Several important components of the epidemiology of KKV infection of wrinkle-lipped free-tailed bat inhabiting Kaeng Khoi cave were studied during 1973–1974. The data presented support the natural circulation of KKV in free-tailed bats at a high prevalence, driven by the integration of immunologically-naïve juvenile bats into the population after maternal antibody protection waned. Importantly, infection of these bats with KKV did not appear to be benign; isolation of KKV was only from moribund or dead bats, and experimentally-infected bats developed pathogenic lesions in the liver and lungs. Virus isolations of KKV were also only made from dead wrinkle-lipped free-tailed bats in Cambodia, with no isolations from apparently healthy bats [
7].
4.1. Recruitment of Susceptible Bats
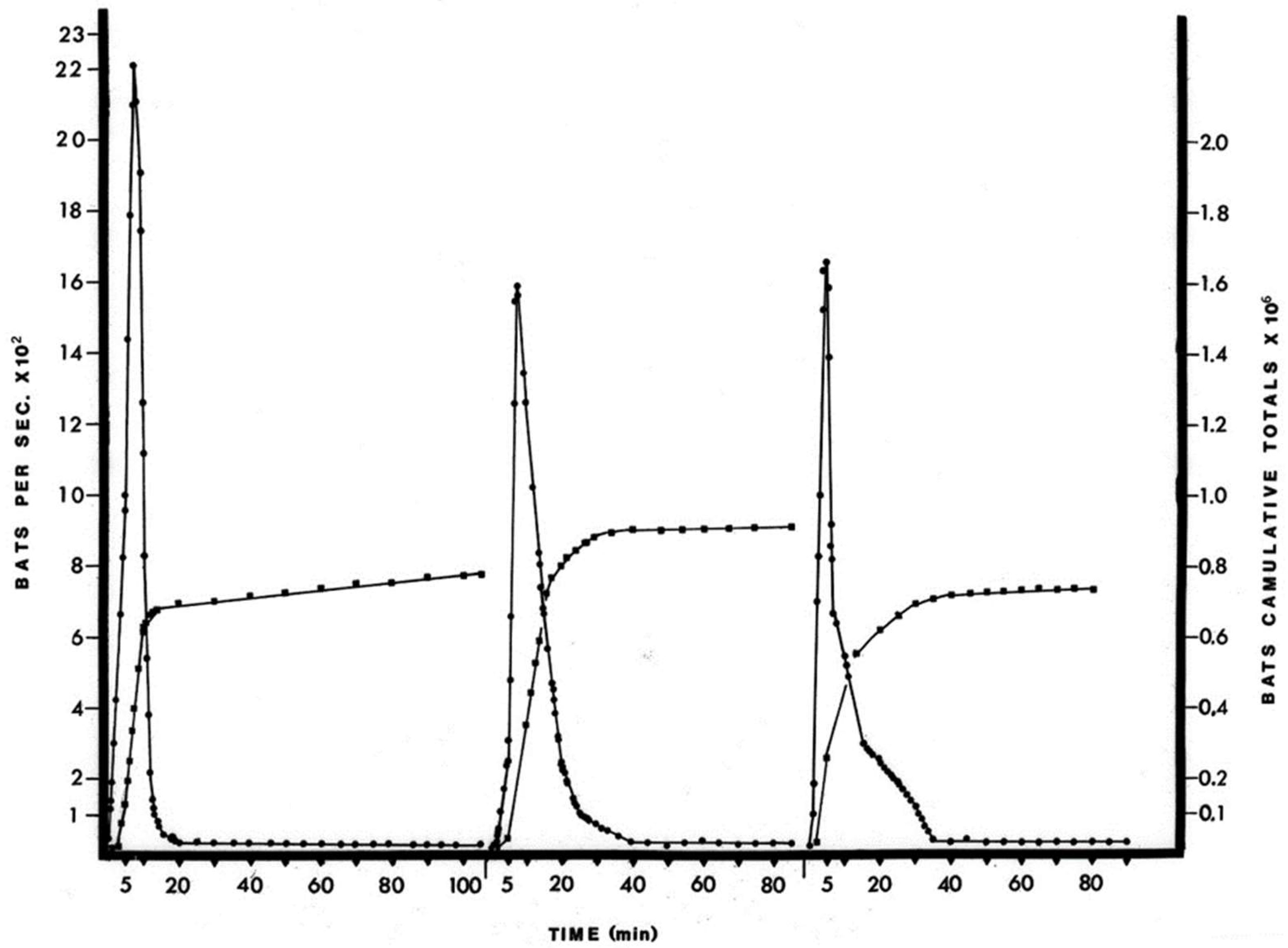
The April birthing period was estimated to be about 900,000 bats or an approximate doubling of the cave population. Although the accuracy of the estimates is difficult to assess without knowing the true population values, the method did record a doubling of the population in June, when these juvenile bats would have become volant (
Figure S5). Alternative methods [
10,
18] for the estimation of large bat populations, i.e., by roost density, exodus trapping and recapture ratio, are problematic because they disrupt either the exodus flow or the roosting bats themselves. The photographic procedure simply records the natural exodus flight, but limitations include not being able to accurately differentiate bat species or age from the photographs. The free-tailed bat population returned to previous levels within two weeks of the initial June foraging flights (
Figure S5). This increase in numbers of bats and the subsequent decrease in number of bats in the cave population represents recruitment through birth and then the dispersal of bats over a wide area and is probably representative over the species home range.
Serological results of the sampled newborns showed that 84% (approximately 700,000 of 900,000 recruited newborns) of pups had neutralizing antibodies to KKV (
Table 2). These antibodies were likely of maternal origin, as this antibody prevalence matched that of the lactating female bats and was detected within days of birth (
Table 2). The sampled newborn bats were too young to have acquired antibodies from an outside infection, and, in addition, attempts to isolate virus from them were consistently negative. These antibodies were most likely transmitted via the placenta, since these bats have a hemochorial placenta which permits antibody passage [
16]. Neutralizing antibodies were not found in the milk of ten seropositive lactating females (data not shown).
The high antibody prevalence rate in the newborn bats may be expected to protect the seropositive young from systemic KKV infection. The length of time maternal antibodies would protect the newborns would depend upon the half-life of the IgG immunoglobulin of bats, the initial titer of the antibodies and perhaps the dosage of KKV that the bats are exposed to in the cave. It is not known at what time after birth the bat would become susceptible to KKV infection. The cohort of baby bats born in mid-April maintained a high antibody prevalence of 77% and 71% at 32–39 and 73–80 days of age, respectively (
Table 2), but with some decrease in titer. This attrition suggests that maternal antibodies were still present at the time of weaning in late May and early June, about 6–7 weeks after birth, and that the majority of bats in the mid-April birth cohort would probably become susceptible to KKV over the next few months (July, August and September). Thus, of the 900,000 bats born in April, over 100,000 seronegative individuals were susceptible to KKV infection at birth and the remainder would provide the susceptible population over a period of several months. Transfer of maternal-derived (MDA) antibodies from female bats to pups, and the persistence of these antibodies has been documented in several other disease systems. Epstein et al. monitored the half-life and maintenance of MDA in both captive
Pteropus hypomelanus bats vaccinated against canine distemper virus (CDV), and Hendra virus antibodies in wild-caught
P. alecto bats [
19]. In both cases, transferred maternal immunity lasted approximately 7.5–8.5 months, with an antibody half-life of 96 days for CDV and 52 days for Hendra. Maternal antibody protection of African straw-colored fruit bats (
Eidolon helvum) exposed to Lagos bat virus or African henipa-virus was about six months, with a low point in seroprevalence at 11–12 months [
20]. The waning of MDA in large bat populations with synchronous birthing is known to contribute to the dynamics of viral transmission and maintenance, as pulses of immunologically-naïve individuals enter the population at once [
20,
21,
22,
23]. Wrinkle-lipped free-tailed bats have two birthing periods per year, an ecological attribute important to the maintenance of filoviruses [
21,
23]. Marburg virus infection rates peaked in older juvenile Egyptian rousette bats in Uganda during the birthing period when the next cohort was born, presumably due to the waning of MDA in those older juveniles [
23].
The antibody prevalence rates of the adult bats sampled from March 1973 through September 1973 are difficult to interpret because reliable methods are not available for the estimation of the age of the free-tailed bats older than about four months. Further, the movement patterns of these bats among caves and other roosting areas are unknown, so it is difficult to appreciate whether or not the samples taken at different times of year represent the same group of individuals. However, it is likely that the presence of the reproductively active females accounted for the high antibody prevalence observed in March, April and May and that the low antibody prevalence rates found in August and September reflect the loss of maternal immunity by the cohort of bats born in mid-April (
Table 3).
4.2. Kaeng Khoi Virus Infection in the Bat
Of the 136 bats initially included in the three experimental groups (
Table 1), 35 bats were studied sufficiently and are discussed in the “Results” Section. The difficulties in conducting the experiment were as follows: (i) the histories and physical condition of the bats were unknown; (ii) many of the bats had naturally acquired antibodies—the bats were acquired from a KKV endemic region and the immune status of some bats was not known at the time of inoculation; (iii) there was considerable nonspecific mortality due to handling and captivity. In retrospect, most of these difficulties could have been avoided, if the sera of the captured bats had been first examined for antibodies and the infection attempted in antibody-negative bats which had survived the first week of captivity. Still, pathology data are presented in terms of the immunological status of the bats, from which some differences were observed (
Table 9). The presence of pre-existing antibodies from KKV exposure did appear to be protective against subsequent infection. By comparison, infection-induced antibody titers decayed much more slowly than MDA, and were highly variable in African straw-colored fruit bats [
22].
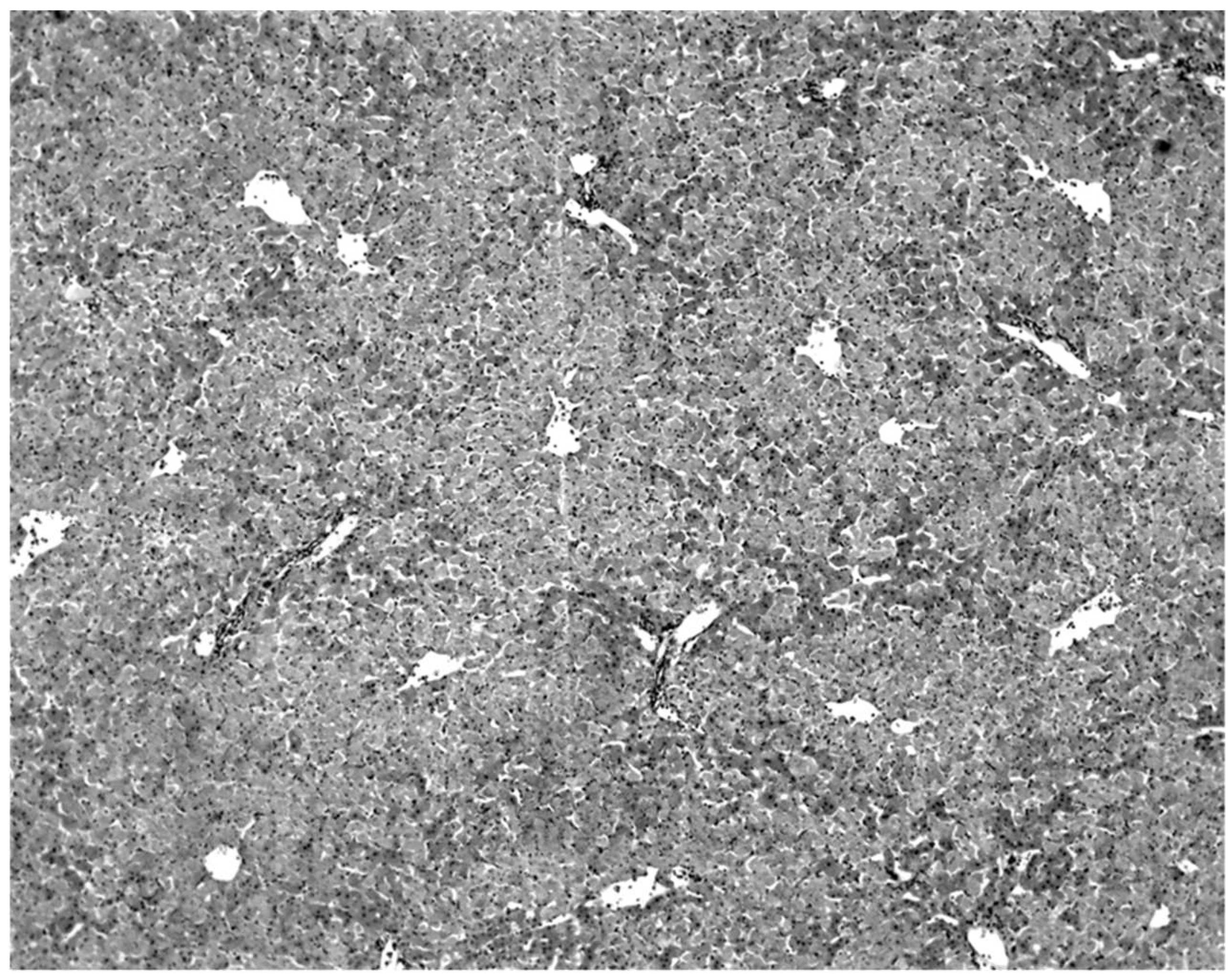
The only characteristic lesion found in naturally infected moribund bats was an acute massive destruction of the liver parenchymal cells. All of the moribund bats found positive for KKV had hepatic lesions which were well advanced and were probably responsible for the mortality and morbidity in the infected bat (
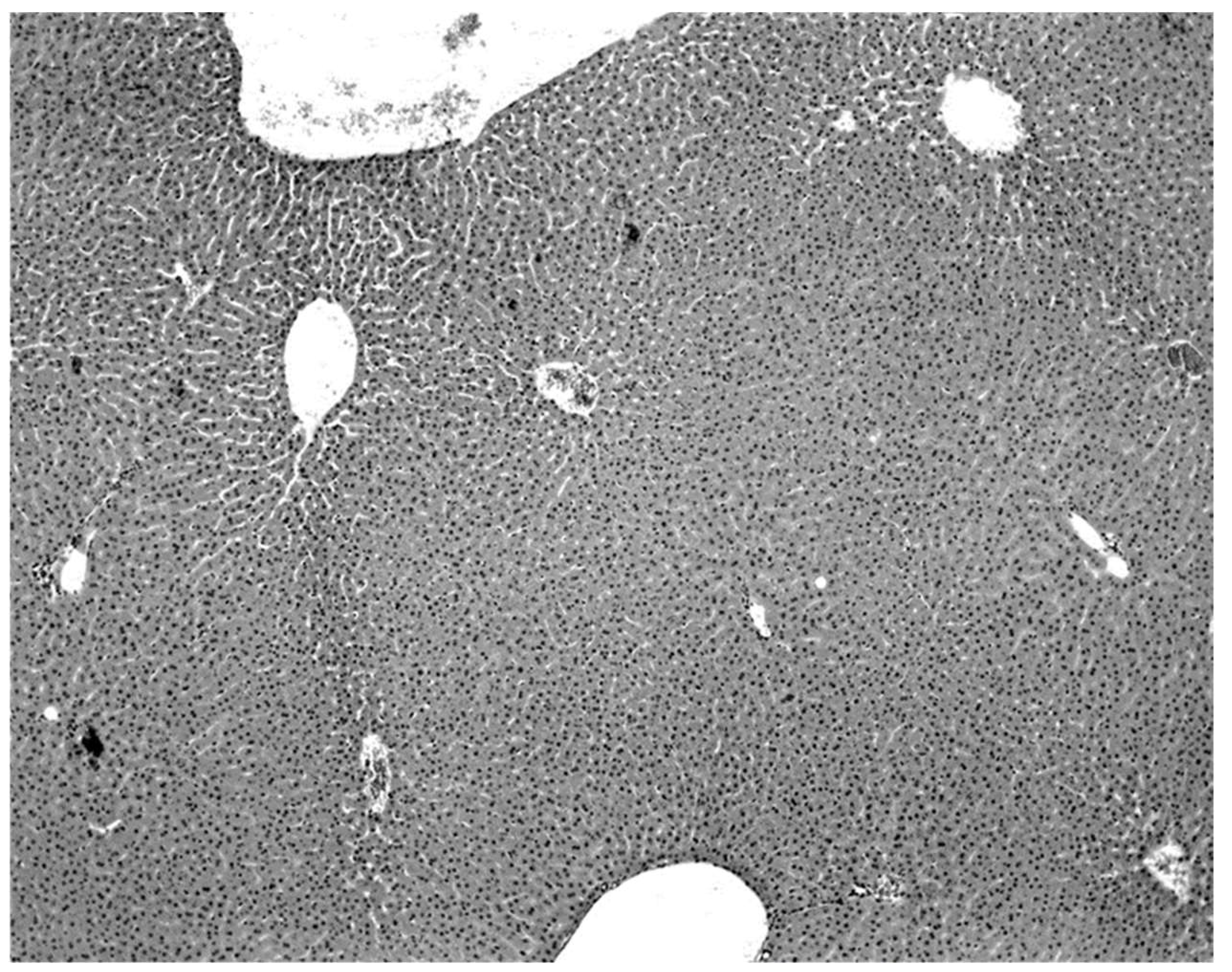
Figure 2). Livers of KKV-negative morbid bats were essentially normal (
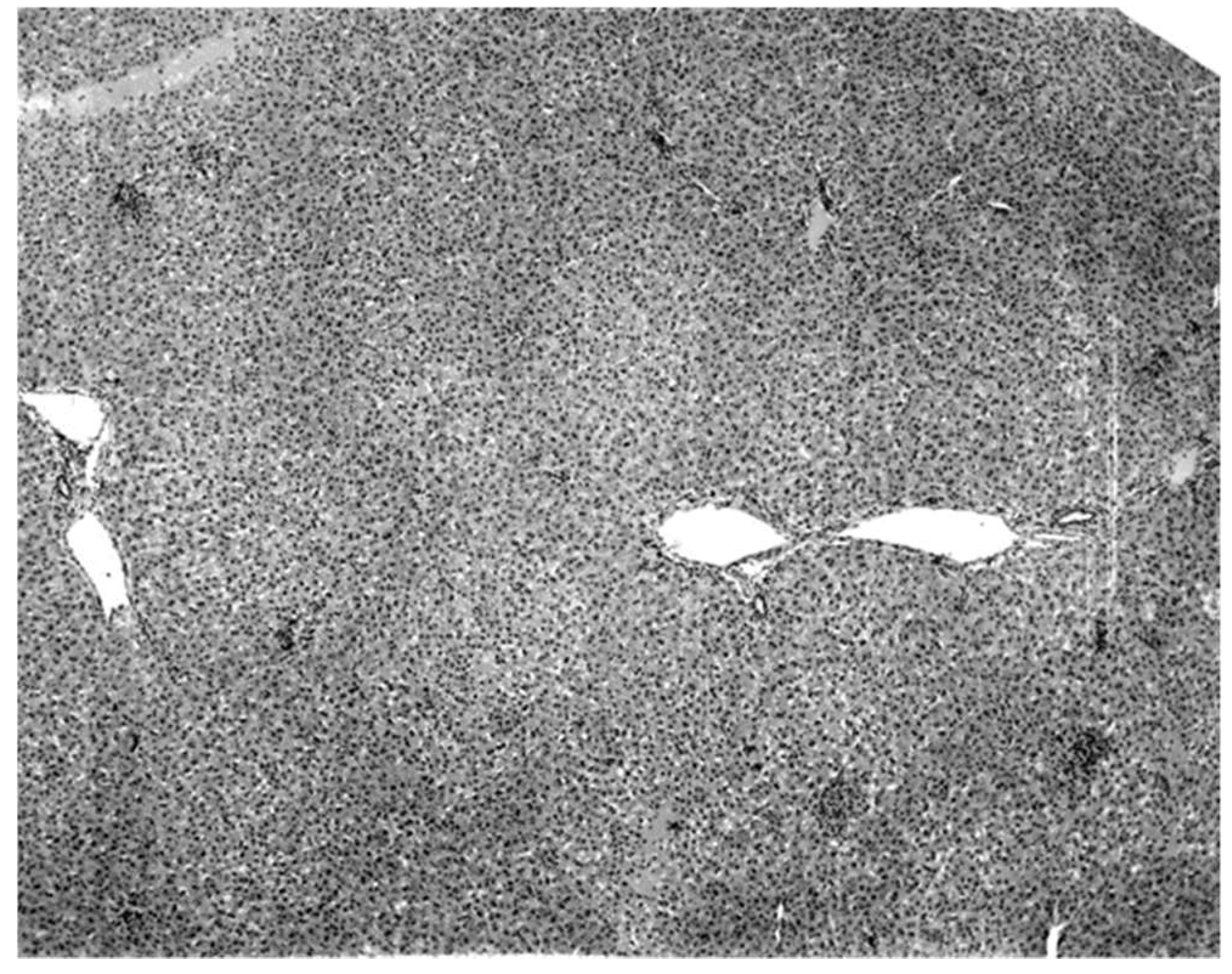
Figure 3). Suckling mice inoculated with KKV also died with a massive destruction of their liver parenchymal cells (data not shown). Furthermore, experimental infection produced an acute hepatitis in the free-tailed bat (
Figure 4). These virus isolation and pathology data show that KKV is hepatotropic and seems to produce hepatitis in the free-tailed bat that may lead to the death (
Table 4,
Table 6 and
Table 9;
Figure 2 and
Figure 4). However, a major limitation of this analysis is that immunohistochemical localization of viral antigen in the affected tissues was not performed to associate the pathology directly with the presence of KKV in the same sample. Hepatitis is characterized by perivascular infiltrates as seen in
Figure 2, but it is unclear what cell types are involved in this case. Viruses from multiple families within the order
Bunyavirales characteristically produce liver destruction, including many viruses in the family
Peribunyaviridae: Caraparu (group C) [
24], Patois [
25], Guama [
26] Oropouche [
27];
Phleboviridae: Rift Valley fever virus [
28,
29,
30];
Nairoviridae: Crimean Congo hemorrhagic fever virus [
29] and
Hantaviridae: hemorrhagic fever with renal syndrome (hantaviruses) [
29].
The high isolation rate of virus from sick and dead bats and the lack of isolation from healthy bats, together with the severe hepatic lesions in the sick bats, strongly indicate that KKV infection produces mortality and that it may be responsible for as many as 50–75% of the deaths occurring in the cave (
Table 4). These observations were made during March 1973 and January–April 1974, but they are probably representative of what occurs throughout the year. February–April also represents the dry season, in which food is less available and energetic stresses were noted among bats in this species in Cambodia [
11]. The relationship between reproductive phenology, virus shedding, and energetic reserves over the wet and dry seasons should be further investigated. Patterns and duration of viral shedding by bats over time are also not yet known.
In 1969 an attempt was made to estimate the number of bats dying in the cave. All dead bats found on the floor of the cave were collected three times a day, for one week of each month, for twelve months. The highest number of daily deaths for any one month did not exceed 30 bats, giving a maximal estimate of annual deaths in the cave of about 11,000, or between 1–2% of the population in the cave. This estimate seems to be very low, and suggests that a large majority of deaths are occurring outside the cave. However, the age structure and movement patterns of the bat population in the cave during this period of time were not known and, therefore, this estimate only indicates the number of deaths occurring in the cave and cannot be interpreted as a mortality rate or to indicate what proportion of this mortality outside the cave is due to KKV. The significance of mortality due to KKV on the bat population warrants further investigation.
Although there is no good basis to calculate the mortality rate in bats infected with KKV, the large numbers of bats with immunity and the high proportion of newborns with maternal immunity suggest that the mortality due to this infection may be lower than indicated by the isolation rates from dead and moribund bats.