Characterization of Modified Mechanically Activated Cassava Starch Magnetic Porous Microspheres and Its Adsorption for Cd(II) Ions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the AAM-MSMPM Absorbent
2.3. Characterization of the AAM-MSMPM Absorbent
2.4. Adsorption of the AAM-MSMPM for Cd(II) Ions
3. Results and Discussion
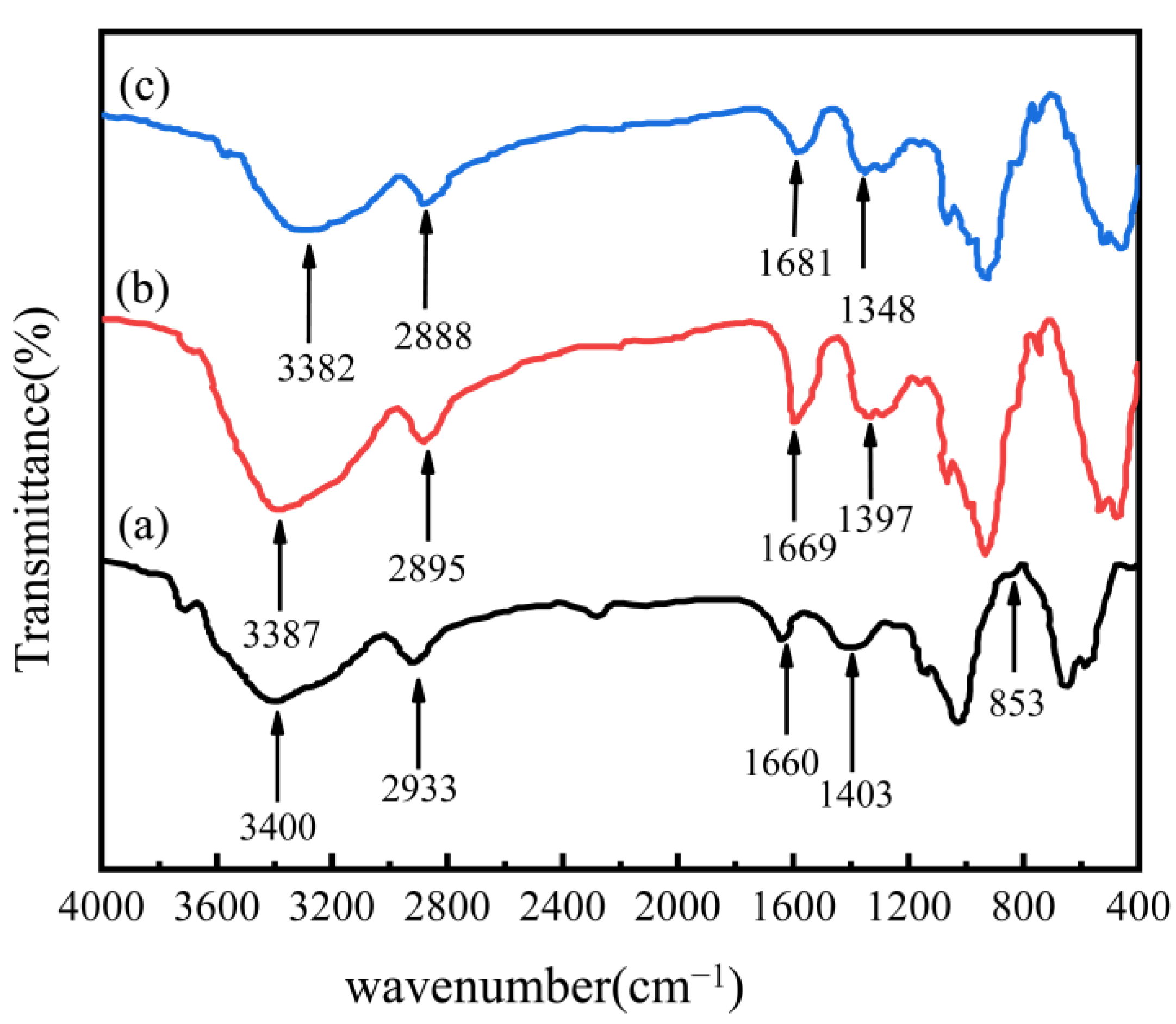
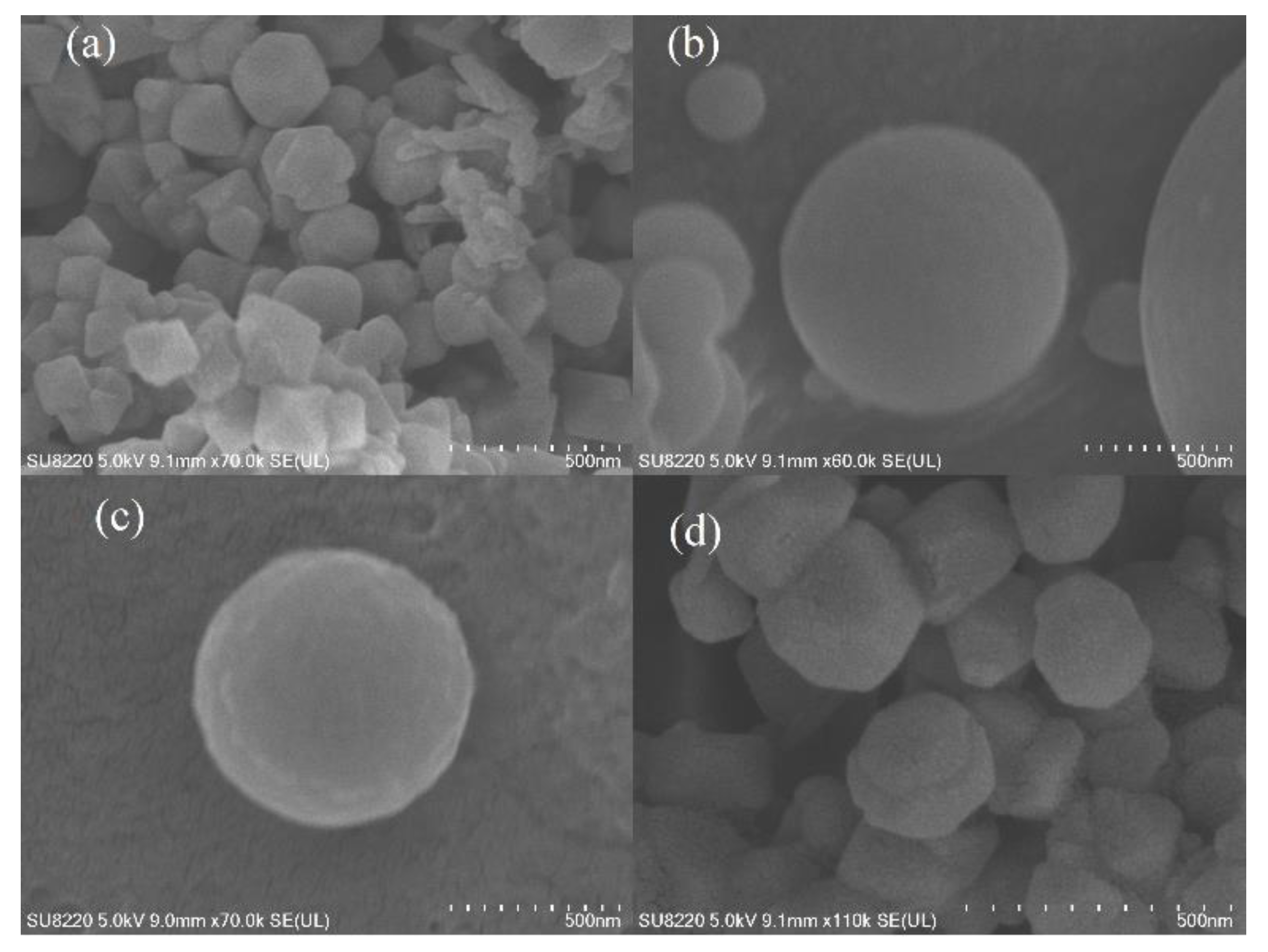
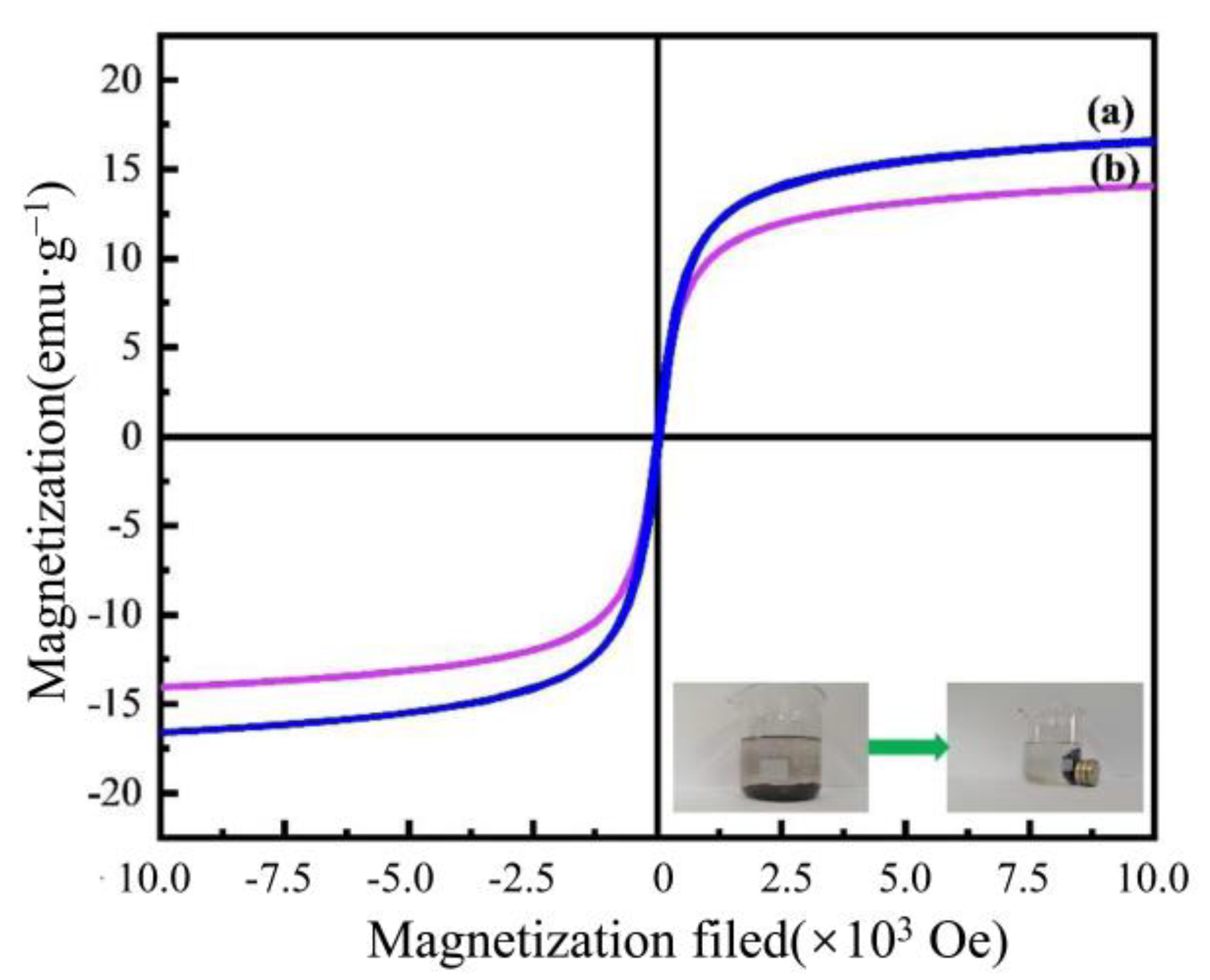
3.1. Adsorbent Characterization
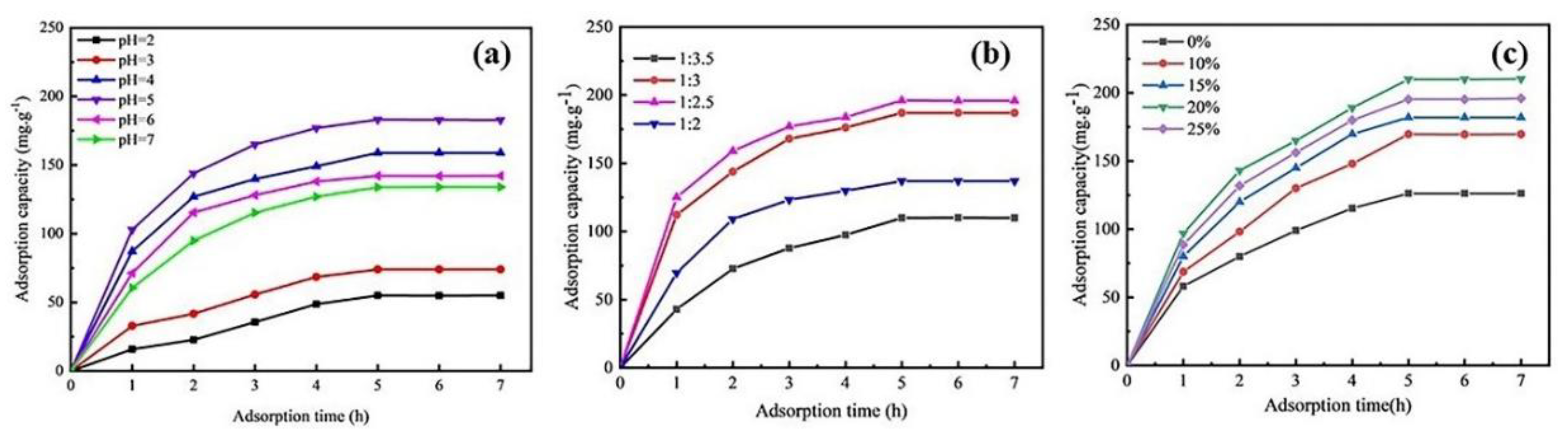
3.2. Effects of Adsorption Conditions
3.3. Adsorption Isotherms of Cd(II) Ions on the AAM-MSMPM
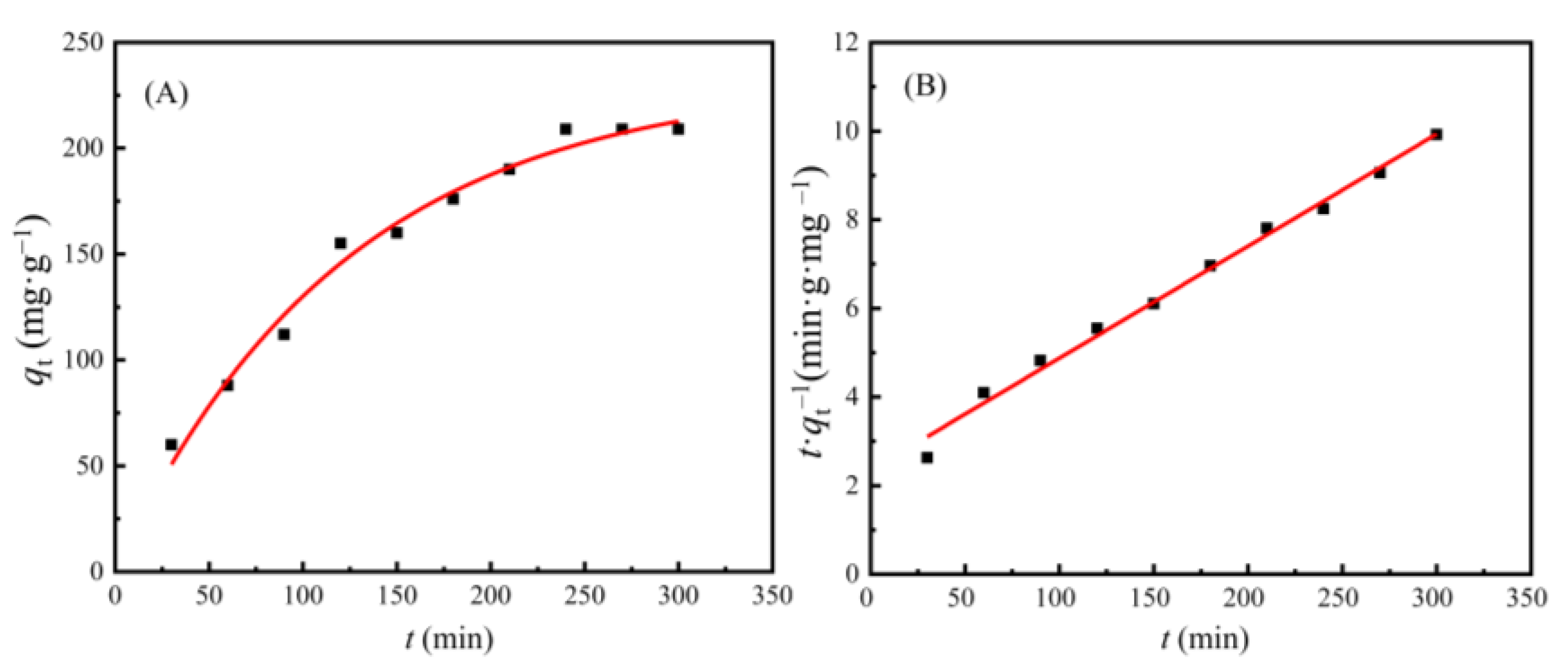
3.4. Adsorption Kinetics of Cd(II) Ions on the AAM-MSMPM
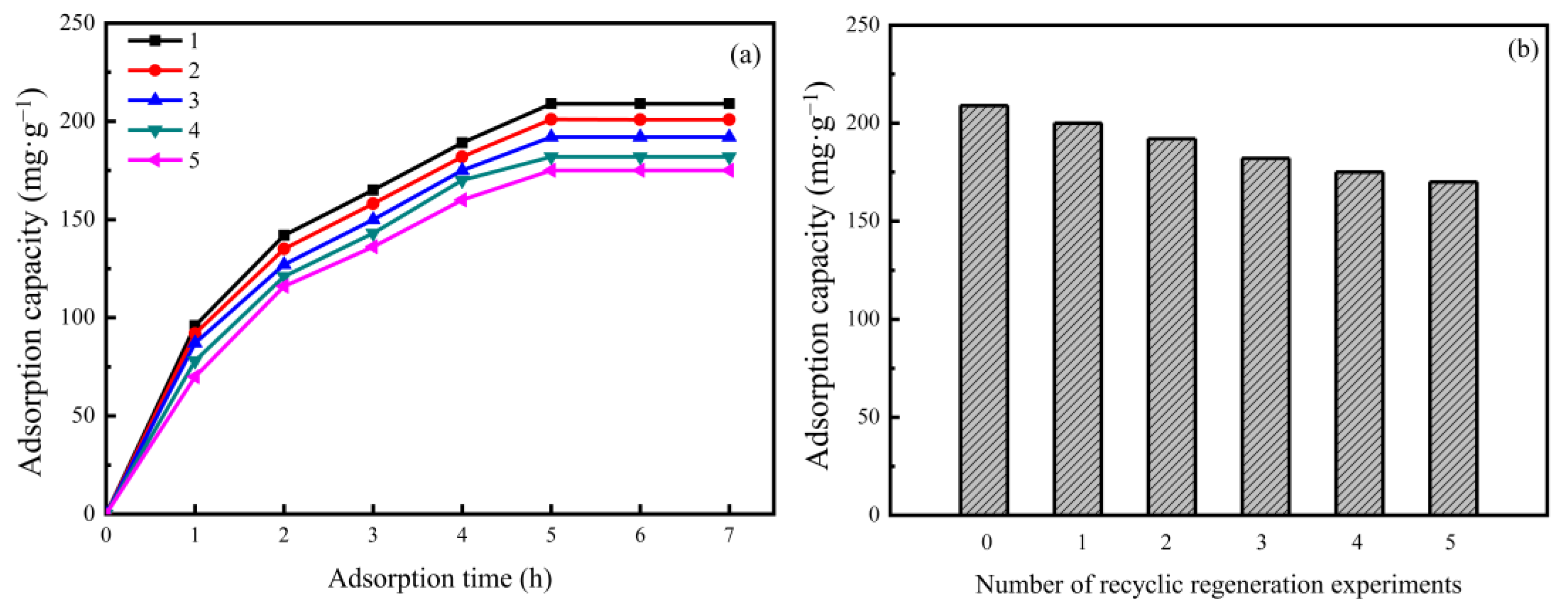
3.5. Adsorption–Desorption Cycle Regeneration Performance of the AAM-MSMPM
3.6. Comparison of the Adsorption Capacity of AAM-MSMPM with That of Other Adsorption Materials
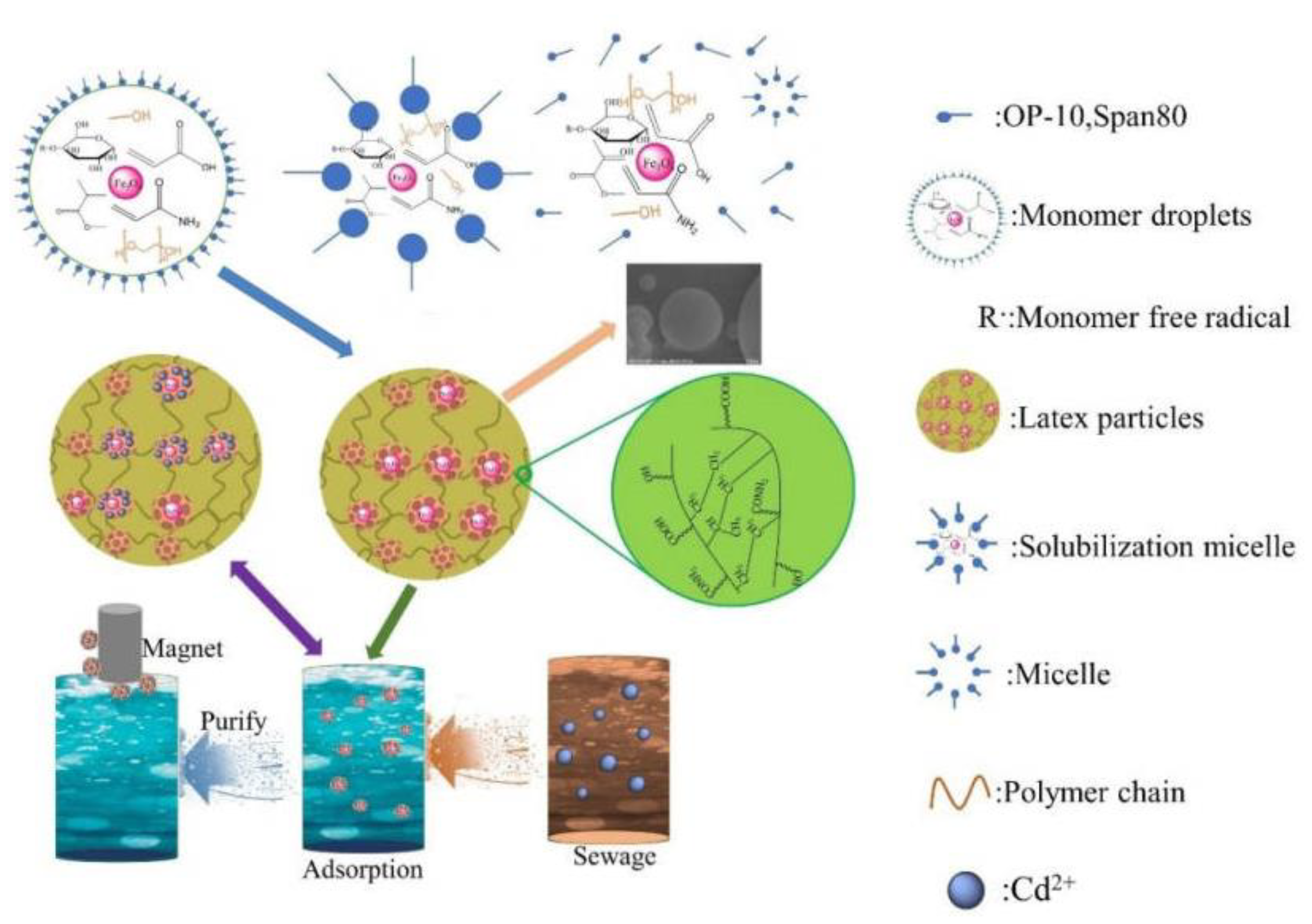
3.7. Adsorption Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, J.; Fu, D.; Hu, W.; Lu, X.; Wu, Y.; Bryan, H. Physiological responses and accumulation ability of Microcystis aeruginosa to zinc and cadmium: Implications for bioremediation of heavy metal pollution. Bioresour. Technol. 2020, 303, 122963. [Google Scholar] [CrossRef]
- Bortone, I.; Santonastaso, G.; Erto, A.; Chianese, S.; Di Nardo, A.; Musmarra, D. An innovative in-situ DRAINage system for advanced groundwater reactive TREATment (in-DRAIN-TREAT). Chemosphere 2021, 270, 129412. [Google Scholar] [CrossRef]
- Lekshmi, R.; Rejiniemon, T.S.; Sathya, R.; Kuppusamy, P.; Al-mekhlafi, F.A.; Wadaan, M.A.; Rajendran, P. Adsorption of heavy metals from the aqueous solution using activated biomass from Ulva flexuosa. Chemosphere 2022, 306, 135479. [Google Scholar] [CrossRef]
- Liu, K.; Fan, L.; Li, Y.; Zhou, Z.; Chen, C.; Chen, B.; Yu, F. Concentrations and health risks of heavy metals in soils and crops around the Pingle manganese (Mn) mine area in Guangxi Province, China. Environ. Sci. Pollut. Res. 2018, 25, 30180–30190. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Arsad, A.; Abbas, A.; Gbadamosi, A.; Azli, N.B.; Oseh, J. Ultrasound-assisted weak-acid hydrolysis of crystalline starch nanoparticles for chemical enhanced oil recovery. Int. J. Biol. Macromol. 2020, 148, 1251–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, B.; Liu, H.; Liang, X.; Ma, W.; Shi, Z.; Yang, S. Zinc effects on cadmium toxicity in two wheat varieties (Triticum aestivum L.) differing in grain cadmium accumulation. Ecotoxicol. Environ. Saf. 2019, 183, 109562. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Y.; Chen, G.; Cai, M. Enhanced mechanical and hydrophobic properties of composite cassava starch films with stearic acid modified MCC (microcrystalline cellulose)/NCC (nanocellulose) as strength agent. Int. J. Biol. Macromol. 2020, 142, 846–854. [Google Scholar] [CrossRef]
- Zhang, A.; Yin, X.; Shen, X.; Liu, Y. Removal of Fluticasone Propionate and Clobetasol Propionate by Calcium Peroxide: Synergistic Effects of Oxidation, Adsorption, and Base Catalysis. ES Energy Environ. 2018, 1, 89–98. [Google Scholar] [CrossRef]
- Batista, R.D.; de Cássia Sousa Mendes, D.; Morais, C.C.; Thomaz, D.V.; Ramirez Ascheri, D.P.; Damiani, C.; Asquieri, E.R. Physicochemical, functional and rheological properties of fermented and non-fermented starch from canary seed (Phalaris canariensis). Food Hydrocoll. 2020, 99, 105346. [Google Scholar] [CrossRef]
- Gu, H.; Xu, X.; Zhang, H.; Liang, C.; Lou, H.; Ma, C.; Li, Y.; Guo, Z.; Gu, J. Chitosan-coated-magnetite with Covalently Grafted Polystyrene Based Carbon Nanocomposites for Hexavalent Chromium Adsorption. Eng. Sci. 2018, 1, 46–54. [Google Scholar] [CrossRef]
- Hou, T.; Yu, S.; Zhou, M.; Wu, M.; Liu, J.; Zheng, X.; Li, J.; Wang, J.; Wang, X. Effective removal of inorganic and organic heavy metal pollutants with poly(amino acid)-based micromotors. Nanoscale 2020, 12, 5227–5232. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Huang, J.; Zhang, Y.; Tong, Z.; Liao, A.; Guo, X.; Qin, Z.; Guo, Z. Aminated cassava residue-based magnetic microspheres for Pb(II) adsorption from wastewater. Korean J. Chem. Eng. 2019, 36, 226–235. [Google Scholar] [CrossRef]
- Castelo-Quibén, J.; Pastrana-Martínez, L.M.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. From Polyethylene to Highly Graphitic and Magnetic Carbon Spheres Nanocomposites: Carbonization under Pressure. Nanomaterials 2019, 9, 606. [Google Scholar] [CrossRef]
- Cristescu, N.D.; Craciun, E.-M.; Soós, E. Mechanics of Elastic Composites, 1st ed.; Chapman and Hall/CRC Press: New York, NY, YSA, 2003. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Shahinpour, A.; Tanhaei, B.; Ayati, A.; Beiki, H.; Sillanpää, M. Binary dyes adsorption onto novel designed magnetic clay-biopolymer hydrogel involves characterization and adsorption performance: Kinetic, equilibrium, thermodynamic, and adsorption mechanism. J. Mol. Liq. 2022, 366, 120303. [Google Scholar] [CrossRef]
- Ganguly, S.; Neelam; Grinberg, I.; Margel, S. Layer by layer controlled synthesis at room temperature of tri-modal (MRI, fluorescence and CT) core/shell superparamagnetic IO/human serum albumin nanoparticles for diagnostic applications. Polym. Adv. Technol. 2021, 32, 3909–3921. [Google Scholar] [CrossRef]
- Zhang, M.-K.; Zhang, X.-H.; Han, G.-Z. Magnetic alginate/PVA hydrogel microspheres with selective adsorption performance for aromatic compounds. Sep. Purif. Technol. 2021, 278, 119547. [Google Scholar] [CrossRef]
- Chen, K.; He, J.; Li, Y.; Cai, X.; Zhang, K.; Liu, T.; Hu, Y.; Lin, D.; Kong, L.; Liu, J. Removal of cadmium and lead ions from water by sulfonated magnetic nanoparticle adsorbents. J. Colloid Interface Sci. 2017, 494, 307–316. [Google Scholar] [CrossRef]
- Abass, A.B.; Awoyale, W.; Alamu, E.O. Assessment of the chemical and trace metal composition of dried cassava products from Nigeria. Qual. Assur. Saf. Crops Foods 2019, 11, 43–52. [Google Scholar] [CrossRef]
- Falade, K.O.; Ibanga-Bamijoko, B.; Ayetigbo, O.E. Comparing properties of starch and flour of yellow-flesh cassava cultivars and effects of modifications on properties of their starch. J. Food Meas. Charact. 2019, 13, 2581–2593. [Google Scholar] [CrossRef]
- Hu, H.; Liu, W.; Shi, J.; Huang, Z.; Zhang, Y.; Huang, A.; Yang, M.; Qin, X.; Shen, F. Structure and functional properties of octenyl succinic anhydride modified starch prepared by a non-conventional technology. Starch-Stärke 2016, 68, 151–159. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, Z.; Li, D.; Wu, M. Effects of Ball Milling Processes on the Microstructure and Rheological Properties of Microcrystalline Cellulose as a Sustainable Polymer Additive. Materials 2018, 11, 1057. [Google Scholar] [CrossRef] [PubMed]
- Gilet, A.; Quettier, C.; Wiatz, V.; Bricout, H.; Ferreira, M.; Rousseau, C.; Monflier, E.; Tilloy, S. Unconventional media and technologies for starch etherification and esterification. Green Chem. 2018, 20, 1152–1168. [Google Scholar] [CrossRef]
- Xie, J.; Zhong, G.; Cai, C.; Chen, C.; Chen, X. Rapid and efficient separation of glycoprotein using pH double-responsive imprinted magnetic microsphere. Talanta 2017, 169, 98–103. [Google Scholar] [CrossRef]
- Xie, X.; Xiong, H.; Zhang, Y.; Tong, Z.; Liao, A.; Qin, Z. Preparation magnetic cassava residue microspheres and its application for Cu(II) adsorption. J. Environ. Chem. Eng. 2017, 5, 2800–2806. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, X.; Luo, X.; Su, T.; Zhang, Y.; Qin, Z.; Ji, H. Mechanically activated starch magnetic microspheres for Cd(II) adsorption from aqueous solution. Chin. J. Chem. Eng. 2021, 33, 40–49. [Google Scholar] [CrossRef]
- Ogata, F.; Nagai, N.; Ueta, E.; Nakamura, T.; Kawasaki, N. Biomass Potential of Virgin and Calcined Tapioca (Cassava Starch) for the Removal of Sr(II) and Cs(I) from Aqueous Solutions. Chem. Pharm. Bull. 2018, 66, 295–302. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Oliveira, V.R.L.; Santos, F.K.G.; Barros Neto, E.L.; Leite, R.H.L.; Aroucha, E.M.M.; Silva, R.R.; Silva, K.N.O. Incorporation of bentonite clay in cassava starch films for the reduction of water vapor permeability. Food Res. Int. 2018, 105, 637–644. [Google Scholar] [CrossRef]
- Zhu, F.; Hua, Y.; Li, G. Physicochemical properties of potato, sweet potato and quinoa starch blends. Food Hydrocoll. 2020, 100, 105278. [Google Scholar] [CrossRef]
- Li, H.; Gong, J.; Zhang, S.; Zhang, J.; Hu, Y. Synthesis and adsorpting property of magnetic imprinted crosslinked acrylic acid/acrylamide grafted-esterified cyanoethyl cassava starch microspheres. Chem. Ind. Eng. Prog. 2019, 38, 1930–1940. [Google Scholar] [CrossRef]
- Mulani, K.; Patil, V.; Chavan, N.; Donde, K. Adsorptive removal of chromium(VI) using spherical resorcinol-formaldehyde beads prepared by inverse suspension polymerization. J. Polym. Res. 2019, 26, 41. [Google Scholar] [CrossRef]
- Olojede, A.O.; Sanni, A.I.; Banwo, K. Effect of legume addition on the physiochemical and sensorial attributes of sorghum-based sourdough bread. LWT 2020, 118, 108769. [Google Scholar] [CrossRef]
- Omondi, J.O.; Lazarovitch, N.; Rachmilevitch, S.; Kukew, T.; Yermiyahu, U.; Yasuor, H. Potassium and storage root development: Focusing on photosynthesis, metabolites and soluble carbohydrates in cassava. Physiol. Plant. 2020, 169, 169–178. [Google Scholar] [CrossRef]
- Oyeyinka, S.A.; Adeloye, A.A.; Olaomo, O.O.; Kayitesi, E. Effect of fermentation time on physicochemical properties of starch extracted from cassava root. Food Biosci. 2020, 33, 100485. [Google Scholar] [CrossRef]
- Palavecino, P.M.; Penci, M.C.; Ribotta, P.D. Effect of Sustainable Chemical Modifications on Pasting and Gel Properties of Sorghum and Cassava Starch. Food Bioprocess Technol. 2020, 13, 112–120. [Google Scholar] [CrossRef]
- Mesa, E.; Manjarres-Pinzon, K.; Rodriguez-Sandoval, E. Gluten-free cheese bread from frozen dough: Effect of modified cassava starch. Food Sci. Technol. 2019, 39, 654–661. [Google Scholar] [CrossRef]
- Padilla-Ortega, E.; Leyva-Ramos, R.; Mendoza-Barron, J. Role of electrostatic interactions in the adsorption of cadmium(II) from aqueous solution onto vermiculite. Appl. Clay Sci. 2014, 88–89, 10–17. [Google Scholar] [CrossRef]
- Mondragón, M.; López-Villegas, O.; Sánchez-Valdés, S.; Rodríguez-González, F.J. Effect of Thermoplastic Starch and Photocrosslinking on the Properties and Morphology of Electrospun Poly(ethylene-co-vinyl alcohol) Mats. Polym. Eng. Sci. 2020, 60, 474–480. [Google Scholar] [CrossRef]
- Padi, R.K.; Chimphango, A. Feasibility of commercial waste biorefineries for cassava starch industries: Techno-economic assessment. Bioresour. Technol. 2020, 297, 122461. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, H.; Huang, J.; Ge, Z.; Guo, J.; Feng, X.; Xu, Q. Improvement of the bonding properties of cassava starch-based wood adhesives by using different types of acrylic ester. Int. J. Biol. Macromol. 2019, 126, 603–611. [Google Scholar] [CrossRef]
- Wang, X.; Huang, Y.; Zhong, Z.; Pan, Z.; Liu, C. Theoretical investigation of cadmium vapor adsorption on kaolinite surfaces with DFT calculations. Fuel 2016, 166, 333–339. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]
- Long, X.; Chen, H.; Huang, T.; Zhang, Y.; Lu, Y.; Tan, J.; Chen, R. Removal of Cd(II) from Micro-Polluted Water by Magnetic Core-Shell Fe3O4@Prussian Blue. Molecules 2021, 26, 2497. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, J.; Xing, L.; Wang, D.; Wang, L.; Xiao, H.; Ke, J. Magnetic Fe3O4/attapulgite hybrids for Cd(II) adsorption: Performance, mechanism and recovery. J. Hazard. Mater. 2021, 412, 125237. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-R.; Song, Y.; Chang, C. Adsorption properties and mechanism of ginkgo biloba leaf-based materials for Cd(II) in aqueous solution. Environ. Sci. Pollut. Res. 2022, 29, 78499–78508. [Google Scholar] [CrossRef] [PubMed]
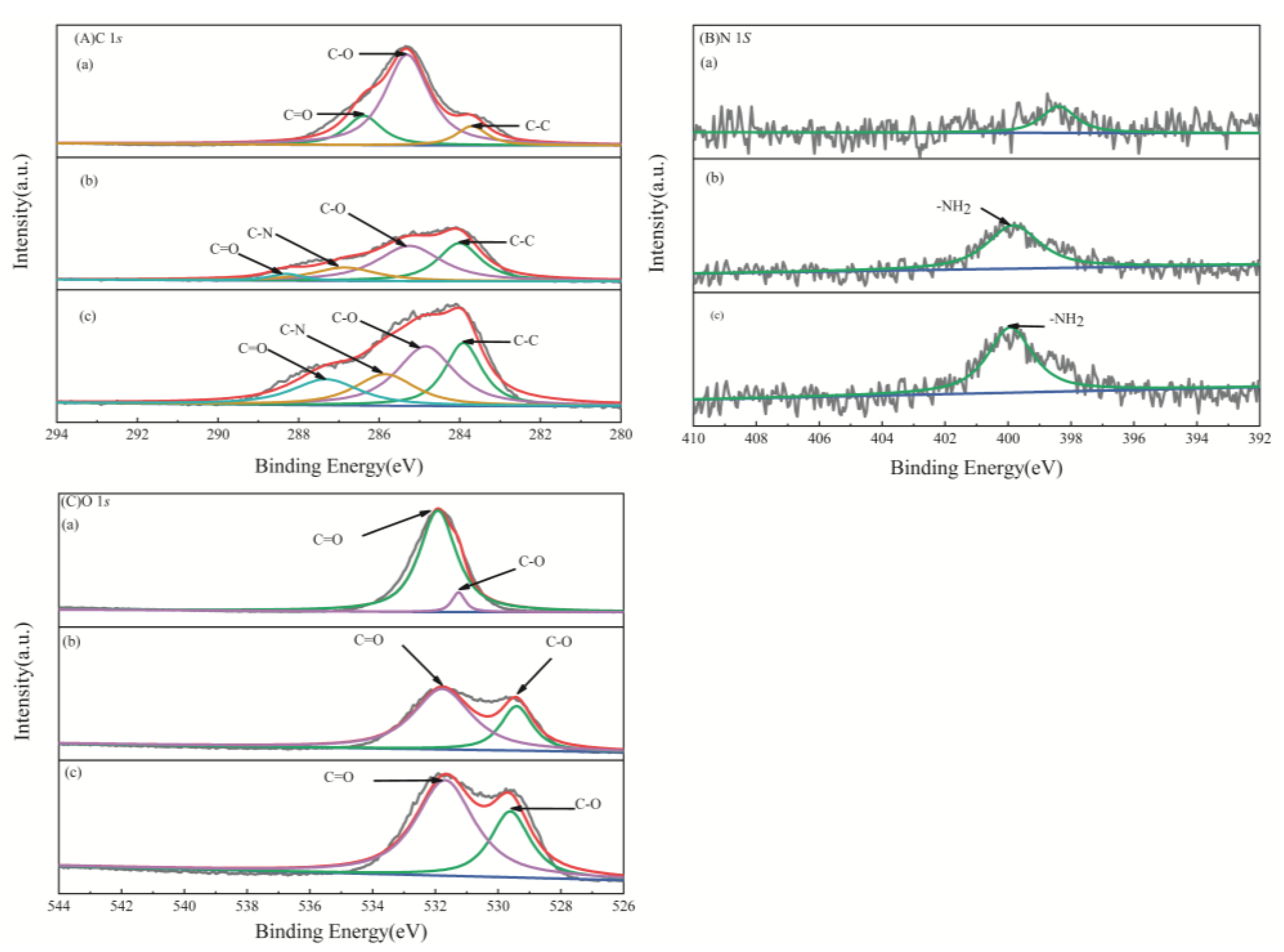


| XPS Profiles | Element Valence | Binding Energy (eV) | ||
|---|---|---|---|---|
| MS | AAM-MSMPM | Cd(II)/AAM-MSMPM | ||
| C 1s | C–C | 283.73 | 284.01 | 283.91 |
| C–O | 285.35 | 285.25 | 284.85 | |
| C–N | - | 288.31 | 287.32 | |
| C=O | 286.42 | 286.85 | 282.82 | |
| N 1s | –NH2 | - | 399.83 | 399.92 |
| O 1s | O=C | 531.28 | 529.41 | 529.61 |
| O–C | 531.96 | 531.78 | 531.69 | |
| Samples | BET Surface Area (m2·g–1) | Pore Volume (cm3·g–1) | Pore Size (nm) |
|---|---|---|---|
| AAM-MSM(a) | 7.00 | 0.03 | 19.00 |
| 10 wt% AAM-MSMPM(b) | 21.00 | 0.09 | 17.00 |
| 15 wt% AAM-MSMPM(c) | 21.00 | 0.10 | 14.00 |
| 10 wt% AAM-MSMPM(d) | 26.00 | 0.12 | 11.00 |
| 25 wt% AAM-MSMPM(e) | 35.00 | 0.13 | 7.00 |
| KL (L·mg–1) | qm (mg·g–1) | KF | n | R2 | |
|---|---|---|---|---|---|
| Langmuir model | 0.0263 | 198.60 | N/A | N/A | 0.9963 |
| Freundlich model | N/A | N/A | 1.9951 | 1.3751 | 0.9782 |
| k1 (min–1) | qe (mg·g–1) | k2 (g·mg–1·min–1) | qe (mg·g–1) | R2 | |
|---|---|---|---|---|---|
| Pseudo-first-order | 0.00891 | 232.6186 | N/A | N/A | 0.9826 |
| Pseudo-second-order | N/A | N/A | 2.7636 × 10−4 | 220.5011 | 0.9965 |
| Different Kinds of Adsorbents | Adsorption Conditions | The Maximal Adsorption Capacity of Cd(II) (mg·g–1) | Ref. | ||
|---|---|---|---|---|---|
| t (h) | pH | T (°C) | |||
| Polyaniline grafted chitosan (PGC) | 1.5 | 6 | 30 | 14.33 | [43] |
| AAM-MSM | 5 | 7 | 25 | 39.98 | [27] |
| Fe3O4@PB | 4 | 6 | 25 ± 1 | 9.25 | [44] |
| Fe3O4/ATP | 2 | 7 | 20 | 141.1 | [45] |
| KMnO4-GL | 5 | 6 | 25 | 48.82 | [46] |
| AAM-MSMPM | 5 | 5 | 55 | 210.68 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Zhao, X.; Luo, X.; Zhang, Y.; Qin, Z.; Ji, H. Characterization of Modified Mechanically Activated Cassava Starch Magnetic Porous Microspheres and Its Adsorption for Cd(II) Ions. Nanomaterials 2023, 13, 513. https://doi.org/10.3390/nano13030513
Xie X, Zhao X, Luo X, Zhang Y, Qin Z, Ji H. Characterization of Modified Mechanically Activated Cassava Starch Magnetic Porous Microspheres and Its Adsorption for Cd(II) Ions. Nanomaterials. 2023; 13(3):513. https://doi.org/10.3390/nano13030513
Chicago/Turabian StyleXie, Xinling, Xiaona Zhao, Xuan Luo, Youquan Zhang, Zuzeng Qin, and Hongbing Ji. 2023. "Characterization of Modified Mechanically Activated Cassava Starch Magnetic Porous Microspheres and Its Adsorption for Cd(II) Ions" Nanomaterials 13, no. 3: 513. https://doi.org/10.3390/nano13030513
APA StyleXie, X., Zhao, X., Luo, X., Zhang, Y., Qin, Z., & Ji, H. (2023). Characterization of Modified Mechanically Activated Cassava Starch Magnetic Porous Microspheres and Its Adsorption for Cd(II) Ions. Nanomaterials, 13(3), 513. https://doi.org/10.3390/nano13030513







