Electrocatalytic Oxygen Reduction Reaction of Graphene Oxide and Metal-Free Graphene in an Alkaline Medium
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
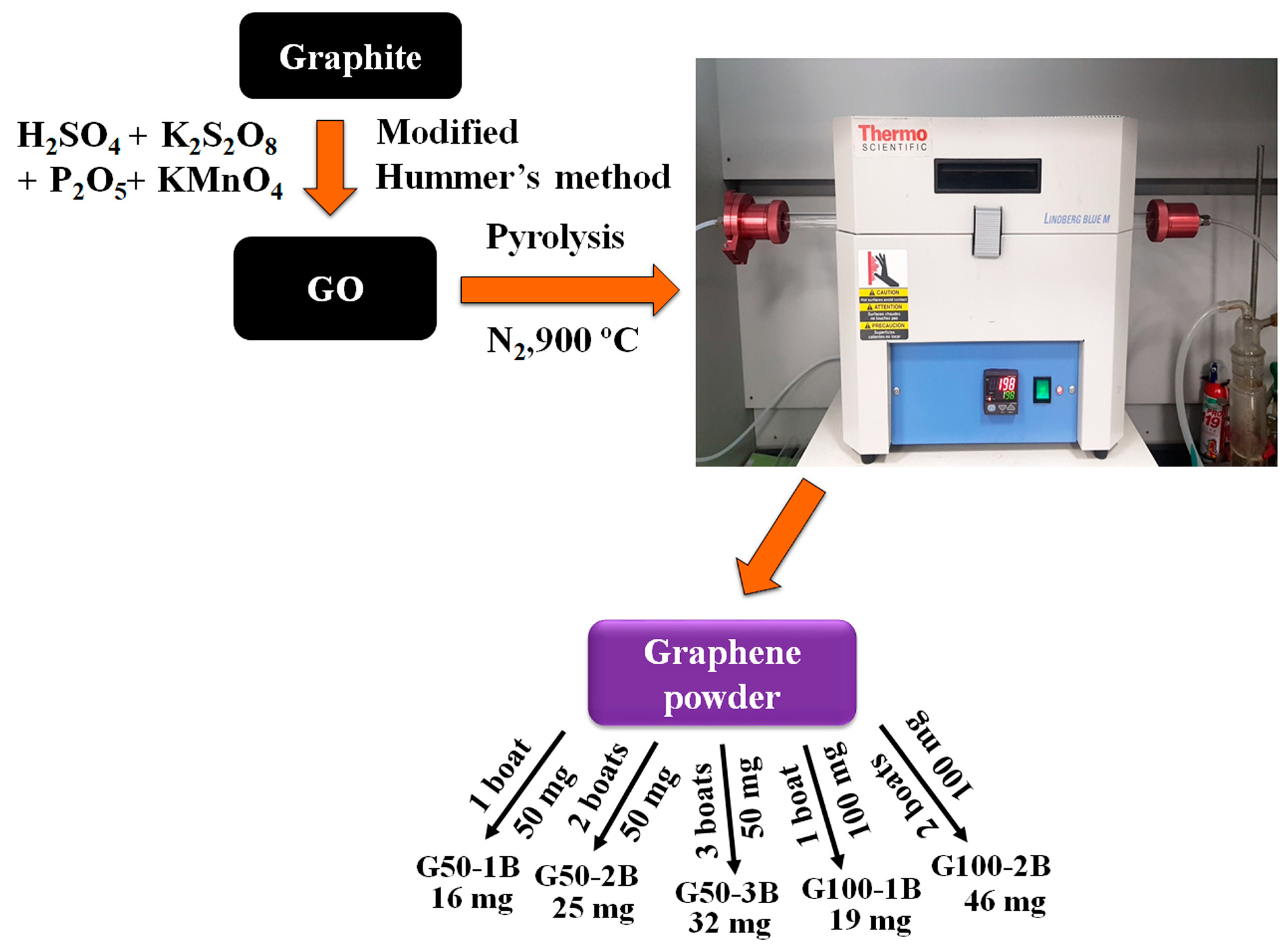
2.2. Synthesis of GO by Modified Hummer’s Method
2.3. Preparation of Graphene
2.4. Characterizations
2.5. Catalyst Ink Preparation and Electrochemical Measurements
3. Results and Discussion
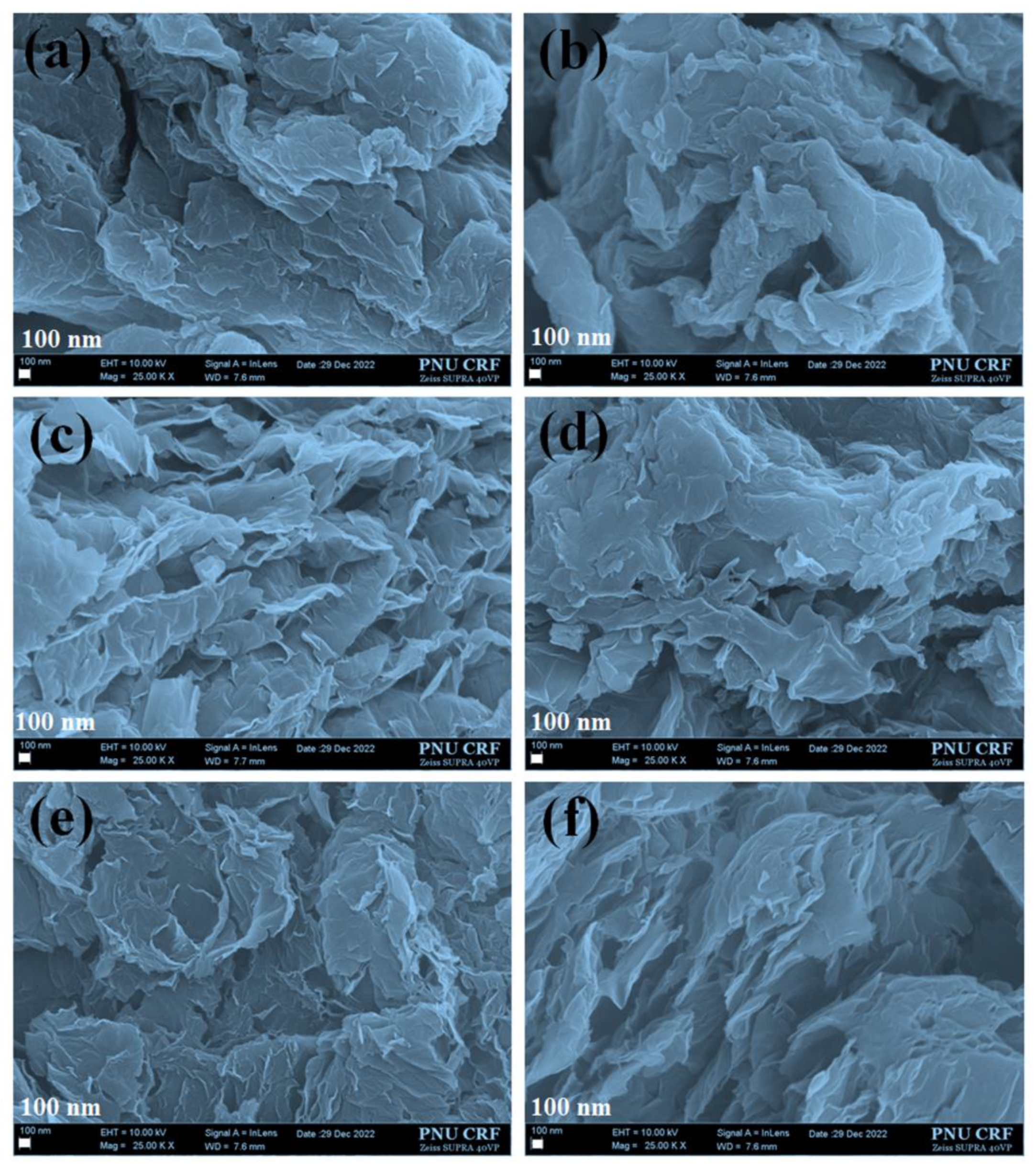
3.1. Surface Morphological Analysis
3.1.1. Field Emission Scanning Electron Microscopy (FESEM) Analysis
3.1.2. Field Emission Transmission Electron Microscopy (FETEM) Analysis
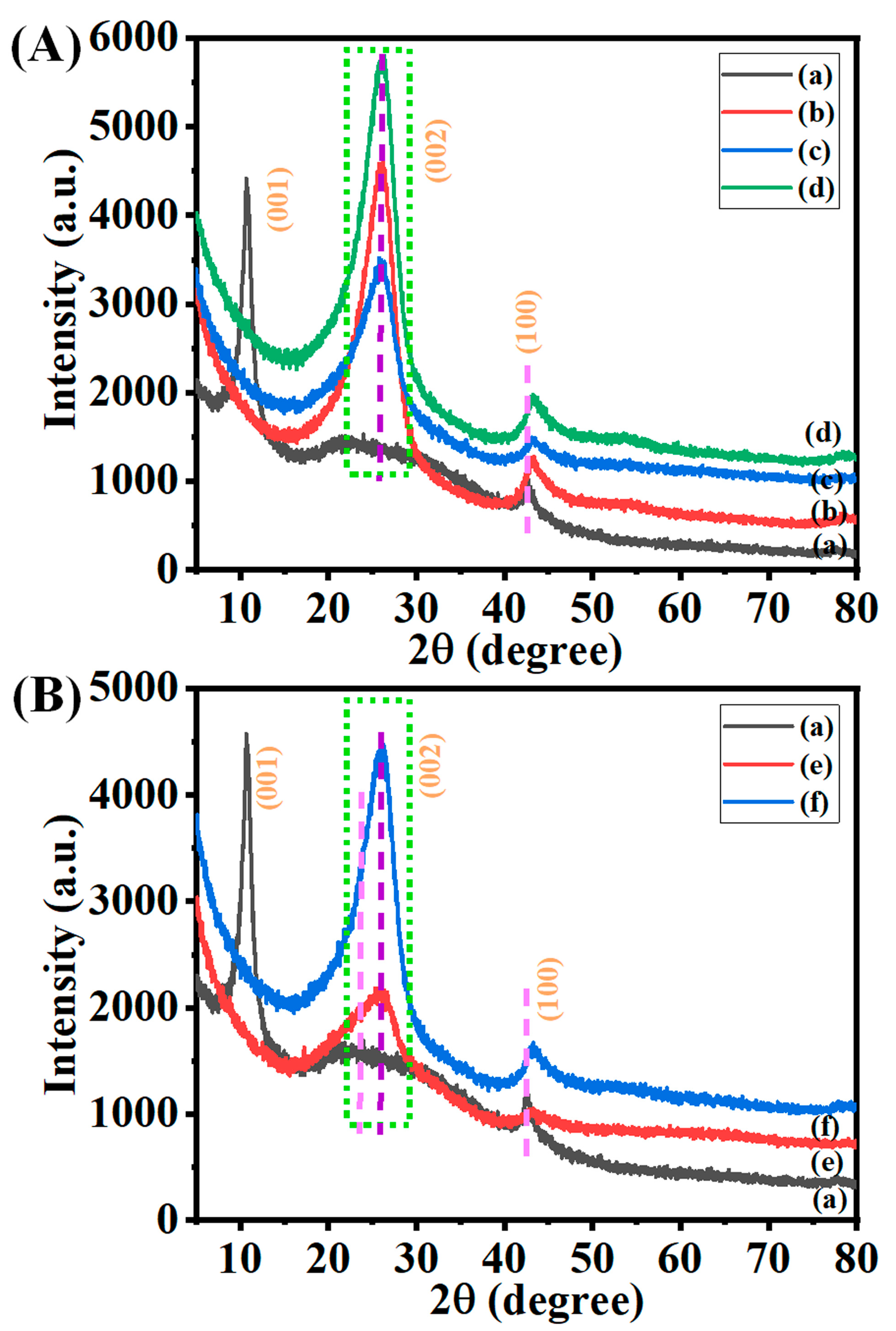
3.2. Crystallographic Studies
3.3. Surface Functionality Analysis
3.3.1. Fourier Infra-Red Spectroscopy (FTIR)
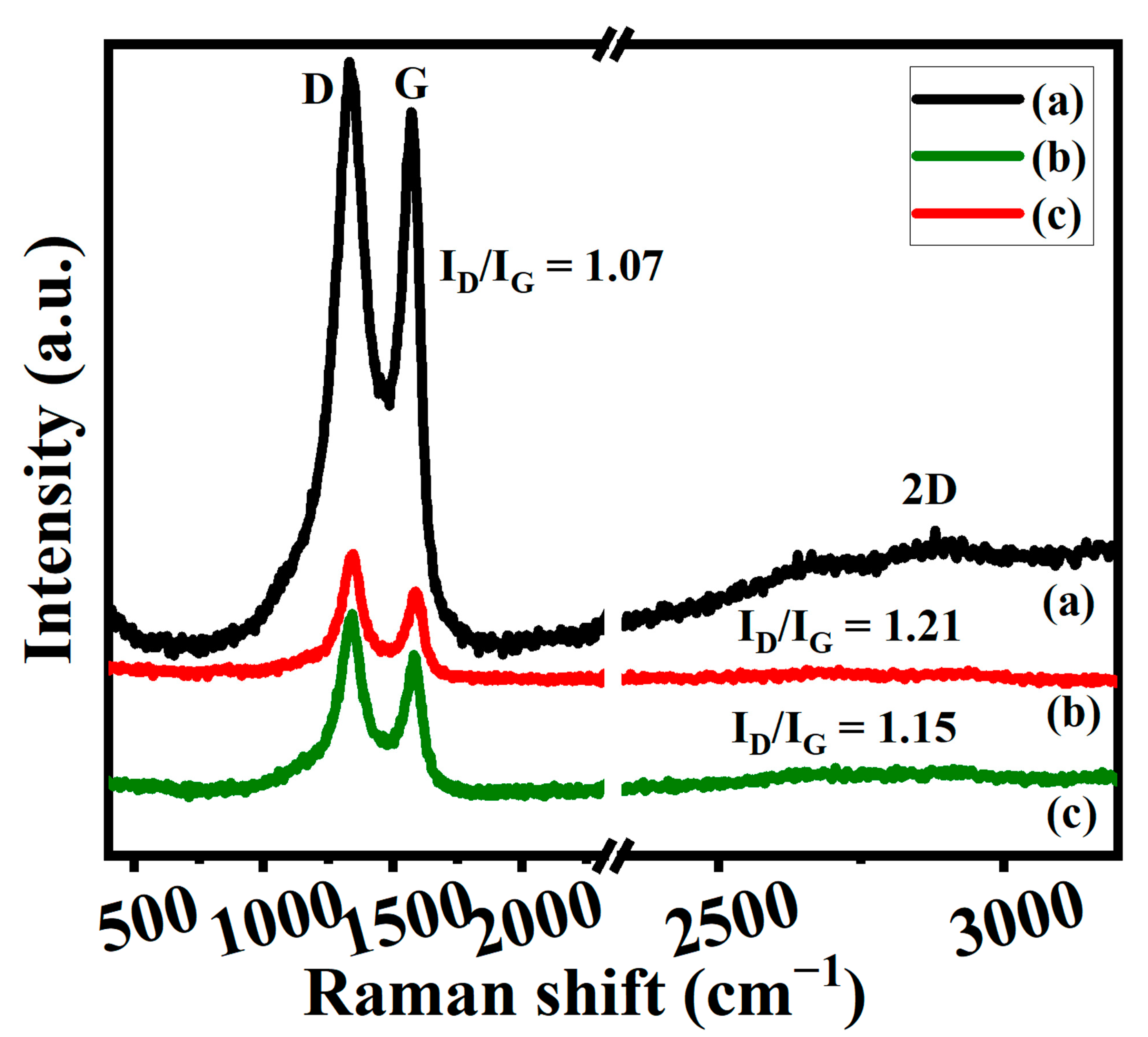
3.3.2. Raman Spectroscopy
3.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
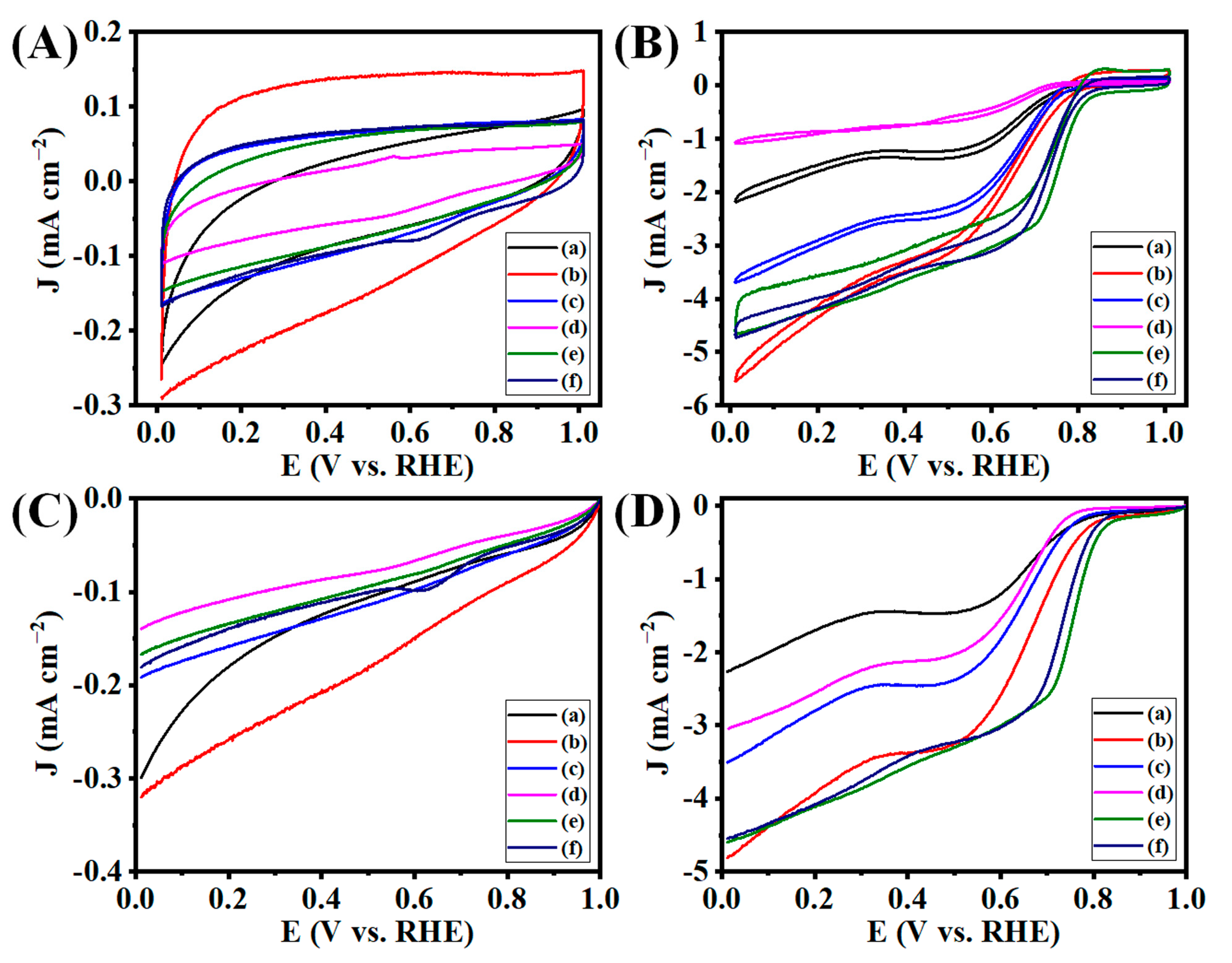
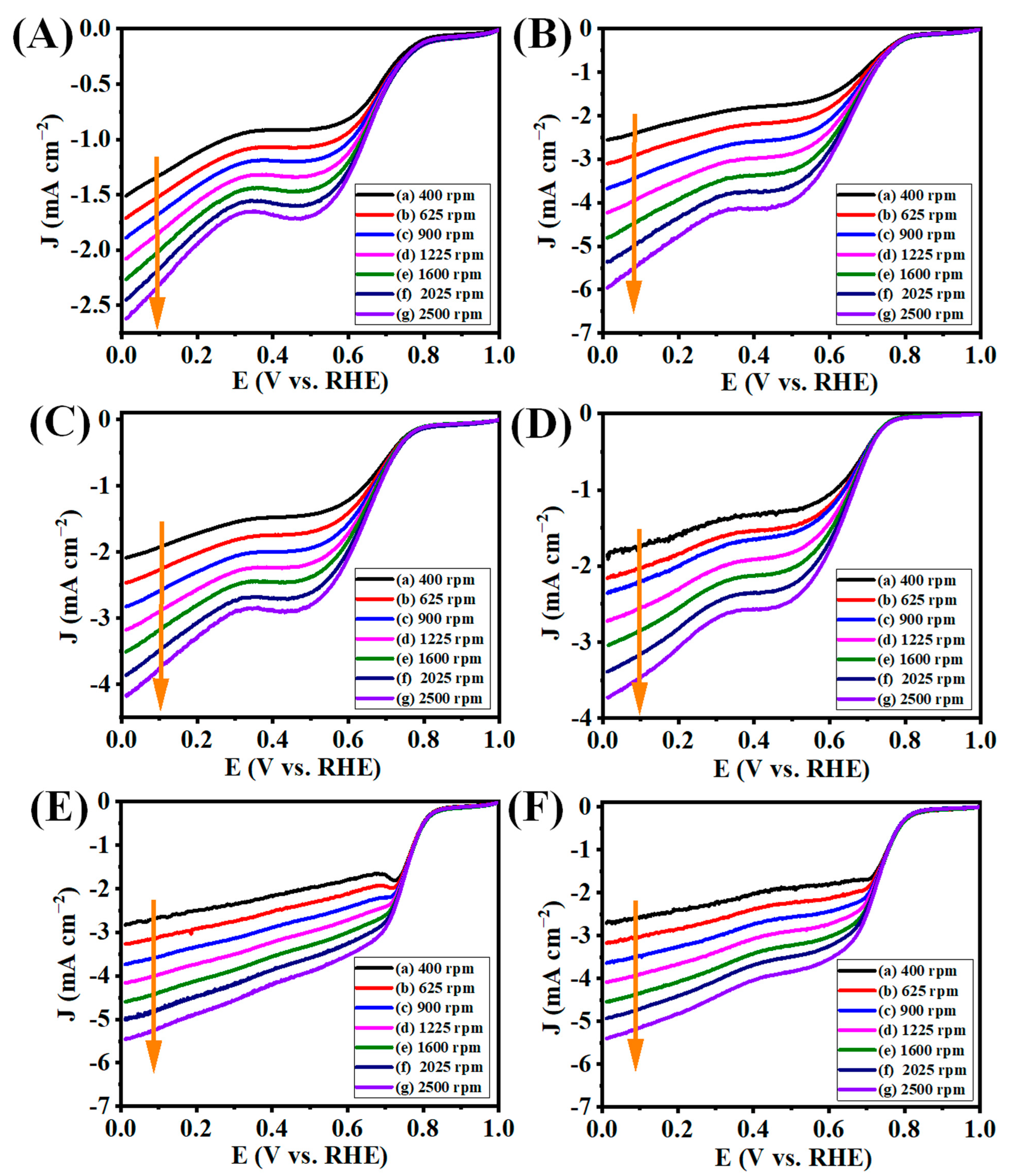
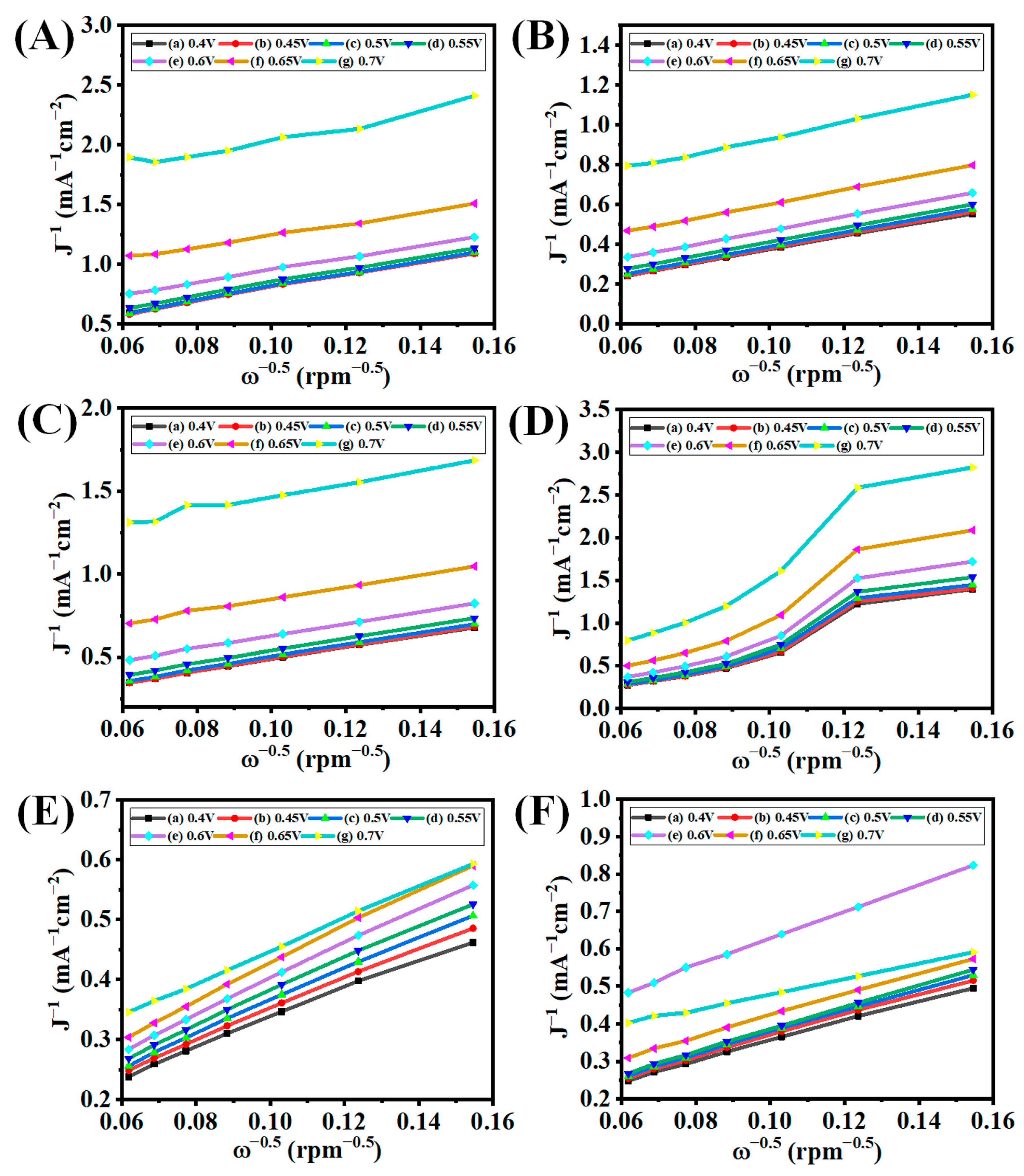
3.5. Electrocatalytic ORR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Xiang, Z.-P.; Tan, A.-D.; Fu, Z.-Y.; Piao, J.-H.; Liang, Z.-X. Oxygen Reduction Reaction on Single Pt Nanoparticle. J. Energy Chem. 2020, 49, 323–326. [Google Scholar] [CrossRef]
- Lei, H.; Piao, J.; Brouzgou, A.; Gorbova, E.; Tsiakaras, P.; Liang, Z. Synthesis of Nitrogen-Doped Mesoporous Carbon Nanosheets for Oxygen Reduction Electrocatalytic Activity Enhancement in Acid and Alkaline Media. Int. J. Hydrogen Energy 2019, 44, 4423–4431. [Google Scholar] [CrossRef]
- Ścigała, A.; Szczęsny, R.; Kamedulski, P.; Trzcinski, M.; Szłyk, E. Copper Nitride/Silver Nanostructures Synthesized via Wet Chemical Reduction Method for the Oxygen Reduction Reaction. J. Nanoparticle Res. 2023, 25, 28. [Google Scholar] [CrossRef]
- Wan, K.; Tan, A.; Yu, Z.; Liang, Z.; Piao, J.; Tsiakaras, P. 2D Nitrogen-Doped Hierarchically Porous Carbon: Key Role of Low Dimensional Structure in Favoring Electrocatalysis and Mass Transfer for Oxygen Reduction Reaction. Appl. Catal. B Environ. 2017, 209, 447–454. [Google Scholar] [CrossRef]
- Nagappan, S.; Duraivel, M.; Elayappan, V.; Muthuchamy, N.; Mohan, B.; Dhakshinamoorthy, A.; Prabakar, K.; Lee, J.-M.; Park, K.H. Metal–Organic Frameworks-Based Cathode Materials for Energy Storage Applications: A Review. Energy Technol. 2023, 11, 2201200. [Google Scholar] [CrossRef]
- Niu, Q.; Chen, B.; Guo, J.; Nie, J.; Guo, X.; Ma, G. Flexible, Porous, and Metal–Heteroatom-Doped Carbon Nanofibers as Efficient ORR Electrocatalysts for Zn–Air Battery. Nano-Micro Lett. 2019, 11, 8. [Google Scholar] [CrossRef]
- Chang, H.; Cong, S.; Wang, L.; Wang, C. Research Progress of Bifunctional Oxygen Reactive Electrocatalysts for Zinc–Air Batteries. Nanomaterials 2022, 12, 3834. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Farkas, A.; Madarász, J.; László, K. Comparison of Thermally and Chemically Reduced Graphene Oxides by Thermal Analysis and Raman Spectroscopy. J. Therm. Anal. Calorim. 2020, 142, 331–337. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and Structural Studies of High Quality Graphene Oxide and Reduced Graphene Oxide Prepared by Different Chemical Oxidation Methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Kamedulski, P.; Skorupska, M.; Binkowski, P.; Arendarska, W.; Ilnicka, A.; Lukaszewicz, J.P. High Surface Area Micro-Mesoporous Graphene for Electrochemical Applications. Sci. Rep. 2021, 11, 22054. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L. Preparation of Graphene by a Low-Temperature Thermal Reduction at Atmosphere Pressure. Nanoscale 2010, 2, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Silva Filho, J.C.; Venancio, E.C.; Silva, S.C.; Takiishi, H.; Martinez, L.G.; Antunes, R.A. A Thermal Method for Obtention of 2 to 3 Reduced Graphene Oxide Layers from Graphene Oxide. SN Appl. Sci. 2020, 2, 1450. [Google Scholar] [CrossRef]
- Han, Y.; Wang, T.; Gao, X.; Li, T.; Zhang, Q. Preparation of Thermally Reduced Graphene Oxide and the Influence of Its Reduction Temperature on the Thermal, Mechanical, Flame Retardant Performances of PS Nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 84, 336–343. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Kim, Y.B. Nitrogen-Rich Graphitic-Carbon@graphene as a Metal-Free Electrocatalyst for Oxygen Reduction Reaction. Sci. Rep. 2020, 10, 12431. [Google Scholar] [CrossRef]
- Dumont, J.H.; Martinez, U.; Artyushkova, K.; Purdy, G.M.; Dattelbaum, A.M.; Zelenay, P.; Mohite, A.; Atanassov, P.; Gupta, G. Nitrogen-Doped Graphene Oxide Electrocatalysts for the Oxygen Reduction Reaction. ACS Appl. Nano Mater. 2019, 2, 1675–1682. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Cui, L.; Zhang, Y.; Fan, K.; Li, B.; Zong, L.; Wang, L. Boosting the Oxygen Reduction Reaction Behaviour of Ru Single Atoms in Porous Carbon Nanospheres via Microscopic Coordination Environment Manipulation. Appl. Surf. Sci. 2023, 615, 156304. [Google Scholar] [CrossRef]
- Wang, G.; Wang, W.; Chen, Y.; Yan, C.; Gao, Z. Fe3C Decorated, N-Doped Porous Carbon as Cost-Effective and Efficient Catalyst for Oxygen Reduction Reaction. Appl. Surf. Sci. 2023, 610, 155456. [Google Scholar] [CrossRef]
- Higgins, D.; Zamani, P.; Yu, A.; Chen, Z. The Application of Graphene and Its Composites in Oxygen Reduction Electrocatalysis: A Perspective and Review of Recent Progress. Energy Environ. Sci. 2016, 9, 357–390. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Karuppannan, M.; Vinothkannan, M.; Ramachandran, K.; Kwon, O.J.; Yoo, D.J. Ultrafine Pt Nanoparticles Stabilized by MoS 2 /N-Doped Reduced Graphene Oxide as a Durable Electrocatalyst for Alcohol Oxidation and Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2019, 11, 12504–12515. [Google Scholar] [CrossRef]
- Iordache, M.; Oubraham, A.; Sorlei, I.-S.; Lungu, F.A.; Capris, C.; Popescu, T.; Marinoiu, A. Noble Metals Functionalized on Graphene Oxide Obtained by Different Methods—New Catalytic Materials. Nanomaterials 2023, 13, 783. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Vásárhelyi, L.; Ballai, G.; Haspel, H.; Oszkó, A.; Kukovecz, Á.; Kónya, Z. Noble-Metal-Free Iron Nitride/Nitrogen-Doped Graphene Composite for the Oxygen Reduction Reaction. ACS Omega 2019, 4, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Duraivel, M.; Hira, S.A.; Prabakar, K.; Ha, C.-S.; Joo, S.H.; Nam, K.M.; Park, K.H. Heteroatom-Doped Nanomaterials/Core–Shell Nanostructure Based Electrocatalysts for the Oxygen Reduction Reaction. J. Mater. Chem. A 2022, 10, 987–1021. [Google Scholar] [CrossRef]
- Fortunato, G.V.; De Lima, F.; Maia, G. Oxygen-Reduction Reaction Strongly Electrocatalyzed by Pt Electrodeposited onto Graphene or Graphene Nanoribbons. J. Power Sources 2016, 302, 247–258. [Google Scholar] [CrossRef]
- Nagappan, S.; Duraivel, M.; Park, N.; Prabakar, K.; Park, K.H. Implementation of Heteroatom-Doped Nanomaterial/Core–Shell Nanostructure Based Electrocatalysts for Fuel Cells and Metal-Ion/Air/Sulfur Batteries. Mater. Adv. 2022, 3, 6096–6124. [Google Scholar] [CrossRef]
- Guo, L.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Cui, L. Inherent N-Doped Honeycomb-like Carbon/Fe3O4 Composites with Versatility for Efficient Microwave Absorption and Wastewater Treatment. ACS Sustain. Chem. Eng. 2019, 7, 9237–9248. [Google Scholar] [CrossRef]
- Tao, H.; Yan, C.; Robertson, A.W.; Gao, Y.; Ding, J.; Zhang, Y.; Ma, T.; Sun, Z. N-Doping of Graphene Oxide at Low Temperature for the Oxygen Reduction Reaction. Chem. Commun. 2017, 53, 873–876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tao, H.; Liu, Y.; Yan, C.; Hong, S.; Masa, J.; Robertson, A.W.; Liu, S.; Qiu, J.; Sun, Z. Ultrasound-Assisted Nitrogen and Boron Codoping of Graphene Oxide for Efficient Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2019, 7, 3434–3442. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, X.; Buba, Y.; Wang, H.; Wang, X.; Zhao, Y. Electrocatalytic Performance of Cobalt/Nickel Nanoparticles Encapsulated by N-Doped Carbon Nanotubes toward the Oxygen Reduction Reaction. Appl. Surf. Sci. 2023, 615, 156317. [Google Scholar] [CrossRef]
- Zhang, T.; He, C.; Sun, F.; Ding, Y.; Wang, M.; Peng, L.; Wang, J.; Lin, Y. Co3O4 Nanoparticles Anchored on Nitrogen-Doped Reduced Graphene Oxide as a Multifunctional Catalyst for H2O2 Reduction, Oxygen Reduction and Evolution Reaction. Sci. Rep. 2017, 7, 43638. [Google Scholar] [CrossRef]
- Kim, H.W.; Park, H.; Roh, J.S.; Shin, J.E.; Lee, T.H.; Zhang, L.; Cho, Y.H.; Yoon, H.W.; Bukas, V.J.; Guo, J.; et al. Carbon Defect Characterization of Nitrogen-Doped Reduced Graphene Oxide Electrocatalysts for the Two-Electron Oxygen Reduction Reaction. Chem. Mater. 2019, 31, 3967–3973. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, M.; Wang, K.; Song, S. Enhanced Electrocatalytic Activity for H2O2 Production by the Oxygen Reduction Reaction: Rational Control of the Structure and Composition of Multi-Walled Carbon Nanotubes. Chin. J. Catal. 2019, 40, 523–533. [Google Scholar] [CrossRef]
- Brouzgou, A.; Song, S.; Liang, Z.-X.; Tsiakaras, P. Non-Precious Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media: Latest Achievements on Novel Carbon Materials. Catalysts 2016, 6, 159. [Google Scholar] [CrossRef]
- Ma, R.; Lin, G.; Zhou, Y.; Liu, Q.; Zhang, T.; Shan, G.; Yang, M.; Wang, J. A Review of Oxygen Reduction Mechanisms for Metal-Free Carbon-Based Electrocatalysts. npj Comput. Mater. 2019, 5, 78. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Chen, H.; Wang, Y.; Song, S. Recent Advances in Electrochemical 2e Oxygen Reduction Reaction for On-Site Hydrogen Peroxide Production and Beyond. Chem. Commun. 2020, 56, 12109–12121. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.H.; Liu, Y.; Liu, M.; Wong, C. ping 3D Nitrogen-Doped Graphene Prepared by Pyrolysis of Graphene Oxide with Polypyrrole for Electrocatalysis of Oxygen Reduction Reaction. Nano Energy 2013, 2, 241–248. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Heo, J.; Choi, M.; Chang, J.; Ji, D.; Kang, S.W.; Hong, J. Highly Permeable Graphene Oxide/Polyelectrolytes Hybrid Thin Films for Enhanced CO2/N2 Separation Performance. Sci. Rep. 2017, 7, 456. [Google Scholar] [CrossRef]
- Ain, Q.T.; Haq, S.H.; Alshammari, A.; Al-Mutlaq, M.A.; Anjum, M.N. The Systemic Effect of PEG-NGO-Induced Oxidative Stress in Vivo in a Rodent Model. Beilstein J. Nanotechnol. 2019, 10, 901–911. [Google Scholar] [CrossRef]
- Slobodian, O.M.; Lytvyn, P.M.; Nikolenko, A.S.; Naseka, V.M.; Khyzhun, O.Y.; Vasin, A.V.; Sevostianov, S.V.; Nazarov, A.N. Low-Temperature Reduction of Graphene Oxide: Electrical Conductance and Scanning Kelvin Probe Force Microscopy. Nanoscale Res. Lett. 2018, 13, 139. [Google Scholar] [CrossRef]
- Kim, H.W.; Bukas, V.J.; Park, H.; Park, S.; Diederichsen, K.M.; Lim, J.; Cho, Y.H.; Kim, J.; Kim, W.; Han, T.H.; et al. Mechanisms of Two-Electron and Four-Electron Electrochemical Oxygen Reduction Reactions at Nitrogen-Doped Reduced Graphene Oxide. ACS Catal. 2020, 10, 852–863. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile Synthesis of Nitrogen-Doped Graphene via Pyrolysis of Graphene Oxide and Urea, and Its Electrocatalytic Activity toward the Oxygen-Reduction Reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; Cumpson, P.; Joseph, P.; Papakonstantinou, P. Oxygen Reduction Reaction by Electrochemically Reduced Graphene Oxide. Faraday Discuss. 2014, 173, 415–428. [Google Scholar] [CrossRef]
- Dave, K.; Park, K.H.; Dhayal, M. Two-Step Process for Programmable Removal of Oxygen Functionalities of Graphene Oxide: Functional, Structural and Electrical Characteristics. RSC Adv. 2015, 5, 95657–95665. [Google Scholar] [CrossRef]
- Li, X.; Wan, K.; Liu, Q.; Piao, J.; Zheng, Y.; Liang, Z. Nitrogen-Doped Ordered Mesoporous Carbon: Effect of Carbon Precursor on Oxygen Reduction Reactions. Chin. J. Catal. 2016, 37, 1562–1567. [Google Scholar] [CrossRef]
- Navaee, A.; Salimi, A. Efficient Amine Functionalization of Graphene Oxide through the Bucherer Reaction: An Extraordinary Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. RSC Adv. 2015, 5, 59874–59880. [Google Scholar] [CrossRef]
- Kumar, M.P.; Kesavan, T.; Kalita, G.; Ragupathy, P.; Narayanan, T.N.; Pattanayak, D.K. On the Large Capacitance of Nitrogen Doped Graphene Derived by a Facile Route. RSC Adv. 2014, 4, 38689–38697. [Google Scholar] [CrossRef]
- Yang, H.B.; Miao, J.; Hung, S.-F.; Chen, J.; Tao, H.B.; Wang, X.; Zhang, L.; Chen, R.; Gao, J.; Chen, H.M.; et al. Identification of Catalytic Sites for Oxygen Reduction and Oxygen Evolution in N-Doped Graphene Materials: Development of Highly Efficient Metal-Free Bifunctional Electrocatalyst. Sci. Adv. 2016, 2, e1501122. [Google Scholar] [CrossRef]
- Tian, G.-L.; Zhao, M.-Q.; Yu, D.; Kong, X.-Y.; Huang, J.-Q.; Zhang, Q.; Wei, F. Nitrogen-Doped Graphene/Carbon Nanotube Hybrids: In Situ Formation on Bifunctional Catalysts and Their Superior Electrocatalytic Activity for Oxygen Evolution/Reduction Reaction. Small 2014, 10, 2251–2259. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.-X.; Gao, X.; Goldsmith, B.R.; Cong, Y.; Zhai, Z.; Miao, S.; Jiang, Q.; Dou, Y.; Wang, J.; et al. Nitrogen-Doped Graphene Layers for Electrochemical Oxygen Reduction Reaction Boosted by Lattice Strain. J. Catal. 2019, 378, 113–120. [Google Scholar] [CrossRef]
- Bang, G.S.; Shim, G.W.; Shin, G.H.; Jung, D.Y.; Park, H.; Hong, W.G.; Choi, J.; Lee, J.; Choi, S.-Y. Pyridinic-N-Doped Graphene Paper from Perforated Graphene Oxide for Efficient Oxygen Reduction. ACS Omega 2018, 3, 5522–5530. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Ding, Y.; Xue, Y.; Li, F.; Chen, P.; Chen, Y. 3D Nitrogen-Doped Graphene Aerogels as Efficient Electrocatalyst for the Oxygen Reduction Reaction. Carbon N. Y. 2018, 139, 137–144. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; Yang, Z.; Wang, N.; Tu, Z.; Yi, Y.; Huang, C.; Jiang, L.; Zhang, M.; He, J.; et al. Nitrogen-Doped Porous Graphdiyne: A Highly Efficient Metal-Free Electrocatalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2017, 9, 29744–29752. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.-F.; Chen, X.; Li, B.-Q.; Hou, T.-Z.; Zhang, B.; Zhang, Q.; Titirici, M.-M.; Wei, F. Topological Defects in Metal-Free Nanocarbon for Oxygen Electrocatalysis. Adv. Mater. 2016, 28, 6845–6851. [Google Scholar] [CrossRef]
- Men, B.; Sun, Y.; Li, M.; Hu, C.; Zhang, M.; Wang, L.; Tang, Y.; Chen, Y.; Wan, P.; Pan, J. Hierarchical Metal-Free Nitrogen-Doped Porous Graphene/Carbon Composites as an Efficient Oxygen Reduction Reaction Catalyst. ACS Appl. Mater. Interfaces 2016, 8, 1415–1423. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagappan, S.; Duraivel, M.; Han, S.; Yusuf, M.; Mahadadalkar, M.; Park, K.; Dhakshinamoorthy, A.; Prabakar, K.; Park, S.; Ha, C.-S.; et al. Electrocatalytic Oxygen Reduction Reaction of Graphene Oxide and Metal-Free Graphene in an Alkaline Medium. Nanomaterials 2023, 13, 1315. https://doi.org/10.3390/nano13081315
Nagappan S, Duraivel M, Han S, Yusuf M, Mahadadalkar M, Park K, Dhakshinamoorthy A, Prabakar K, Park S, Ha C-S, et al. Electrocatalytic Oxygen Reduction Reaction of Graphene Oxide and Metal-Free Graphene in an Alkaline Medium. Nanomaterials. 2023; 13(8):1315. https://doi.org/10.3390/nano13081315
Chicago/Turabian StyleNagappan, Saravanan, Malarkodi Duraivel, SeongHoon Han, Mohammad Yusuf, Manjiri Mahadadalkar, KyeongMun Park, Amarajothi Dhakshinamoorthy, Kandasamy Prabakar, Sungkyun Park, Chang-Sik Ha, and et al. 2023. "Electrocatalytic Oxygen Reduction Reaction of Graphene Oxide and Metal-Free Graphene in an Alkaline Medium" Nanomaterials 13, no. 8: 1315. https://doi.org/10.3390/nano13081315
APA StyleNagappan, S., Duraivel, M., Han, S., Yusuf, M., Mahadadalkar, M., Park, K., Dhakshinamoorthy, A., Prabakar, K., Park, S., Ha, C.-S., Lee, J.-M., & Park, K. H. (2023). Electrocatalytic Oxygen Reduction Reaction of Graphene Oxide and Metal-Free Graphene in an Alkaline Medium. Nanomaterials, 13(8), 1315. https://doi.org/10.3390/nano13081315










