Optimization Preparation and Evaluation of Chitosan Grafted Norfloxacin as a Hemostatic Sponge
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
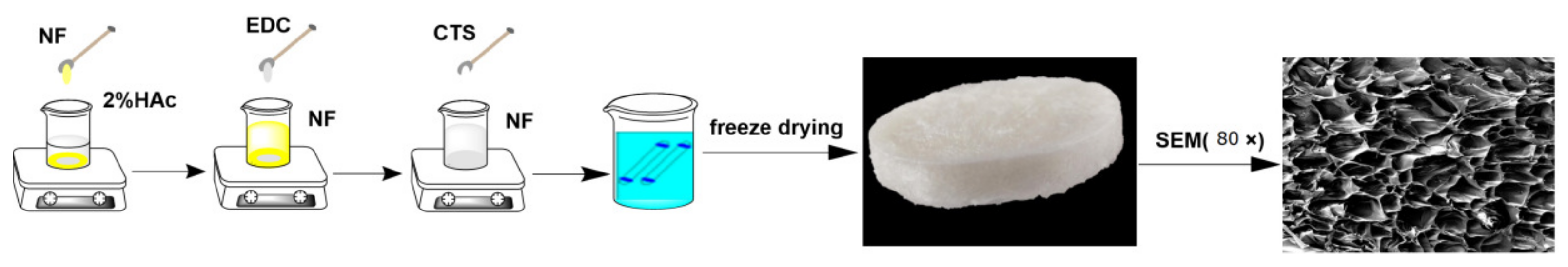
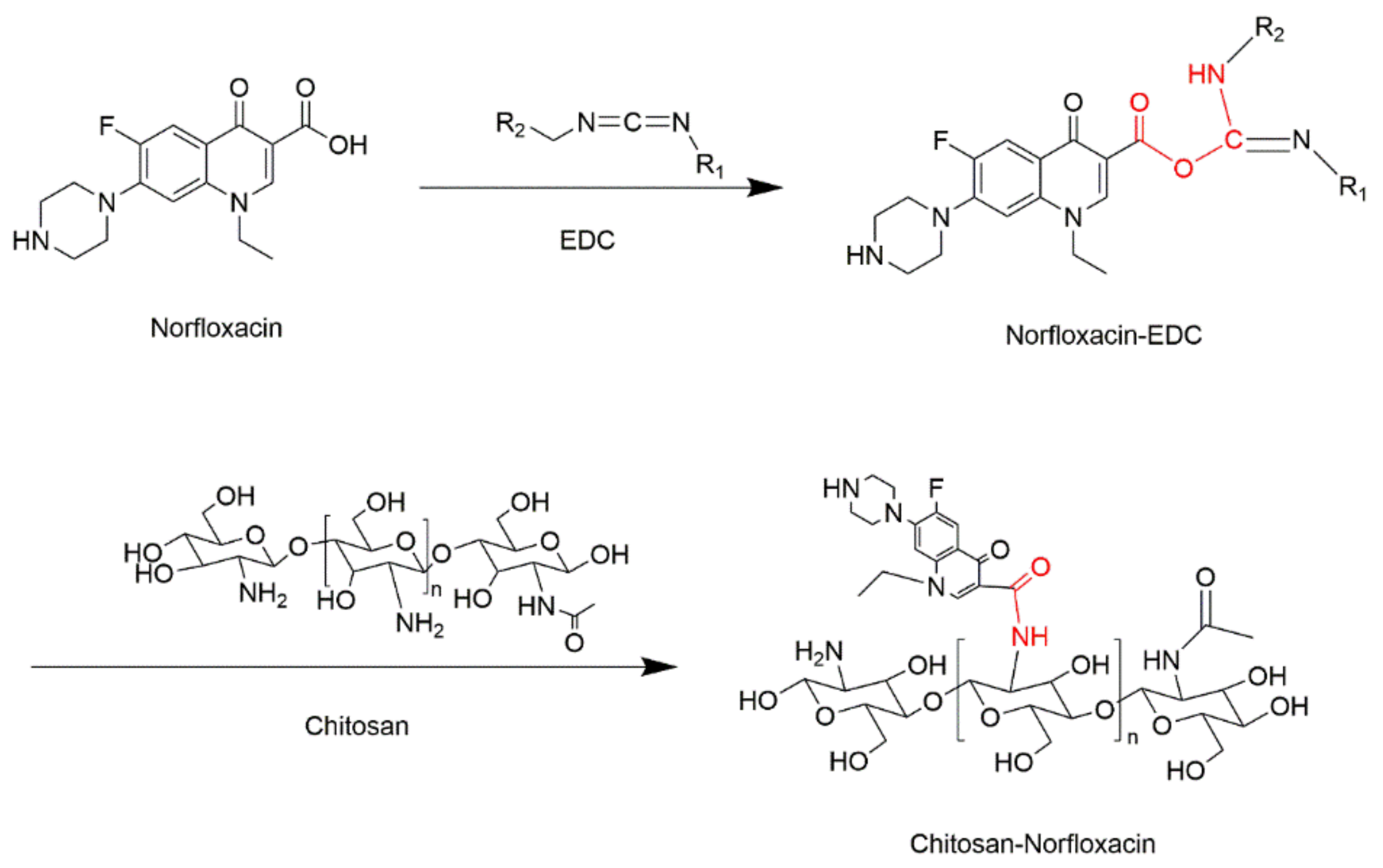
2.2. Preparation of CTS-NF
2.3. Morphology Observation of CTS-NF
2.4. X-ray Diffraction (XRD) Characterization
2.5. Measurement of the Grafting Ratio of CTS-NF
2.6. Single-Factor Tests
2.7. Response Surface Methodology Optimization
2.8. In Vitro Antimicrobial Activity Evaluation by MIC Assay
2.9. Whole Blood Clotting and Red Blood Cell Aggregation Assays
2.10. Blood Plasma Coagulation Assay In Vitro
2.11. Animal Hemostasis Experiments
2.11.1. Hemorrhage Model of Ear Artery
2.11.2. Liver Hemorrhage Model
2.12. Statistical Analysis
3. Results and Discussion
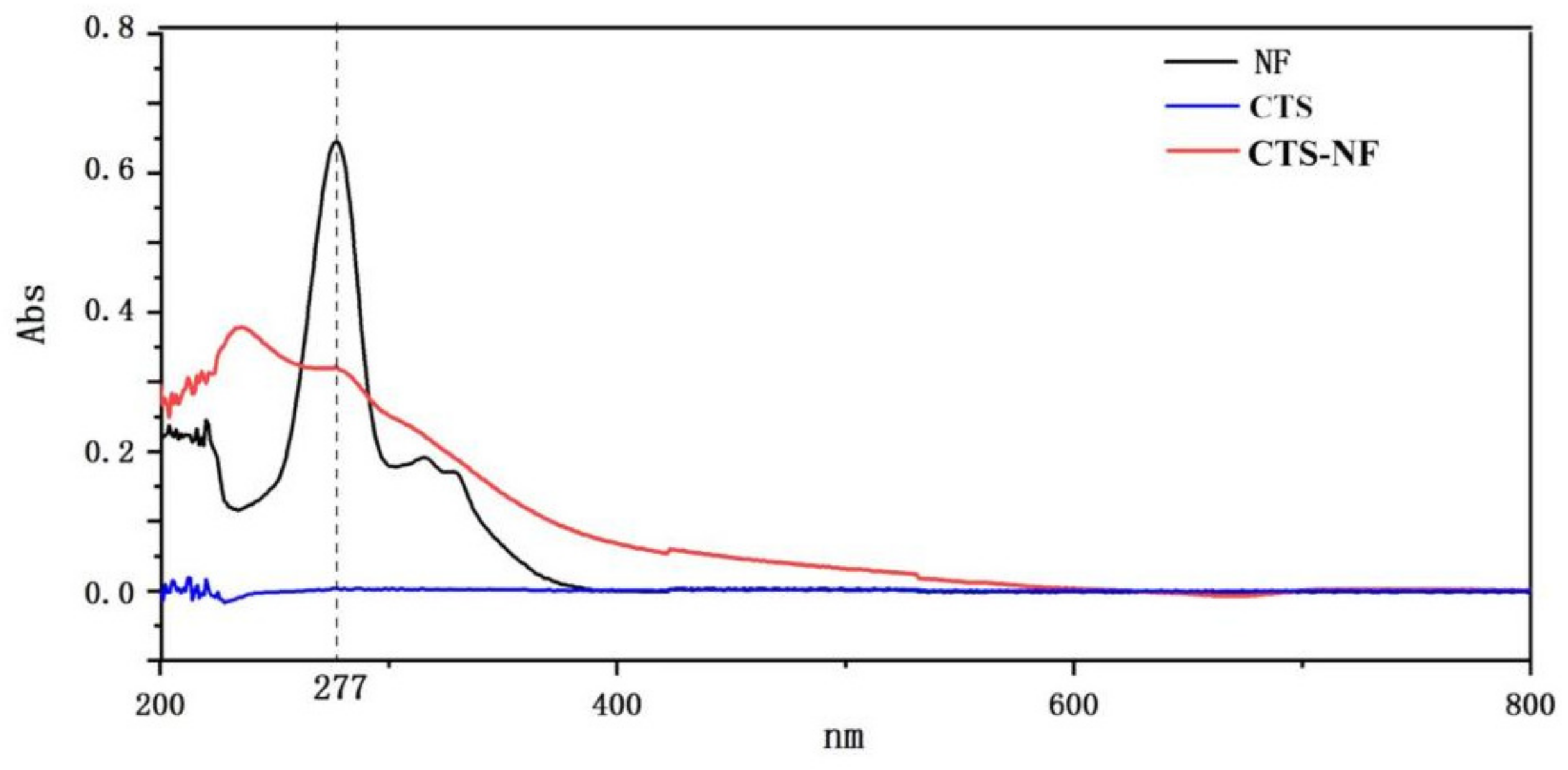
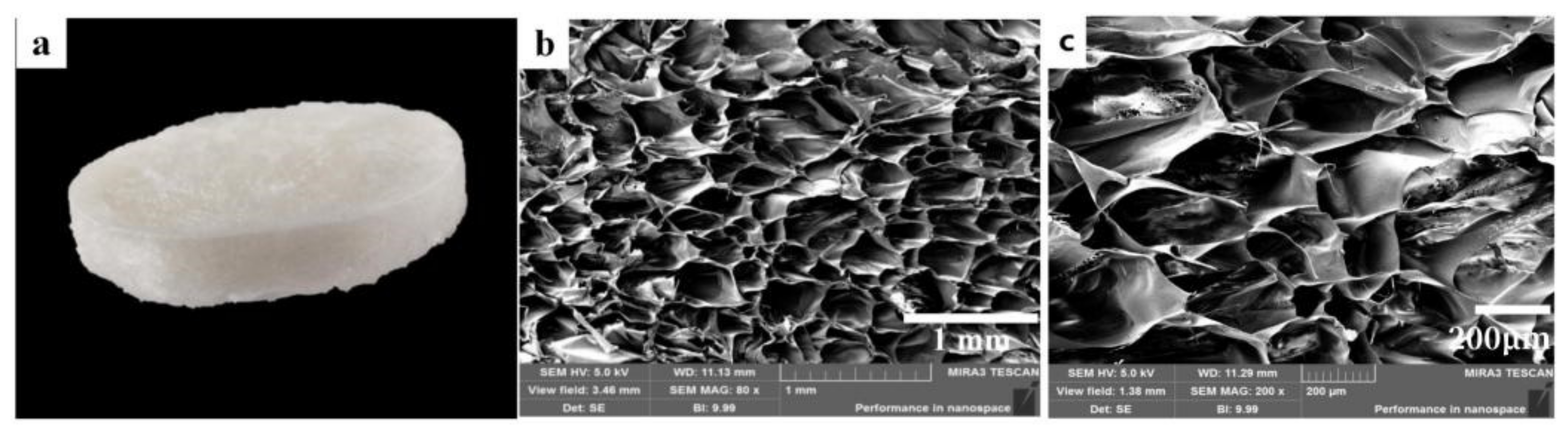
3.1. Characterization of CTS-NF
3.2. XRD Analysis
3.3. Single-Factor Analysis
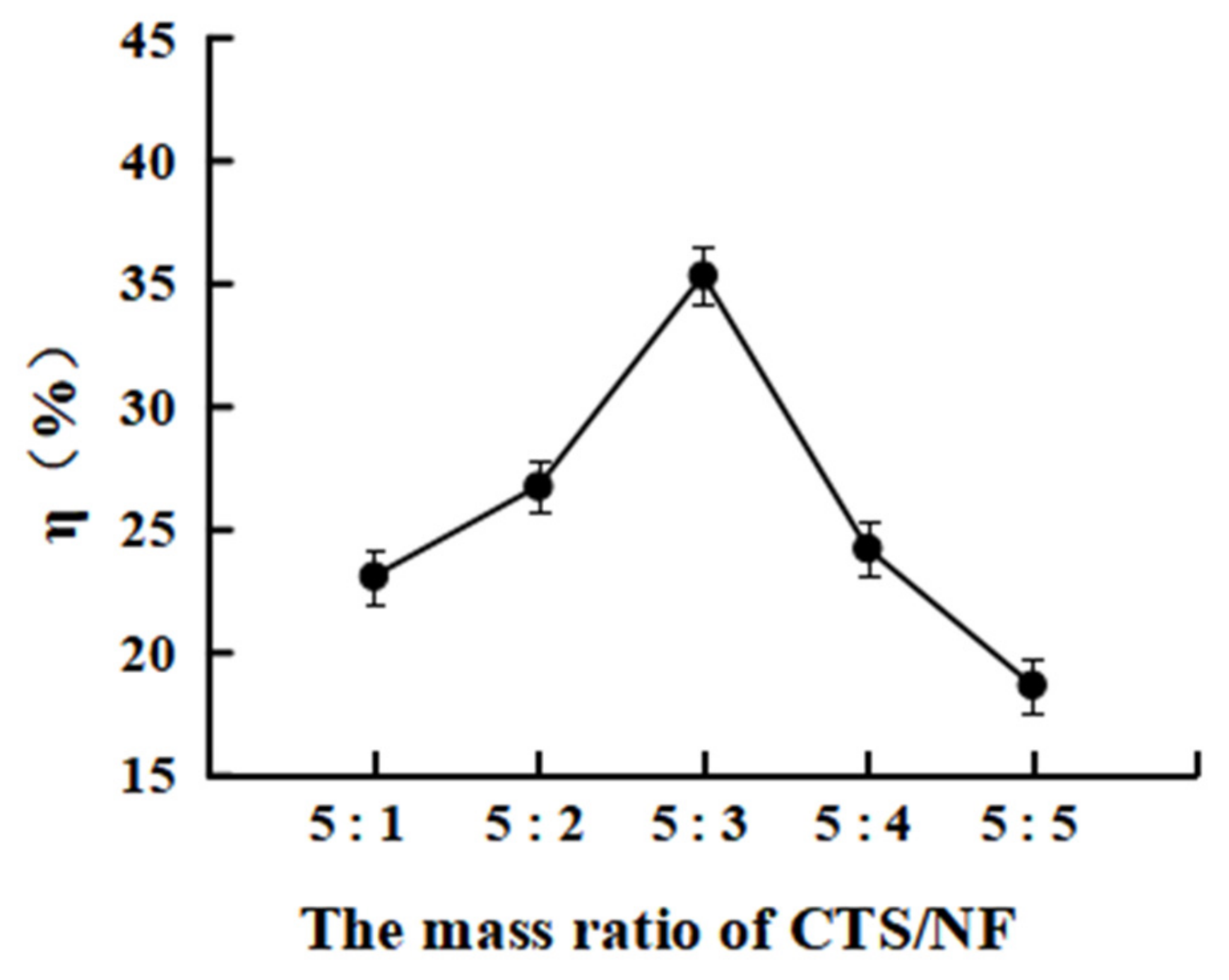
3.3.1. Effect of the Mass Ratio MCTS/MNF on the Grafting Rate η%
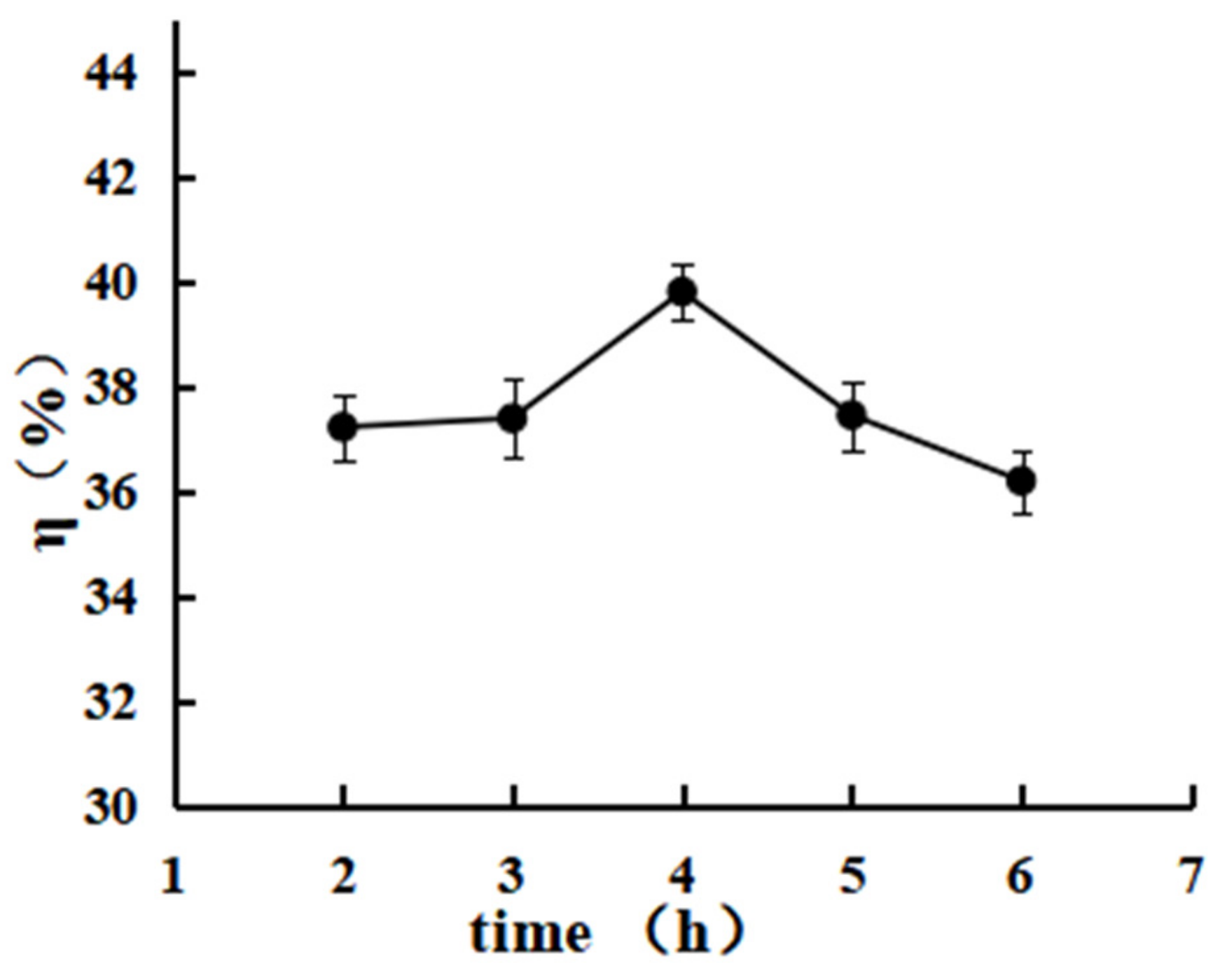
3.3.2. Effect of the Reaction Time on the Grafting Rate
3.3.3. Effect of the Reaction Temperature on the Grafting Rate
3.4. Response Surface Analysis
3.5. Antibacterial Activity
3.6. Whole Blood Clotting and Red Blood Cell Aggregation
3.7. Blood Plasma Coagulation
3.8. Animal Hemostasis Experiment
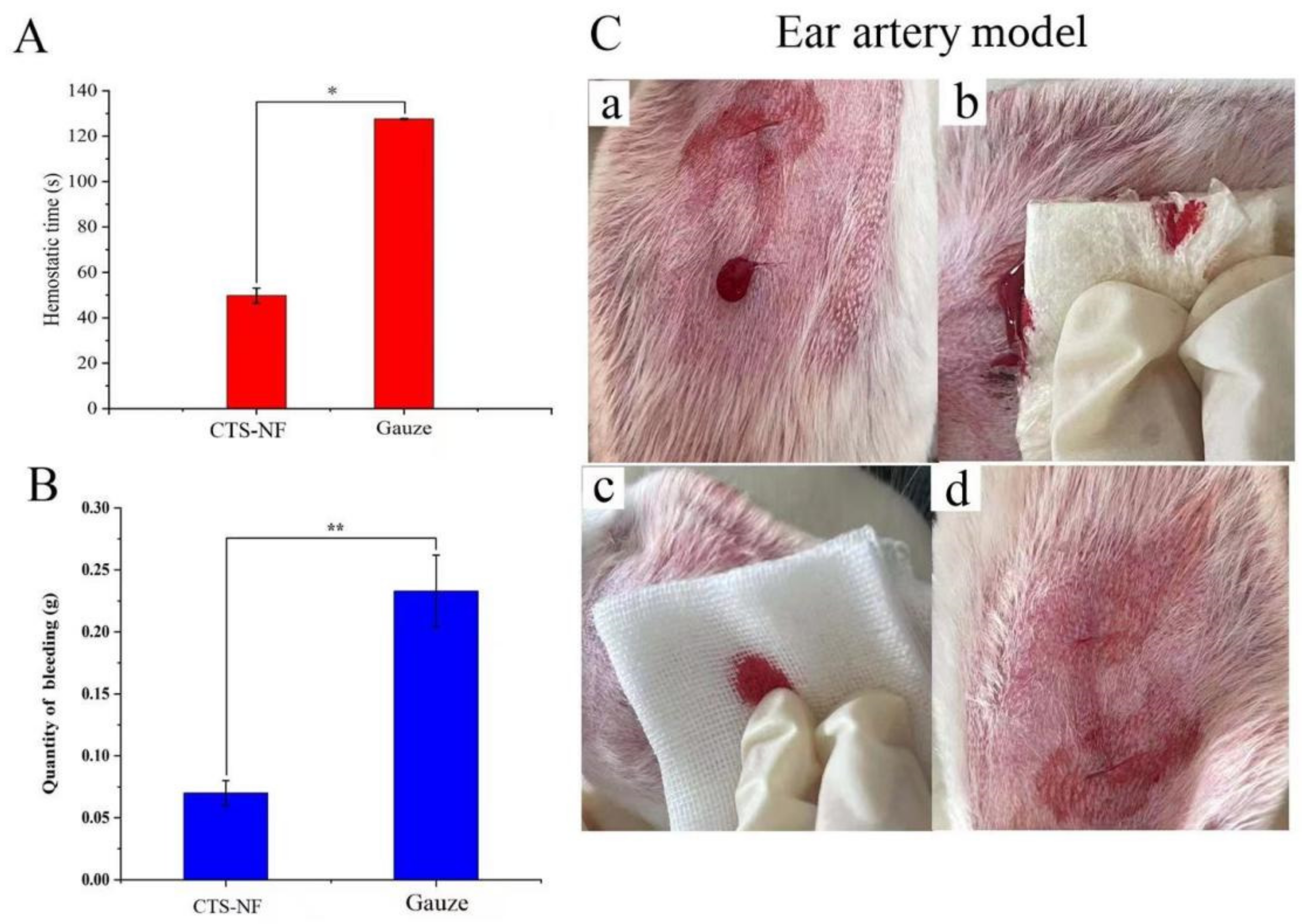
3.8.1. Ear Artery Bleeding
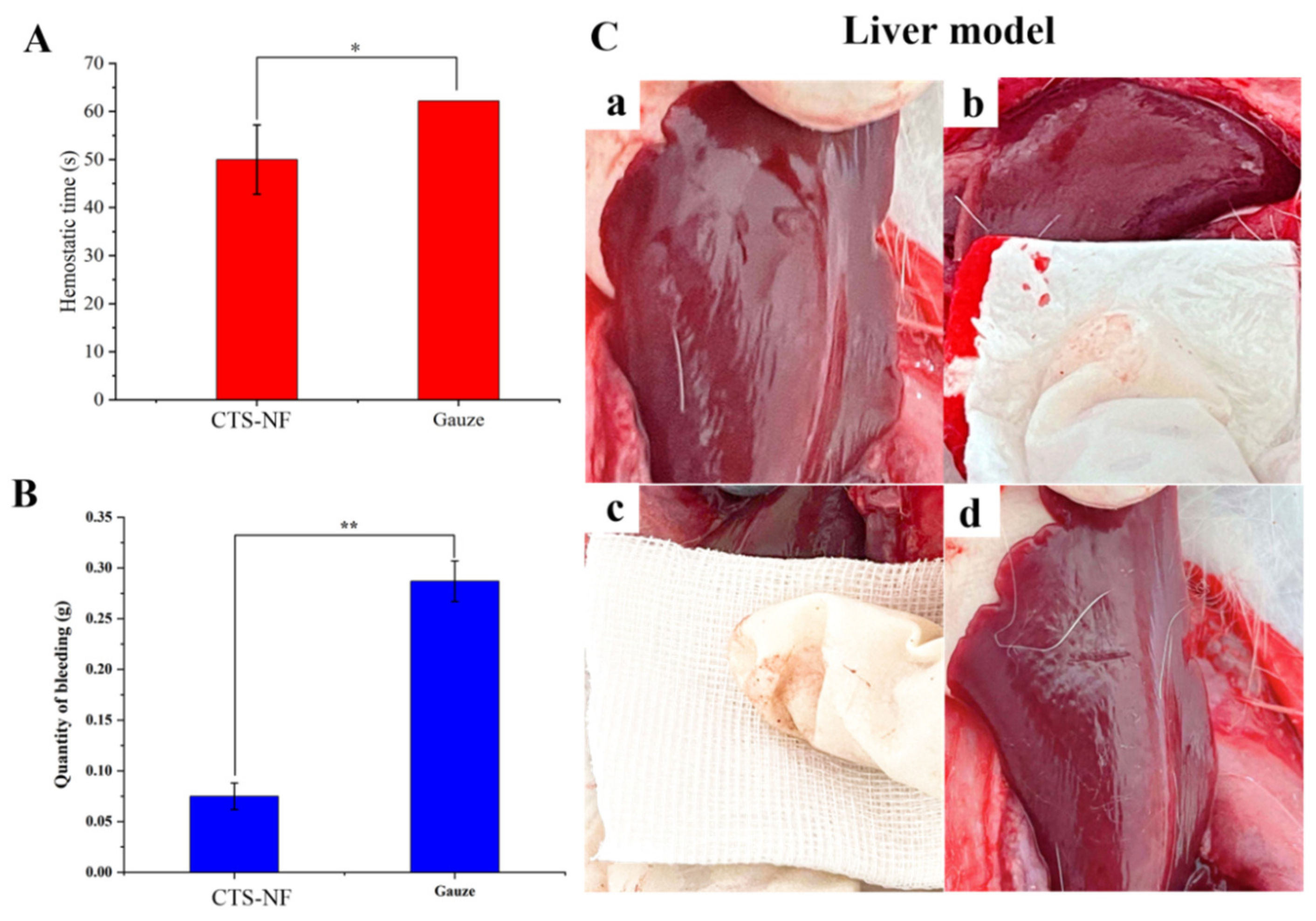
3.8.2. Liver Bleeding
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, X.; Qi, Y.; Ma, X.B.; Wang, Y.; Song, H.Z.; Zhao, Y.L.; Li, W.J.; Qin, J.L. Poly (aspartic acid) based self-healing hydrogel with blood coagulation characteristic for rapid hemostasis and wound healing applications. Colloids. Surf. B 2022, 214, 112430. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.Y.; Yang, N.; Zhao, Y.; Yang, D.P.; Hu, Y.L.; Yang, D.L.; Song, X.J.; Wang, W.J.; Dong, X.C. Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today 2021, 39, 101165. [Google Scholar] [CrossRef]
- Yu, R.; Zhang, H.; Guo, B. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nano-Micro Lett. 2022, 14, 1–46. [Google Scholar] [CrossRef]
- Zheng, Y.; Pan, N.; Liu, Y.; Ren, X. Novel porous chitosan/N-halamine structure with efficient antibacterial and hemostatic properties. Carbohydr. Polym. 2021, 253, 117205. [Google Scholar] [CrossRef]
- Zhang, Y.; Dang, Q.; Liu, C.; Yan, J.; Cha, D.; Liang, S.; Li, X.; Fan, B. Synthesis, characterization, and evaluation of poly(aminoethyl) modified chitosan and its hydrogel used as antibacterial wound dressing. Int. J. Biol. Macromol. 2017, 102, 457–467. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, J.W.; Hu, Z.; Zhong, S.Y.; Huang, N.; Zhao, Y.T.; Tao, Y.; Liang, Y.L. Preparation of norfloxacin-grafted chitosan antimicrobial sponge and its application in wound repair. Int. J. Biol. Macromol. 2022, 210, 243–251. [Google Scholar] [CrossRef]
- Alkabli, J. Progress in preparation of thiolated, crosslinked, and imino-chitosan derivatives targeting specific applications. Eur. Polym. J. 2022, 165, 110998. [Google Scholar] [CrossRef]
- Oryan, A.; Sahvieh, S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017, 104, 1003–1011. [Google Scholar] [CrossRef]
- Azmana, M.; Mahmood, S.; Hilles, A.R.; Rahman, A.; Arifin, M.A.B.; Ahmed, S. A review on chitosan and chitosan-based bionanocomposites: Promising material for combatting global issues and its applications. Int. J. Biolo. Macromol. 2021, 185, 832–848. [Google Scholar] [CrossRef]
- Cao, S.J.; Xu, G.; Li, Q.J.; Zhang, S.K.; Yang, Y.F.; Chen, J.D. Double crosslinking chitosan sponge with antibacterial and hemostatic properties for accelerating wound repair. Compos. Part B 2022, 234, 109746. [Google Scholar] [CrossRef]
- Atashgahi, M.; Ghaemi, B.; Valizadeh, A.; Moshirib, A.; Nekoofar, M.H.; Amanid, A. Epinephrine-entrapped chitosan nanoparticles covered by gelatin nanofibers: A bi-layer nano-biomaterial for rapid hemostasis. Int. J. Pharm. 2021, 608, 121074. [Google Scholar] [CrossRef]
- Lestari, W.; Yusry, W.N.A.W.; Haris, M.S.; Jaswir, I.; Idrus, E. A glimpse on the function of chitosan as a dental hemostatic agent. Jpn. Dent. Sci. Rev. 2020, 56, 147–154. [Google Scholar] [CrossRef]
- Lowrence, R.C.; Ramakrishnan, A.; Sundaramoorthy, N.S. Norfloxacin salts of carboxylic acids curtail planktonic and biofilm mode of growth in ESKAPE pathogens. J. Appl. Microbiol. 2018, 124, 408–422. [Google Scholar] [CrossRef]
- Ismail, N.A.; Amin, K.A.M.; Razali, M.H. Antibacterial Study of Gellan Gum (GG) Film Incorporated Norfloxacin. J. Pure. Appl. Microbiol. 2019, 13, 1095–1102. [Google Scholar] [CrossRef]
- Ismail, N.A.; Amin, K.; Razali, M.H. Mechanical and Antibacterial Activities Study of Gellan Gum/Virgin Coconut Oil Film Embedded Norfloxacin. IOP Conf. Ser.: Mater. Sci. Eng. 2018, 440, 25–26. [Google Scholar] [CrossRef]
- Dua, K.; Malipeddi, V.R.; Madan, J.; Gupta, G.; Chakravarthi, S.; Awasthi, R.; Kikuchi, I.S.; De, T. Jesus Andreoli Pinto, Norfloxacin and metronidazole topical formulations for effective treatment of bacterial infections and burn wounds. Interv. Med. Appl. Sci. 2018, 440, 012001. [Google Scholar]
- Khan, M.A.; Khan, S.; Kazi, M.; Alshehri, S.M.; Shahid, M.; Khan, S.U.; Hussain, Z.; Sohail, M.; Shafique, M.; Hamid, H.A.; et al. Norfloxacin loaded lipid polymer hybrid nanoparticles for oral administration: Fabrication, characterization, in silico modelling and toxicity evaluation. Pharmaceutics 2021, 13, 1632. [Google Scholar] [CrossRef]
- Ghafar, A.; Rozaik, S.; Akef, A. Rifaximin plus norfloxacin versus norfloxacin alone in primary prophylaxis of spontaneous bacterial peritonitis in patients with variceal bleeding. Egypt. J. Int. Med. 2019, 31, 281–287. [Google Scholar] [CrossRef]
- Goller, S.; Turner, N.J. The antimicrobial effectiveness and cytotoxicity of the antibiotic-loaded chitosan: ECM scaffolds. Appl. Sci. 2020, 10, 3446. [Google Scholar] [CrossRef]
- Buyana, B.; Aderibigbe, B.A.; Ndinteh, D.T.; Fonkui, Y.T.; Kumar, P. Alginate-pluronic topical gels loaded with thymol, norfloxacin and ZnO nanoparticles as potential wound dressings. J. Drug Delivery Sci. Technol. 2020, 60, 101960. [Google Scholar] [CrossRef]
- Koumentakou, I.; Terzopoulou, Z.; Michopoulou, A.; Kalafatakis, I.; Theodorakis, K.; Tzetzis, D.; Bikiaris, D. Chitosan dressings containing inorganic additives and levofloxacin as potential wound care products with enhanced hemostatic properties. Int. J. Biol. Macromol. 2020, 162, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.L.; Wang, Y.F.; Zhang, Y.; Ren, X.H.; Qiu, Y.Y.; Huang, T.S. Novel quaternarized N-halamine chitosan and polyvinyl alcohol nanofibrous membranes as hemostatic materials with excellent antibacterial properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Li, Q.L.; Lu, H.B.; Liu, Z.; Wu, Y.X.; Mao, J.; Gong, S.Q. Effects of the Combined Application of Trimethylated Chitosan and Carbodiimide on the Biostability and Antibacterial Activity of Dentin Collagen Matrix. Polymers 2022, 14, 3166. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of fish collagen as a scaffold for regenerative medicine. Biomed. Res. Int. 2014, 4976, 302932. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2017, 103, 234–241. [Google Scholar] [CrossRef]
- Catanzano, O.; D’Esposito, V.; Formisano, P.; Boatenget, J.S.; Quaglia, F. Composite Alginate-Hyaluronan Sponges for the Delivery of Tranexamic Acid in Post-Extractive Alveolar Wounds. J. Pharmacol. Sci. 2018, 107, 654–661. [Google Scholar] [CrossRef]
- Yan, S.Z.; Xie, F.Y.; Zhang, S.; Jiang, L.Z.; Qi, B.K.; Li, Y. Effects of soybean protein isolate− polyphenol conjugate formation on the protein structure and emulsifying properties: Protein−polyphenol emulsification performance in the presence of chitosan. Colloids Surf. A 2021, 609, 125641. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef]
- Ning, Z.; Liu, Z.; Song, Z.; Wang, C.; Liu, Y.; Gan, J.; Ma, X.; Lu, A. Application of a strategy based on metabolomics guided promoting blood circulation bioactivity compounds screening of vinegar. Chem. Cent. J. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.W.; Liu, C.C.; Cherng, J.H.; Lin, C.S.; Chang, S.J.; Hong, Z.J.; Liu, C.C.; Chiu, Y.K.; Hsu, S.D.; Chang, H. Biological effects of chitosan-based dressing on hemostasis mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Mishra, B.; Hossain, S.; Mohanty, S.; Gupta, M.K.; Verma, D. Fast acting hemostatic agent based on self-assembled hybrid nanofibers from chitosan and casein. Int. J. Biol. Macromol. 2021, 185, 525–534. [Google Scholar] [CrossRef]
- Chen, J.W.; Ai, J.; Chen, S.N.; Xu, Z.Y.; Lin, J.H.; Liu, H.Q.; Chen, Q.H. Synergistic enhancement of hemostatic performance of mesoporous silica by hydrocaffeic acid and chitosan. Int. J. Biol. Macromol. 2019, 139, 1203–1211. [Google Scholar] [CrossRef]
- Fischer, T.H.; Thatte, H.S.; Nichols, T.C.; Bender-Neal, D.E.; Bellinger, D.A.; Vournakis, J.N. Synergistic platelet integrin signaling and factor XII activation in poly-N-acetyl glucosamine fiber-mediated hemostasis. Biomaterials 2005, 26, 5433–5443. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Nor, J.M.S.; Khairul, A.M.A. Composite Film of Chitosan Loaded Norfloxacin with Improved Flexibility and Antibacterial Activity for Wound Dressing Application. Orient. J. Chem. 2017, 33, 628–636. [Google Scholar]




| Factor | Code | Standard | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| MCTS/MNF | A | 0.4 | 0.6 | 0.8 |
| Reaction time (h) | B | 3 | 4 | 5 |
| Temperature (°C) | C | 60 | 65 | 70 |
| Run | A | B | C | η(%) |
|---|---|---|---|---|
| MCTS/MNF | Reaction Time (h) | Temperature (°C) | ||
| 1 | 1 | −1 | 0 | 28.6 |
| 2 | 1 | 1 | 0 | 26.2 |
| 3 | −1 | 0 | −1 | 27.3 |
| 4 | 0 | 0 | 0 | 45.4 |
| 5 | 0 | 0 | 0 | 45.5 |
| 6 | 0 | 0 | 0 | 45.5 |
| 7 | 1 | 0 | −1 | 24.3 |
| 8 | 0 | 0 | 0 | 44.9 |
| 9 | 0 | −1 | −1 | 37.0 |
| 10 | 0 | −1 | 1 | 35.5 |
| 11 | 0 | 1 | −1 | 37.0 |
| 12 | −1 | −1 | 0 | 30.9 |
| 13 | −1 | 1 | 0 | 29.9 |
| 14 | 0 | 0 | 0 | 44.5 |
| 15 | 1 | 0 | 1 | 27.8 |
| 16 | −1 | 0 | 1 | 28.8 |
| 17 | 0 | 1 | 1 | 35.1 |
| Source of Variance | Sum of Square | Degrees of Freedom | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Model | 972.61 | 9 | 108.07 | 57.16 | <0.0001 |
| A | 12.58 | 1 | 12.58 | 6.66 | 0.0365 |
| B | 1.60 | 1 | 1.60 | 0.85 | 0.3883 |
| C | 0.33 | 1 | 0.33 | 0.18 | 0.6865 |
| AB | 0.50 | 1 | 0.50 | 0.26 | 0.6231 |
| AC | 1.02 | 1 | 1.02 | 0.54 | 0.4865 |
| BC | 0.023 | 1 | 0.023 | 0.012 | 0.9153 |
| A2 | 693.50 | 1 | 693.50 | 366.83 | <0.0001 |
| B2 | 59.78 | 1 | 59.78 | 31.62 | 0.0008 |
| C2 | 131.63 | 1 | 131.63 | 69.63 | <0.0001 |
| Residual | 13.23 | 7 | 1.89 | ||
| Lack of fit | 10.32 | 3 | 3.44 | 4.73 | 0.0838 |
| Pure error | 2.91 | 4 | 0.73 | ||
| Total deviation | 985.84 | 16 | |||
| Coefficient of determination R2 = 0.9693 | |||||
| Microorganism | MIC (mg/mL) | ||
|---|---|---|---|
| CTS-NF | CTS | NF | |
| E. coli ATCC 25922 | 0.3125 | 2.5000 | 0.0008 |
| S. aureus ATCC 29213 | 0.1562 | 1.2500 | 0.0003 |
| Sample | PT (s) | APTT (s) | TT (s) | FIB (g/L) |
|---|---|---|---|---|
| Control | 21.32 ± 4.20 | 45.07 ± 10.71 | 24.67 ± 5.77 | 7.18 ± 1.45 |
| CTS-NF | 14.33 ± 2.37 * | 33.08 ± 6.68 * | 13.17 ± 3.91 * | 3.07 ± 0.53 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Yang, Q.; Wang, J.; Hu, Z.; Li, C.; Zhong, S.; Huang, N. Optimization Preparation and Evaluation of Chitosan Grafted Norfloxacin as a Hemostatic Sponge. Polymers 2023, 15, 672. https://doi.org/10.3390/polym15030672
Cheng Y, Yang Q, Wang J, Hu Z, Li C, Zhong S, Huang N. Optimization Preparation and Evaluation of Chitosan Grafted Norfloxacin as a Hemostatic Sponge. Polymers. 2023; 15(3):672. https://doi.org/10.3390/polym15030672
Chicago/Turabian StyleCheng, Yu, Qian Yang, Jiyuan Wang, Zhang Hu, Chengpeng Li, Saiyi Zhong, and Na Huang. 2023. "Optimization Preparation and Evaluation of Chitosan Grafted Norfloxacin as a Hemostatic Sponge" Polymers 15, no. 3: 672. https://doi.org/10.3390/polym15030672
APA StyleCheng, Y., Yang, Q., Wang, J., Hu, Z., Li, C., Zhong, S., & Huang, N. (2023). Optimization Preparation and Evaluation of Chitosan Grafted Norfloxacin as a Hemostatic Sponge. Polymers, 15(3), 672. https://doi.org/10.3390/polym15030672







