Synthesis of DOPO-Based Phosphorus-Nitrogen Containing Hyperbranched Flame Retardant and Its Effective Application for Poly(ethylene terephthalate) via Synergistic Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of hbDT
2.3. Preparation of hbDT/PET Composites
2.4. Characterization
3. Results and Discussion
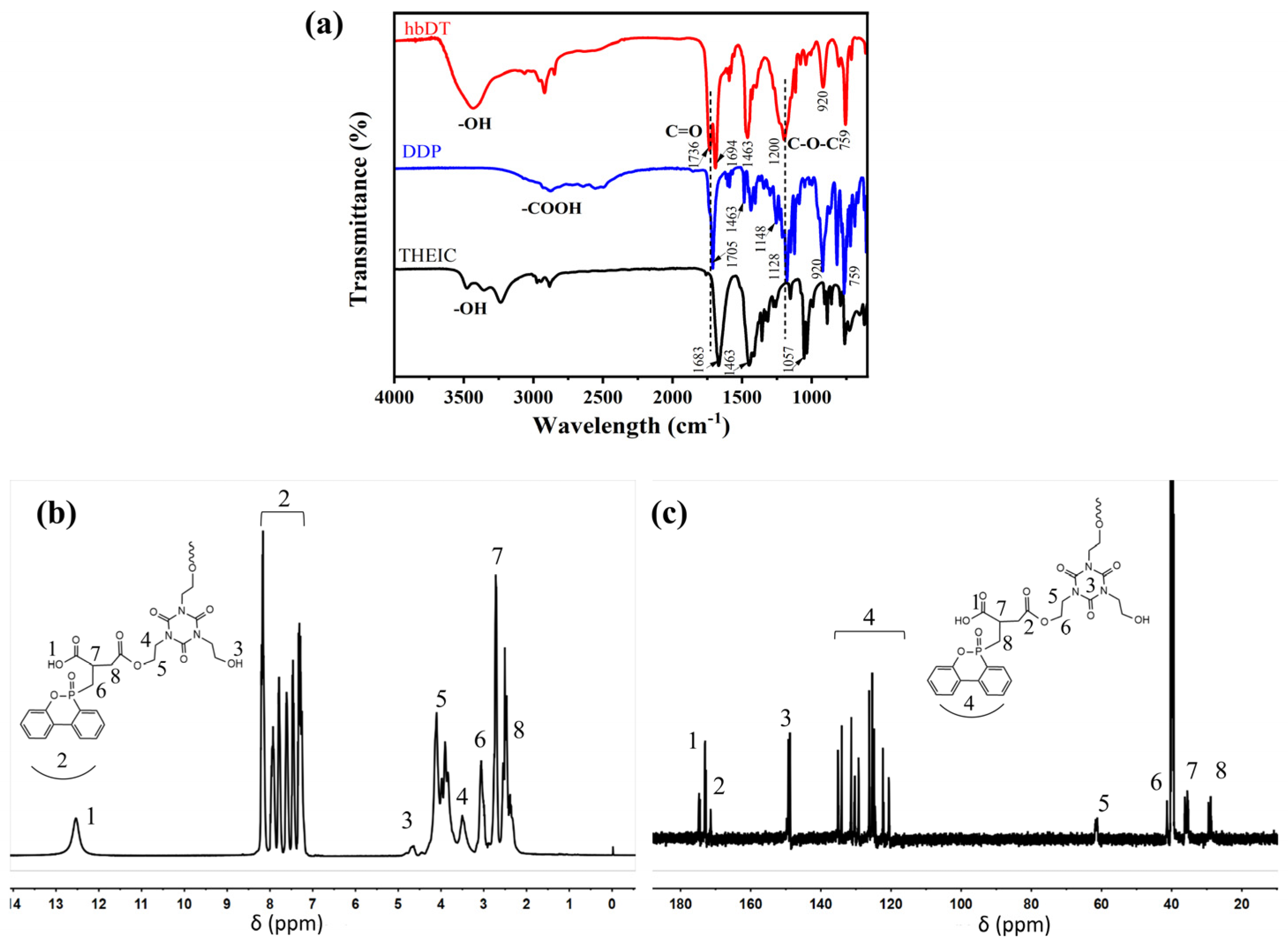
3.1. Structure Characterization of hbDT
3.2. Thermal Performance of hbDT
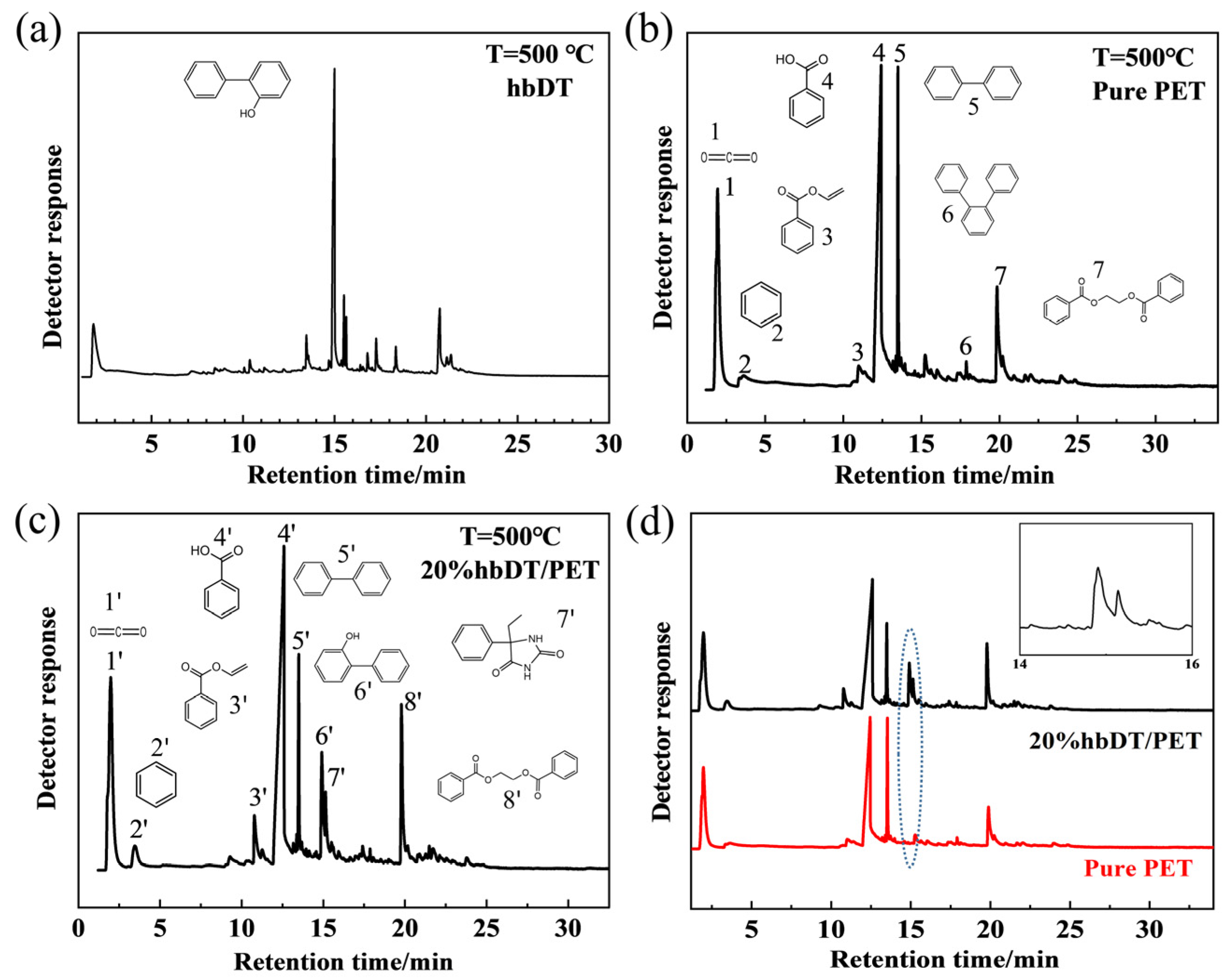
3.3. Thermal Degradation Behavior of hbDT/PET Composites
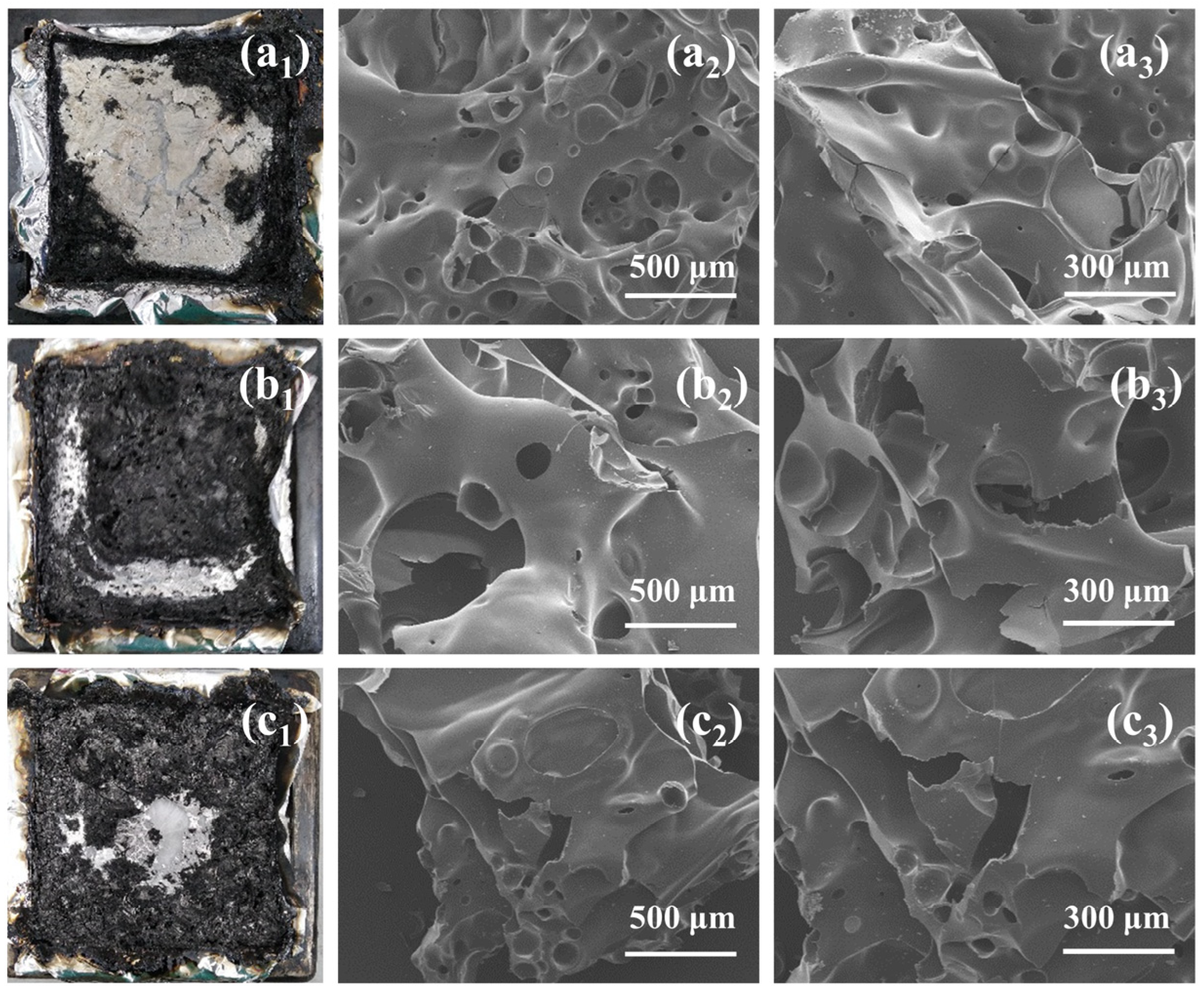
3.4. Flame Retardance and Combustion Behavior of hbDT/PET Composites
3.5. Combustion Properties of hbDT/PET
3.6. Comparison of Flame Retardancy with Other PET Composites
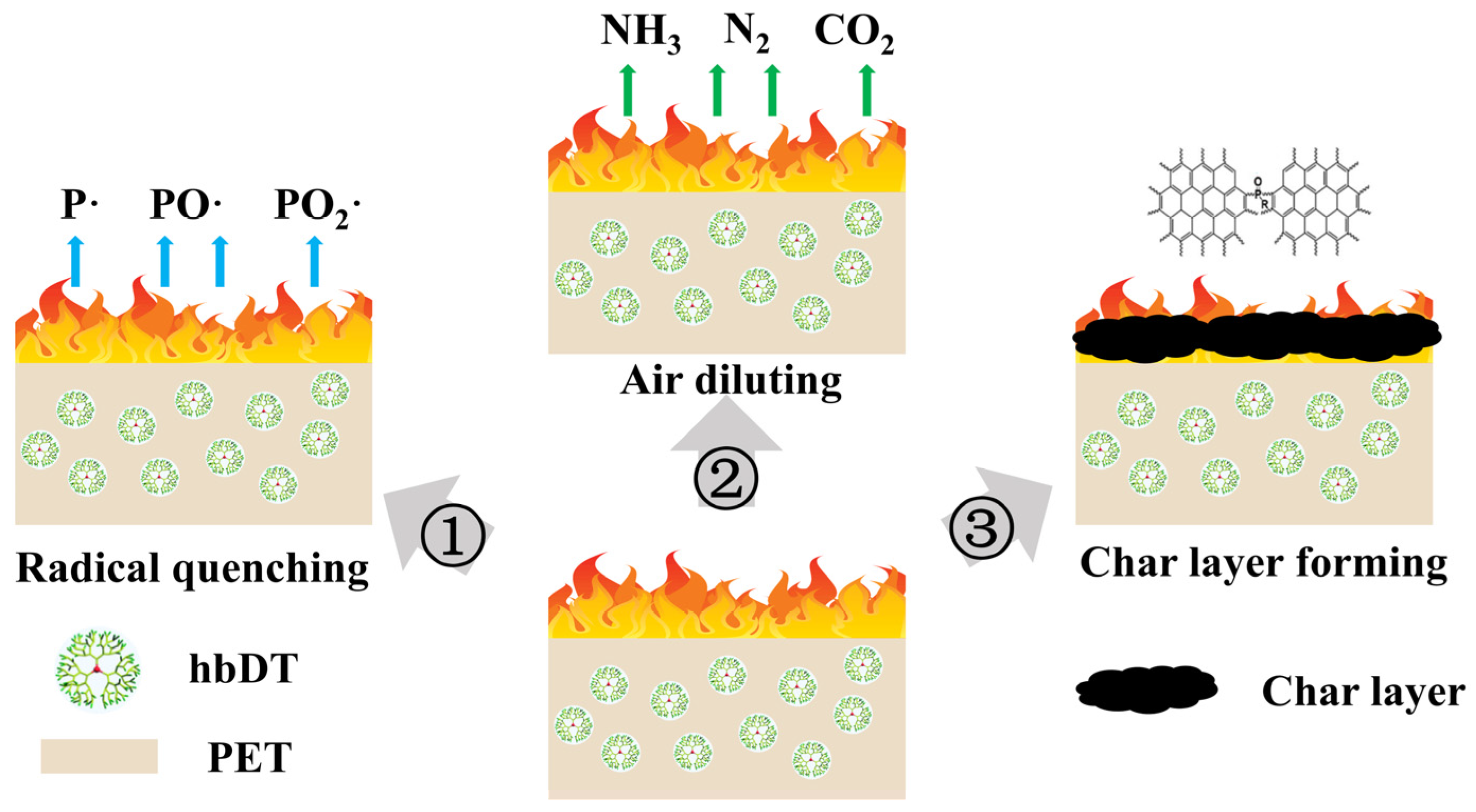
3.7. Flame Retardant Mechanism of hbDT/PET
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Biron, M. 3-Thermoplastics: Economic Overview. Mater. Sel. Thermoplast. Parts 2016, 3, 77–111. [Google Scholar] [CrossRef]
- Visakh, P. Polyethylene Terephthalate: Blends, Composites, and Nanocomposites—State of Art, New Challenges, and Opportunities. Poly(Ethyl. Terephthalate) Based Blends Compos. Nanocomposites 2015, 1, 1–14. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Flame retardancy of thermoplastic polyesters? A review of the recent literature. Polym. Int. 2004, 54, 11–35. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Wang, Y. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2021, 34, 2107905. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Niu, M.; Dai, J.; Bai, J.; Xue, B.; Song, Y.; Peng, Y. Flame-Retarded polyethylene terephthalate with carbon microspheres/magnesium hydroxide compound flame retardant. Fire Mater. 2018, 42, 794–804. [Google Scholar] [CrossRef]
- Salaün, F.; Lemort, G.; Butstraen, C.; Devaux, E.; Capon, G. Influence of silica nanoparticles combined with zinc phosphinate on flame retardant properties of PET. Polym. Adv. Technol. 2017, 28, 1919–1928. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, N.H.; Park, J.; Lim, J.C.; Park, Y.W. Miscibility of flame retardant epoxy resin with poly(ethylene terephthalate) and the characterizations of the blends. Fibers Polym. 2009, 10, 594–600. [Google Scholar] [CrossRef]
- Czégény, Z.; Jakab, E.; Blazsó, M.; Bhaskar, T.; Sakata, Y. Thermal decomposition of polymer mixtures of PVC, PET and ABS containing brominated flame retardant: Formation of chlorinated and brominated organic compounds. J. Anal. Appl. Pyrolysis 2012, 96, 69–77. [Google Scholar] [CrossRef]
- Adner, D.; Helmy, M.; Otto, T.; Schellenberg, J.; Schadewald, A. A macromolecular halogen-free flame retardant and its effect on the properties of thermoplastic polyesters. Fire Mater. 2018, 43, 169–174. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, W.; Yu, X.; Zhang, J.; Ren, Y.; Liu, X.; Qu, H.; Wang, J. Preparation of dyeing, flame retardant and anti-dripping polyethylene terephthalate fibers based on natural sodium copper chlorophyll dyeing and intercalation of phosphorylated sucrose fatty acid ester. Compos. Part B Eng. 2022, 245, 110194. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Cai, W.; Wang, J.; Song, L.; Hu, Y. Recent advances in construction of hybrid nano-structures for flame retardant polymers application. Appl. Mater. Today 2020, 20, 100762. [Google Scholar] [CrossRef]
- Wang, S.; Du, X.; Jiang, Y.; Xu, J.; Zhou, M.; Wang, H.; Cheng, X.; Du, Z. Synergetic enhancement of mechanical and fire-resistance performance of waterborne polyurethane by introducing two kinds of phosphorus–nitrogen flame retardant. J. Colloid Interface Sci. 2018, 537, 197–205. [Google Scholar] [CrossRef]
- Ding, F.; Zhang, S.; Chen, X.; Li, R.; Ren, X. PET fabric treated with environmental-friendly phosphorus-based compounds for enhanced flame retardancy, thermal stability and anti-dripping performance. Compos. Part B Eng. 2022, 235, 109791. [Google Scholar] [CrossRef]
- Zhao, C.-S.; Huang, F.-L.; Xiong, W.-C.; Wang, Y.-Z. A novel halogen-free flame retardant for glass-fiber-reinforced poly(ethylene terephthalate). Polym. Degrad. Stab. 2008, 93, 1188–1193. [Google Scholar] [CrossRef]
- Xia, J.; Su, Y.; Li, W. Post-polymerization functionalization to a novel phosphorus- and nitrogen-containing polyether coating for flame retardant treatment of PET fabric. J. Appl. Polym. Sci. 2019, 136, 47299. [Google Scholar] [CrossRef]
- Li, B.; Zhu, Y.; Wang, X.; Xu, H.; Zhong, Y.; Zhang, L.; Ma, Y.; Sui, X.; Wang, B.; Feng, X.; et al. Synthesis and application of poly (cyclotriphosphazene-resveratrol) microspheres for enhancing flame retardancy of poly (ethylene terephthalate). Polym. Adv. Technol. 2021, 33, 658–671. [Google Scholar] [CrossRef]
- Jang, J.; Oh, J.H.; Moon, S.I. Crystallization Behavior of Poly(ethylene terephthalate) Blended with Hyperbranched Polymers: The Effect of Terminal Groups and Composition of Hyperbranched Polymers. Macromolecules 2000, 33, 1864–1870. [Google Scholar] [CrossRef]
- Kil, S.B.; Augros, Y.; Leterrier, Y.; Månson, J.-A.E.; Christel, A.; Borer, C. Rheological properties of hyperbranched polymer/poly(ethylene terephthalate) reactive blends. Polym. Eng. Sci. 2003, 43, 329–343. [Google Scholar] [CrossRef]
- Ahani, M.; Khatibzadeh, M.; Mohseni, M. Preparation and characterization of Poly(ethylene terephthalate)/hyperbranched polymer nanocomposites by melt blending. Nanocomposites 2016, 2, 29–36. [Google Scholar] [CrossRef]
- Ao, X.; Du, Y.; Yu, D.; Wang, W.; Yang, W.; Sun, B.; Zhu, M. Synthesis, characterization of a DOPO-based polymeric flame retardant and its application in polyethylene terephthalate. Prog. Nat. Sci. 2020, 30, 200–207. [Google Scholar] [CrossRef]
- Privalko, V.P.; Lipatov, Y.S. Glass transition and chain flexibility of linear polymers. J. Macromol. Sci. Part B 1974, 9, 551–564. [Google Scholar] [CrossRef]
- Dudowicz, J.; Freed, K.F.; Douglas, J.F. The Glass Transition Temperature of Polymer Melts. J. Phys. Chem. B 2005, 109, 21285–21292. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.-Y.; Wang, C.-S. Synthesis of novel flame retardant epoxy hardeners and properties of cured products. Polymer 2001, 42, 7617–7625. [Google Scholar] [CrossRef]
- Ciesielski, M.; Schäfer, A.; Döring, M. Novel efficient DOPO-based flame-retardants for PWB relevant epoxy resins with high glass transition temperatures. Polym. Adv. Technol. 2008, 19, 507–515. [Google Scholar] [CrossRef]
- Hawker, C.J.; Lee, R.; Frechet, J.M.J. One-step synthesis of hyperbranched dendritic polyesters. J. Am. Chem. Soc. 1991, 113, 4583–4588. [Google Scholar] [CrossRef]
- Chen, H.; Kong, J. Hyperbranched polymers from A2 + B3 strategy: Recent advances in description and control of fine topology. Polym. Chem. 2016, 7, 3643–3663. [Google Scholar] [CrossRef]
- Duan, H.; Chen, Y.; Ji, S.; Hu, R.; Ma, H. A novel phosphorus/nitrogen-containing polycarboxylic acid endowing epoxy resin with excellent flame retardance and mechanical properties. Chem. Eng. J. 2019, 375, 121916. [Google Scholar] [CrossRef]
- Lin, H.-J.; Liu, S.-R.; Han, L.-J.; Wang, X.-M.; Bian, Y.-J.; Dong, L.-S. Effect of a phosphorus-containing oligomer on flame-retardant, rheological and mechanical properties of poly (lactic acid). Polym. Degrad. Stab. 2013, 98, 1389–1396. [Google Scholar] [CrossRef]
- Duan, L.; Yang, H.; Song, L.; Hou, Y.; Wang, W.; Gui, Z.; Hu, Y. Hyperbranched phosphorus/nitrogen-containing polymer in combination with ammonium polyphosphate as a novel flame retardant system for polypropylene. Polym. Degrad. Stab. 2016, 134, 179–185. [Google Scholar] [CrossRef]
- Kashiwagi, T. Polymer combustion and flammability—Role of the condensed phase. Symp. (Int.) Combust. 1994, 25, 1423–1437. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Wurm, A.; Herrmann, A.; Cornelius, M.; Zhuravlev, E.; Pospiech, D.; Nicula, R.; Schick, C. Temperature Dependency of Nucleation Efficiency of Carbon Nanotubes in PET and PBT. Macromol. Mater. Eng. 2015, 300, 637–649. [Google Scholar] [CrossRef]
- Hu, X.; Yang, H.; Jiang, Y.; He, H.; Liu, H.; Huang, H.; Wan, C. Facile synthesis of a novel transparent hyperbranched phosphorous/nitrogen-containing flame retardant and its application in reducing the fire hazard of epoxy resin. J. Hazard. Mater. 2019, 379, 120793. [Google Scholar] [CrossRef]
- Jin, S.; Qian, L.; Qiu, Y.; Chen, Y.; Xin, F. High-efficiency flame retardant behavior of bi-DOPO compound with hydroxyl group on epoxy resin. Polym. Degrad. Stab. 2019, 166, 344–352. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Guo, D.; Liu, X.; Lei, Y.; Ding, X.; Wang, Y. Fire-Safe Polyesters Enabled by End-Group Capturing Chemistry. Angew. Chem. Int. Ed. 2019, 58, 9188–9193. [Google Scholar] [CrossRef]
- Cheng, J.; Duan, H.; Yang, S.; Wang, J.; Zhang, Q.; Ding, G.; Hu, Y.; Huo, S. A P/N-containing flame retardant constructed by phosphaphenanthrene, phosphonate, and triazole and its flame retardant mechanism in reducing fire hazards of epoxy resin. J. Appl. Polym. Sci. 2020, 137, 49090. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S. An overview of some recent advances in DOPO-derivatives: Chemistry and flame retardant applications. Polym. Degrad. Stab. 2015, 113, 119–134. [Google Scholar] [CrossRef]
- Peng, X.; Liu, Q.; Wang, D.; Liu, C.; Zhao, Y.; Wang, R.; Zheng, P. A hyperbranched structure formed by in-situ crosslinking of additive flame retardant endows epoxy resins with great flame retardancy improvement. Compos. Part B Eng. 2021, 224, 109162. [Google Scholar] [CrossRef]
- Battig, A.; Markwart, J.C.; Wurm, F.R.; Schartel, B. Matrix matters: Hyperbranched flame retardants in aliphatic and aromatic epoxy resins. Polym. Degrad. Stab. 2019, 170, 108986. [Google Scholar] [CrossRef]
- Schartel, B.; Perret, B.; Dittrich, B.; Ciesielski, M.; Krämer, J.; Müller, P.; Altstädt, V.; Zang, L.; Döring, M. Flame Retardancy of Polymers: The Role of Specific Reactions in the Condensed Phase. Macromol. Mater. Eng. 2016, 301, 9–35. [Google Scholar] [CrossRef]







| Sample | T5% (°C) | Tmax (°C) | CY700 °C/wt% | Tg (°C) |
|---|---|---|---|---|
| Pure PET | 403.2 | 457.2 | 8.33 | 80.6 |
| 2%hbDT/PET | 408.7 | 449.0 | 9.23 | 81.8 |
| 5%hbDT/PET | 410.4 | 452.8 | 10.39 | 83.3 |
| Sample | LOI/% | UL-94 | Melt Dripping |
|---|---|---|---|
| Pure PET | 22.1 | NR | Severe |
| 2%hbDT/PET | 28.9 | V-2 | Moderate |
| 5%hbDT/PET | 30.2 | V-0 | Slow |
| Sample | TTI/s | TpHRR/s | pHRR/kW·m−2 | THR/MJ·m−2 | TSP/MJ·m−2 | AEHC/MJ·kg−1 | Residue/wt% |
|---|---|---|---|---|---|---|---|
| Pure PET | 49 | 105 | 1281.6 | 73.2 | 20.5 | 17.4 | 2.6 |
| 2%hbDT/PET | 42 | 95 | 719.1 | 65.7 | 19.3 | 16.9 | 3.5 |
| 5%hbDT/PET | 45 | 80 | 578.1 | 61.3 | 19.0 | 15.8 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalrhem, H.A.O.; Pan, Y.; Gu, H.; Ao, X.; Ji, X.; Jiang, X.; Sun, B. Synthesis of DOPO-Based Phosphorus-Nitrogen Containing Hyperbranched Flame Retardant and Its Effective Application for Poly(ethylene terephthalate) via Synergistic Effect. Polymers 2023, 15, 662. https://doi.org/10.3390/polym15030662
Abdalrhem HAO, Pan Y, Gu H, Ao X, Ji X, Jiang X, Sun B. Synthesis of DOPO-Based Phosphorus-Nitrogen Containing Hyperbranched Flame Retardant and Its Effective Application for Poly(ethylene terephthalate) via Synergistic Effect. Polymers. 2023; 15(3):662. https://doi.org/10.3390/polym15030662
Chicago/Turabian StyleAbdalrhem, Hossamaldin Ahmed Omer, Yueyue Pan, Hongda Gu, Xiang Ao, Xiaohuan Ji, Xiaoze Jiang, and Bin Sun. 2023. "Synthesis of DOPO-Based Phosphorus-Nitrogen Containing Hyperbranched Flame Retardant and Its Effective Application for Poly(ethylene terephthalate) via Synergistic Effect" Polymers 15, no. 3: 662. https://doi.org/10.3390/polym15030662
APA StyleAbdalrhem, H. A. O., Pan, Y., Gu, H., Ao, X., Ji, X., Jiang, X., & Sun, B. (2023). Synthesis of DOPO-Based Phosphorus-Nitrogen Containing Hyperbranched Flame Retardant and Its Effective Application for Poly(ethylene terephthalate) via Synergistic Effect. Polymers, 15(3), 662. https://doi.org/10.3390/polym15030662






