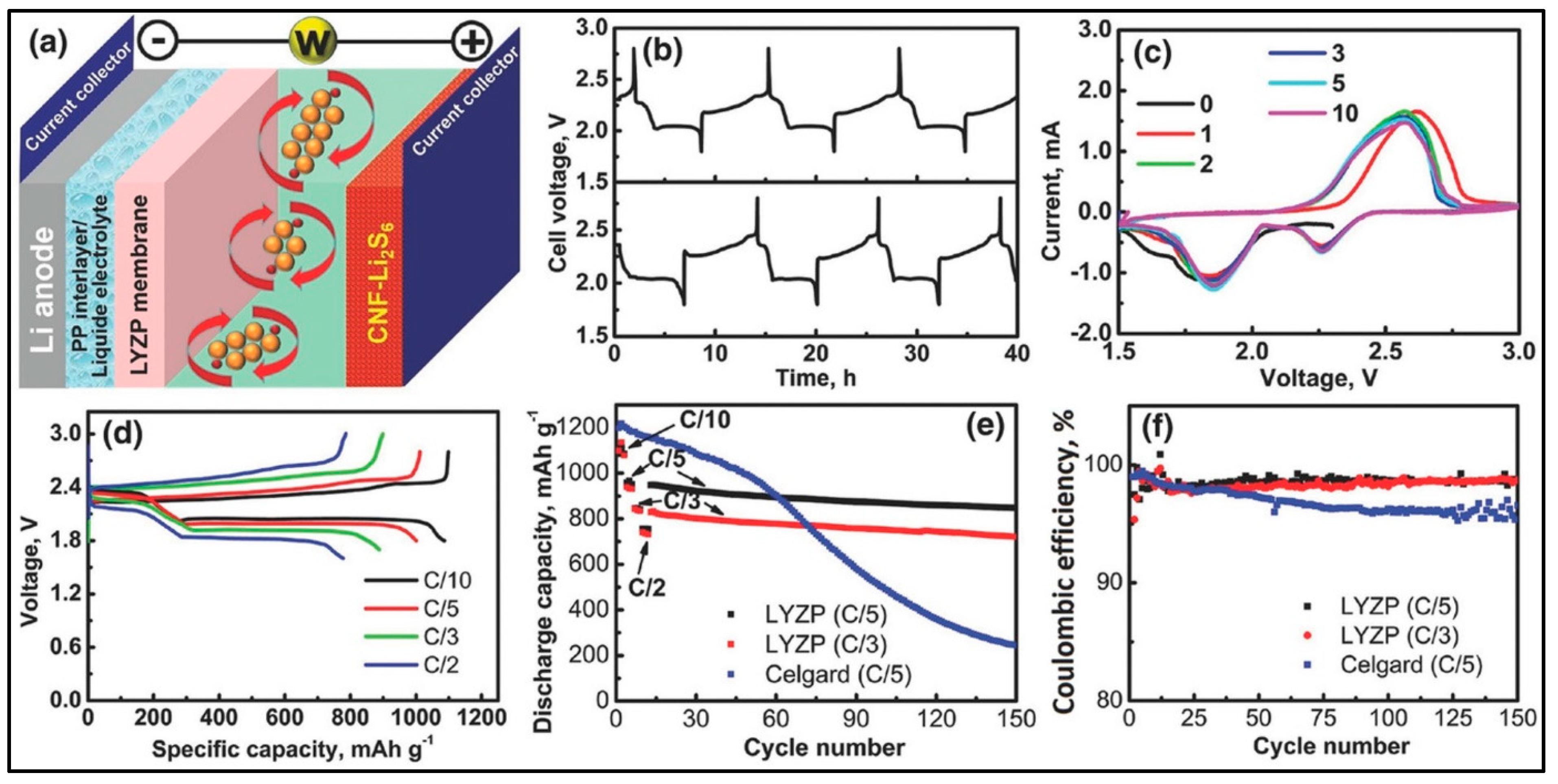
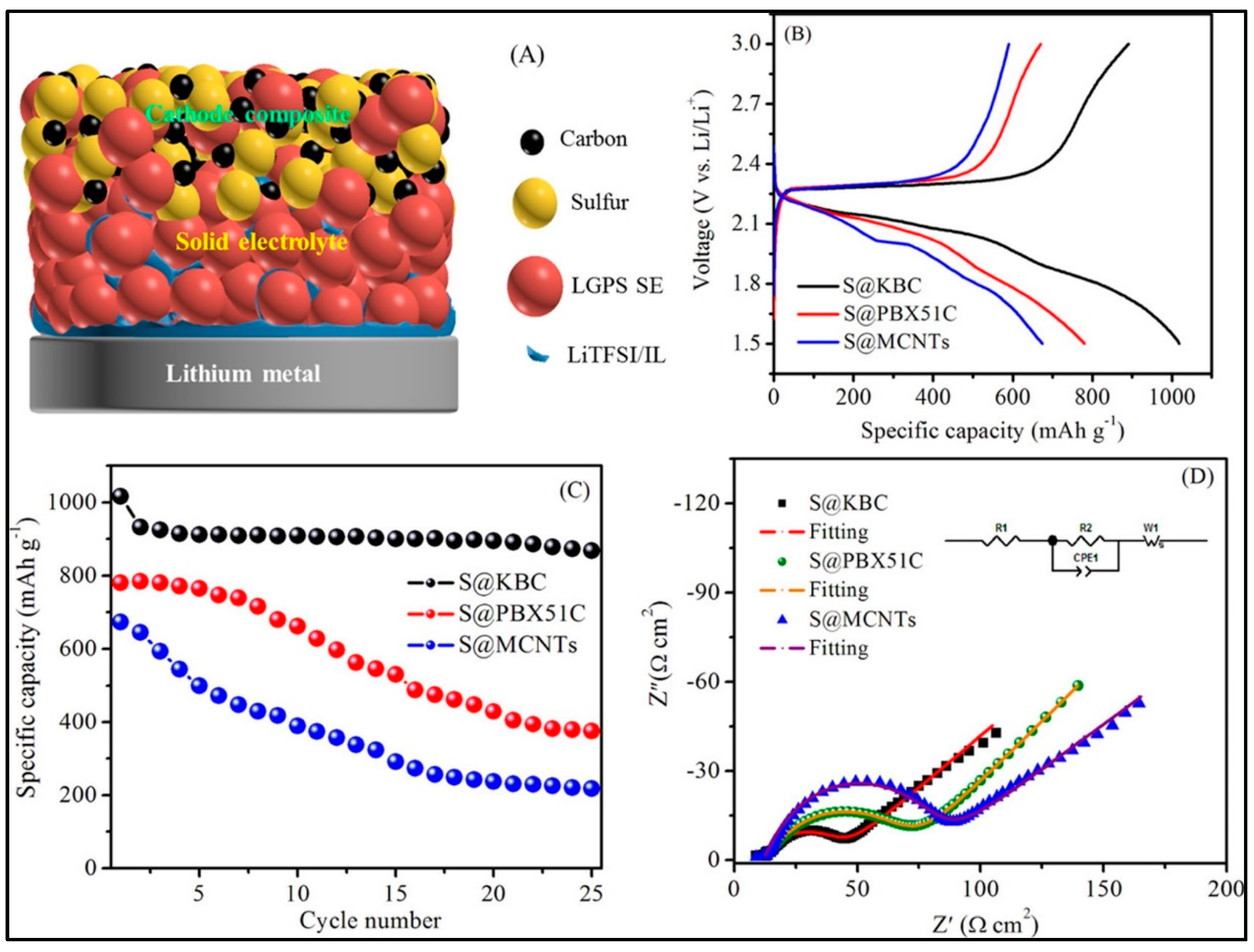

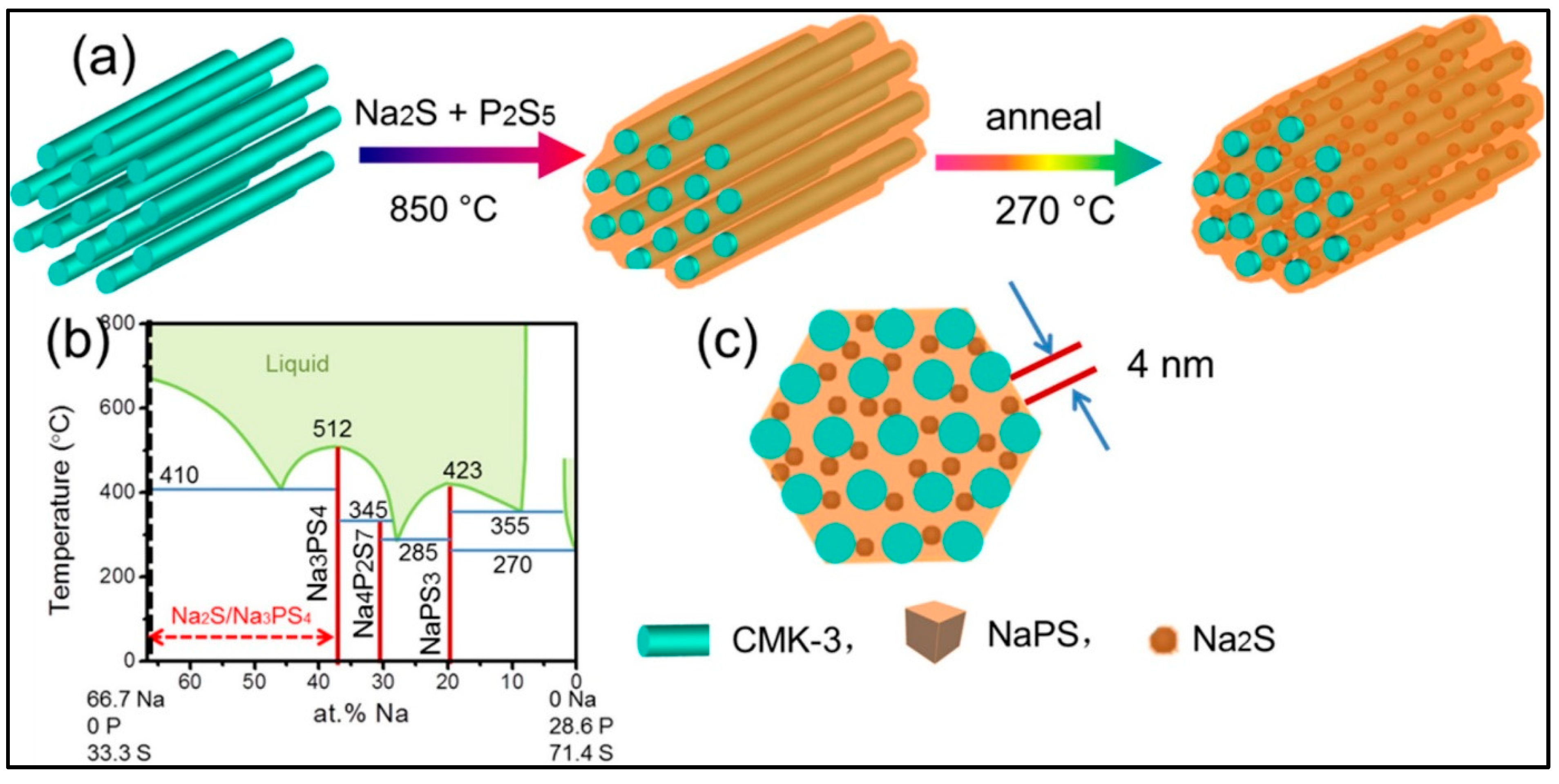
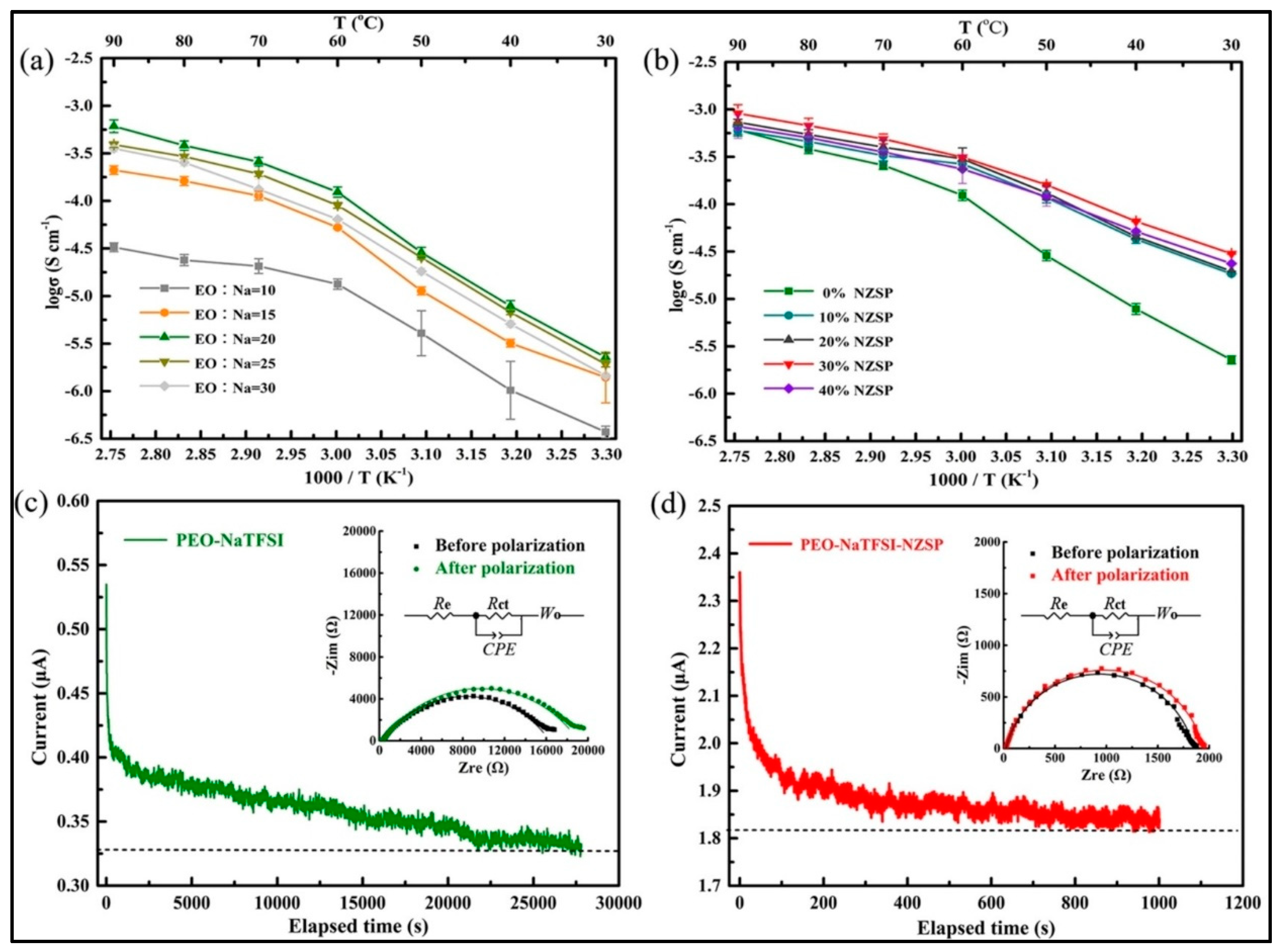

| Electrolyte | Composition | Ionic Conductivity (Scm−1) | Mechanical Properties | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Inorganic solid electrolyte | Oxide-Based (LISICON, NASICON, Garnet etc.) Sulfide-Based (Argyrodite, Thio-LISICON, Halide-Based etc.) | 10−5–10−4 | Strong | Wide electrochemical window, High ionic conductivity, excellent thermal stability, | Poor interface |
| Gel-Polymer electrolyte | Liquid electrolyte confined inside polymer matrix | ~10−3 | soft | High ionic conductivity, good interfacial property. Reduction of polysulfide dissolution to some extent | Less mechanical stability, less thermal stability |
| Solid-Polymer Electrolyte | Polymer + salt + additive | <10−5 | Good | Poor interfacial property, thermally stable, Reduces polysulfide dissolution | Less ionic conductivity; Less mechanical stability |
| Composite electrolyte | Polymer/organic fiber + inorganic | 10−5–10−4 | Strong | Poor interface, thermally stable, reduction of polysulfide dissolution, reduction in dendrite growth | Low ionic conductivity poor thermal stability |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhardwaj, R.K.; Zitoun, D. Recent Progress in Solid Electrolytes for All-Solid-State Metal(Li/Na)–Sulfur Batteries. Batteries 2023, 9, 110. https://doi.org/10.3390/batteries9020110
Bhardwaj RK, Zitoun D. Recent Progress in Solid Electrolytes for All-Solid-State Metal(Li/Na)–Sulfur Batteries. Batteries. 2023; 9(2):110. https://doi.org/10.3390/batteries9020110
Chicago/Turabian StyleBhardwaj, Ravindra Kumar, and David Zitoun. 2023. "Recent Progress in Solid Electrolytes for All-Solid-State Metal(Li/Na)–Sulfur Batteries" Batteries 9, no. 2: 110. https://doi.org/10.3390/batteries9020110
APA StyleBhardwaj, R. K., & Zitoun, D. (2023). Recent Progress in Solid Electrolytes for All-Solid-State Metal(Li/Na)–Sulfur Batteries. Batteries, 9(2), 110. https://doi.org/10.3390/batteries9020110