Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies
Abstract
1. Introduction
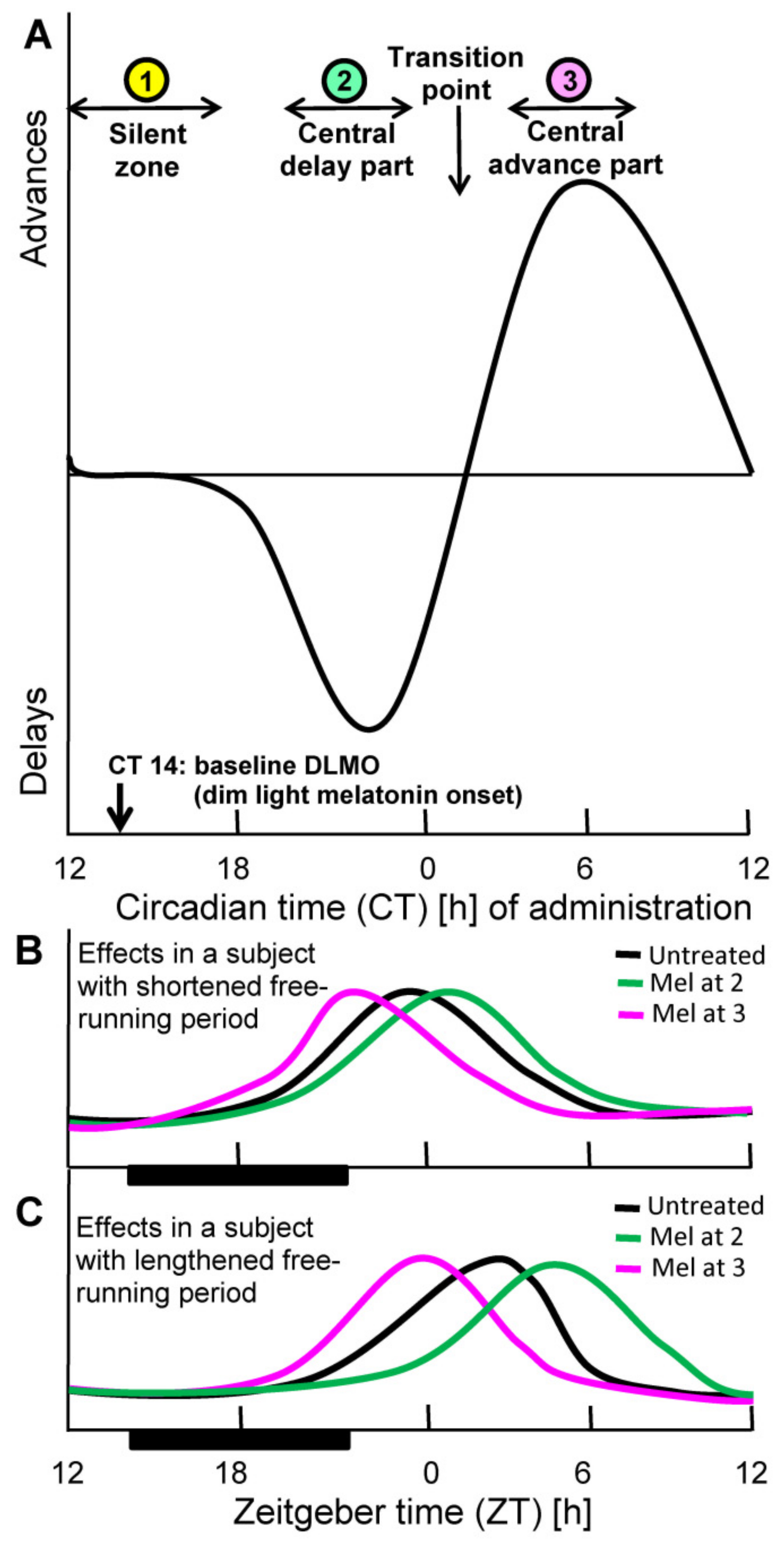
2. Basis of Chronobiotic Treatment: Receptor Affinity, Pharmacokinetics, and Phase Response Curve
3. Tissue and Organellar Melatonin
4. Contrasting Judgments on the Tolerability of Melatonin by Agencies and Researchers
5. The Rationale for Chronobiotic and Nonchronobiotic Treatments
6. Conclusions
Funding
Conflicts of Interest
References
- Hardeland, R.; Coto-Montes, A.; Poeggeler, B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol. Int. 2003, 20, 921–962. [Google Scholar] [CrossRef] [PubMed]
- Acuña-Castroviejo, D.; Rahim, I.; Acuña-Fernández, C.; Fernández-Ortiz, M.; Solera-Marín, J.; Sayed, R.K.A.; Díaz-Casado, M.E.; Rusanova, I.; López, L.C.; Escames, G. Melatonin, clock genes and mitochondria in sepsis. Cell. Mol. Life Sci. 2017, 74, 3965–3987. [Google Scholar] [CrossRef]
- Quay, W.B. Circadian and estrous rhythms in pineal melatonin and 5-hydroxy indole-3-acetic acid. Proc. Soc. Exp. Biol. Med. 1964, 115, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J. Normal patterns of melatonin levels in the pineal gland and body fluids of humans and experimental animals. J. Neural Transm. Suppl. 1986, 21, 35–54. [Google Scholar]
- Reiter, R.J. The melatonin rhythm: Both a clock and a calendar. Experientia 1993, 49, 654–664. [Google Scholar] [CrossRef]
- Bubenik, G.A.; Konturek, S.J. Melatonin and aging: Prospects for human treatment. J. Physiol. Pharmacol. 2011, 62, 13–19. [Google Scholar]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Perreau-Lenz, S.; Kalsbeek, A.; Garidou, M.L.; Wortel, J.; van der Vliet, J.; van Heijningen, C.; Simonneaux, V.; Pévet, P.; Buijs, R.M. Suprachiasmatic control of melatonin synthesis in rats: Inhibitory and stimulatory mechanisms. Eur. J. Neurosci. 2003, 17, 221–228. [Google Scholar] [CrossRef]
- Hastings, M.H.; Duffield, G.E.; Ebling, F.J.; Kidd, A.; Maywood, E.S.; Schurov, I. Non-photic signalling in the suprachiasmatic nucleus. Biol. Cell 1997, 89, 495–503. [Google Scholar] [CrossRef]
- Illnerová, H.; Trávnícková, Z.; Jác, M.; Sumová, A. Comparison of the pineal and SCN rhythmicity. Effect of photic and non-photic stimuli, photoperiod, and age. Adv. Exp Med. Biol. 1999, 460, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin, hormone of darkness and more: Occurrence, control mechanisms, actions and bioactive metabolites. Cell. Mol. Life Sci. 2008, 65, 2001–2018. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, H.; Locatelli, A.; Chemineau, P.; Malpaux, B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 2002, 143, 84–90. [Google Scholar] [CrossRef]
- Tricoire, H.; Malpaux, B.; Møller, M. Cellular lining of the sheep pineal recess studied by light-, transmission-, and scanning electron microscopy: Morphologic indications for a direct secretion of melatonin from the pineal gland to the cerebrospinal fluid. J. Comp. Neurol. 2003, 456, 39–47. [Google Scholar] [CrossRef]
- Tricoire, H.; Møller, M.; Chemineau, P.; Malpaux, B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod. Suppl. 2003, 61, 311–321. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: The chemical expression of darkness. Mol. Cell. Endocrinol. 1991, 79, C153–C158. [Google Scholar] [CrossRef]
- Erren, T.C.; Reiter, R.J. Melatonin: A universal time messenger. Neuro Endocrinol. Lett. 2015, 36, 187–192. [Google Scholar]
- Hardeland, R.; Madrid, J.A; Tan, D.-X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef] [PubMed]
- Pévet, P. The internal time-giver role of melatonin. A key for our health. Rev. Neurol. 2014, 170, 646–652. [Google Scholar] [CrossRef]
- Lewy, A.J.; Ahmed, S.; Jackson, J.M.; Sack, R.L. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int. 1992, 9, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Arendt, J.; Skene, D.J. Melatonin as a chronobiotic. Sleep Med. Rev. 2005, 9, 25–39. [Google Scholar] [CrossRef]
- Pfeffer, M.; Korf, H.W.; Wicht, H. Synchronizing effects of melatonin on diurnal and circadian rhythms. Gen. Comp. Endocrinol. 2018, 258, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonergic sleep promotion: Fundamental chronobiological issues concerning sleep onset and maintenance, dose and duration of action. Sleep Vigil. 2018, 2, 5–11. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H.C. Delivery of pineal melatonin to the brain and SCN: Role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J. CSF generation by pineal gland results in a robust melatonin circadian rhythm in the third ventricle as an unique light/dark signal. Med. Hypotheses 2016, 86, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin―a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.-X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Huether, G. The contribution of extrapineal sites of melatonin synthesis to circulating melatonin levels in higher vertebrates. Experientia 1993, 49, 665–670. [Google Scholar] [CrossRef]
- Bubenik, G.A. Gastrointestinal melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Konturek, S.J.; Konturek, P.C.; Brzozowski, T.; Bubenik, G.A. Role of melatonin in upper gastrointestinal tract. J. Physiol. Pharmacol. 2007, 58 (Suppl. 6), 23–52. [Google Scholar]
- Hardeland, R.; Poeggeler, B. Melatonin beyond its classical functions. Open Physiol. J. 2008, 1, 1–23. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, E7997–E8006. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185. [Google Scholar] [CrossRef]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.A.; Gusella, J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 8734–8738. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Rivera-Bermúdez, M.A.; Gerdin, M.J.; Masana, M.I. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003, 8, d1093–d1108. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin: Signaling mechanisms of a pleiotropic agent. Biofactors 2009, 35, 183–192. [Google Scholar] [CrossRef]
- Kato, K.; Hirai, K.; Nishiyama, K.; Uchikawa, O.; Fukatsu, K.; Ohkawa, S.; Kawamata, Y.; Hinuma, S.; Miyamoto, M. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 2005, 48, 301–310. [Google Scholar] [CrossRef]
- Hardeland, R. Investigational melatonin receptor agonists. Expert Opin. Investig. Drugs 2010, 19, 747–764. [Google Scholar] [CrossRef]
- Claustrat, B.; Brun, J.; Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005, 9, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Srinivasan, V.; Brzezinski, A.; Brown, G.M. Melatonin and its analogs in insomnia and depression. J. Pineal Res. 2012, 52, 365–375. [Google Scholar] [CrossRef]
- Andersen, L.P.H.; Werner, M.U.; Rosenkilde, M.M.; Harpsøe, N.G.; Fuglsang, H.; Rosenberg, J.; Gögenur, I. Pharmacokinetics of oral and intravenous melatonin in healthy volunteers. BMC Pharmacol. Toxicol. 2016, 17, 8. [Google Scholar] [CrossRef]
- Zetner, D.; Andersen, L.P.K.; Alder, R.; Jessen, M.L.; Tolstrup, A.; Rosenberg, J. Pharmacokinetics and safety of intravenous, intravesical, rectal, transdermal, and vaginal melatonin in healthy female volunteers: A cross-over study. Pharmacology 2020, 16, 1–8. [Google Scholar] [CrossRef]
- Nosjean, O.; Ferro, M.; Cogé, F.; Beauverger, P.; Henlin, J.M.; Lefoulon, F.; Fauchère, J.L.; Delagrange, P.; Canet, E.; Boutin, J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000, 275, 31311–31317. [Google Scholar] [CrossRef]
- Nosjean, O.; Nicolas, J.P.; Klupsch, F.; Delagrange, P.; Canet, E.; Boutin, J.A. Comparative pharmacological studies of melatonin receptors: MT1, MT2 and MT3/QR2. Tissue distribution of MT3/QR2. Biochem. Pharmacol. 2001, 61, 1369–1379. [Google Scholar] [CrossRef]
- Mailliet, F.; Ferry, G.; Vella, F.; Berger, S.; Cogé, F.; Chomarat, P.; Mallet, C.; Guenin, S.P.; Guillaumet, G.; Viaud-Massuard, M.C.; et al. Characterization of the melatoninergic MT3 binding site on the NRH:quinone oxidoreductase 2 enzyme. Biochem. Pharmacol. 2005, 71, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.K.; Takeda, Y.; Janjetovic, Z.; Brozyna, A.A.; Skobowiat, C.; Wang, J.; Postlethwaite, A.; Li, W.; Tuckey, R.C.; et al. RORα and RORγ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 2014, 28, 2775–2789. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Zmijewski, M.A.; Jetten, A.M. RORα is not a receptor for melatonin (response to DOI 10.1002/bies.201600018). Bioessays 2016, 38, 1193–1194. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and retinoid orphan receptors: Demand for new interpretations after their exclusion as nuclear melatonin receptors. Melatonin Res. 2018, 1, 77–92. [Google Scholar] [CrossRef]
- Macías, M.; Escames, G.; León, J.; Coto, A.; Sbihi, Y.; Osuna, A.; Acuña-Castroviejo, D. Calreticulin ― melatonin. An unexpected relationship. Eur. J. Biochem. 2003, 270, 832–840. [Google Scholar] [CrossRef]
- Cardinali, D.P.; Freire, F. Melatonin effects on brain. Interaction with microtubule protein, inhibition of fast axoplasmic flow and induction of crystaloid and tubular formations in the hypothalamus. Mol. Cell. Endocrinol. 1975, 2, 317–330. [Google Scholar] [CrossRef]
- Cardinali, D.P. Molecular biology of melatonin: Assessment of the “microtubule hypothesis of melatonin action”. In Melatonin: Current Status and Perspectives; Birau, N., Schlott, W., Eds.; Pergamon: London, UK, 1980; pp. 247–256. [Google Scholar]
- Benítez-King, G.; Antón-Tay, F. Calmodulin mediates melatonin cytoskeletal effects. Experientia 1993, 49, 635–641. [Google Scholar] [CrossRef]
- Landau, M.; Zisapel, N. The low affinity binding of melatonin to calmodulin: Use of computational methods to explain its physiological relevance. In Melatonin—From Molecules to Therapy; Pandi-Perumal, S.R., Cardinali, D.P., Eds.; Nova Science: New York, NY, USA, 2007; pp. 69–79. [Google Scholar]
- Hack, L.M.; Lockley, S.W.; Arendt, J.; Skene, D.J. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J. Biol. Rhythms 2003, 18, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Emens, J.S.; Lefler, B.J.; Yuhas, K.; Jackman, A.R. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol. Int. 2005, 22, 1093–1106. [Google Scholar] [CrossRef]
- Burgess, H.J.; Revell, V.L.; Eastman, C.I. A three pulse phase response curve to three milligrams of melatonin in humans. J. Physiol. 2008, 586, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, R.S.; Trakht, I.; Spence, D.W.; Hardeland, R.; Poeggeler, B.; Cardinali, D.P. Pathophysiology of depression: Role of sleep and the melatonergic system. Psychiatry Res. 2009, 165, 201–214. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pandi-Perumal, S.R.; Trahkt, I.; Spence, D.W.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Melatonin and melatonergic drugs on sleep: Possible mechanisms of action. Int. J. Neurosci. 2009, 119, 821–846. [Google Scholar] [CrossRef]
- Hardeland, R. New approaches in the management of insomnia: Weighing the advantages of prolonged release melatonin and synthetic melatoninergic agonists. Neuropsychiatr. Dis. Treat. 2009, 5, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Messner, M.; Hardeland, R.; Rodenbeck, A.; Huether, G. Tissue retention and subcellular distribution of continuously infused melatonin in rats under near physiological conditions. J. Pineal Res. 1998, 25, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Lewy, A.J.; Emens, J.S.; Sack, R.L.; Hasler, B.P.; Bernert, R.A. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol. Int. 2002, 19, 649–658. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuña-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef]
- He, C.; Wang, J.; Zhang, Z.; Yang, M.; Li, Y.; Tian, X.; Ma, T.; Tao, J.; Zhu, K.; Song, Y.; et al. Mitochondria synthesize melatonin to ameliorate its function and improve mice oocyte’s quality under in vitro conditions. Int. J. Mol. Sci. 2016, 17, 939. [Google Scholar] [CrossRef]
- Quintela, T.; Gonçalves, I.; Silva, M.; Duarte, A.C.; Guedes, P.; Andrade, K.; Freitas, F.; Talhada, D.; Albuquerque, T.; Tavares, S.; et al. Choroid plexus is an additional source of melatonin in the brain. J. Pineal Res. 2018, 65, e12528. [Google Scholar] [CrossRef]
- Slominski, A.T.; Zmijewski, M.A.; Semak, I.; Kim, T.K.; Janjetovic, Z.; Slominski, R.M.; Zmijewski, J.W. Melatonin, mitochondria, and the skin. Cell. Mol. Life Sci. 2017, 74, 3913–3925. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed]
- Venegas, C.; Garcia, J.A.; Escames, G.; Ortiz, F.; Lopez, A.; Doerrier, C.; Garcia-Corzo, L.; Lopez, L.C.; Reiter, R.J.; Acuna-Castroviejo, D. Extrapineal melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules 2020, 25, 4410. [Google Scholar] [CrossRef]
- Costa, E.J.X.; Lopes, R.H.; Lamy-Freund, M.T. Permeability of pure lipid bilayers to melatonin. J. Pineal Res. 1995, 19, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.J.X.; Shida, C.S.; Biaggi, M.H.; Ito, A.S.; Lamy-Freund, M.T. How melatonin interacts with lipid bilayers: A study by fluorescence and ESR spectroscopies. FEBS Lett. 1997, 416, 103–106. [Google Scholar] [CrossRef]
- Hevia, D.; Gonzalez-Menendez, P.; Quiros-Gonzales, I.; Miar, A.; Rodriguez-Garcia, A.; Tan, D.-X.; Reiter, R.J.; Mayo, J.C.; Sainz, R.M. Melatonin uptake through glucose transporters: A new target for melatonin inhibition of cancer. J. Pineal Res. 2015, 58, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; Gonzalez-Menendez, P.; Hevia, D.; Cermuda-Cermuda, R. Melatonin transport into mitochondria. Cell. Mol. Life Sci. 2017, 74, 3927–3940. [Google Scholar] [CrossRef]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human transporters, PEPT 1/2, facilitate melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res. 2017, 62, e12390. [Google Scholar] [CrossRef]
- Ahluwalia, A.; Brzozowska, I.M.; Hoa, N.; Jones, M.K.; Tarnawski, A.S. Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. USA 2018, 115, E1942–E1943. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and the electron transport chain. Cell. Mol. Life Sci. 2017, 74, 3883–3896. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.A.; Sayeed, I.; Siemen, D.; Wolf, G.; Horn, T.F. Direct inhibition of the mitochondrial permeability transition pore: A possible mechanism responsible for anti-apoptotic effects of melatonin. FASEB J. 2004, 18, 869–871. [Google Scholar] [CrossRef]
- Jou, M.J.; Peng, T.I.; Hsu, L.F.; Jou, S.B.; Reiter, R.J.; Yang, C.M.; Chiao, C.C.; Lin, Y.F.; Chen, C.C. Visualization of melatonin’s multiple mitochondrial levels of protection against mitochondrial Ca2+-mediated permeability transition and beyond in rat brain astrocytes. J. Pineal Res. 2010, 48, 20–38. [Google Scholar] [CrossRef]
- Jou, M.J. Melatonin preserves the transient mitochondrial permeability transition for protection during mitochondrial Ca2+ stress in astrocyte. J. Pineal Res. 2011, 50, 427–435. [Google Scholar] [CrossRef]
- Tan, D.-X.; Chen, L.-D.; Poeggeler, B.; Manchester, L.C.; Reiter, R.J. Melatonin: A potent, endogenous hydroxyl radical scavenger. Endocr. J. 1993, 1, 57–60. [Google Scholar]
- Reiter, R.J.; Poeggeler, B.; Tan, D.-X.; Chen, L.-D.; Manchester, L.C.; Guerrero, J.M. Antioxidant capacity of melatonin: A novel action not requiring a receptor. Neuro Endocrinol. Lett. 1993, 15, 103–116. [Google Scholar]
- Reiter, R.J. The role of the neurohormone melatonin as a buffer against macromolecular oxidative damage. Neurochem. Int. 1995, 27, 453–460. [Google Scholar] [CrossRef]
- Tan, D.-X.; Reiter, R.J.; Manchester, L.C.; Yan, M.T.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Antioxidative protection by melatonin: Multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005, 27, 119–130. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Kilic, U.; Yilmaz, B.; Ugur, M.; Yüksel, A.; Reiter, R.J.; Hermann, D.M.; Kilic, E. Evidence that membrane-bound G protein-coupled melatonin receptors MT1 and MT2 are not involved in the neuroprotective effects of melatonin in focal cerebral ischemia. J. Pineal Res. 2012, 52, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Voordouw, B.C.; Euser, R.; Verdonk, R.E.; Alberda, B.T.; de Jong, F.H.; Drogendijk, A.C.; Fauser, B.C.; Cohen, M. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J. Clin. Endocrinol. Metab. 1992, 74, 108–117. [Google Scholar] [CrossRef]
- Silman, R.E. Melatonin: A contraceptive for the nineties. Eur. J. Obstet. Gynecol. Reprod. Biol. 1993, 49, 3–9. [Google Scholar] [CrossRef]
- Reiter, R.J. Pineal control of reproduction. Prog. Clin. Biol. Res. 1981, 59B, 349–355. [Google Scholar]
- Arendt, J.; Symons, A.M.; English, J.; Poulton, A.L.; Tobler, I. How does melatonin control seasonal reproductive cycles? Reprod. Nutr. Dev. 1988, 28, 387–397. [Google Scholar] [CrossRef]
- Malpaux, B.; Thiéry, J.C.; Chemineau, P. Melatonin and the seasonal control of reproduction. Reprod. Nutr. Dev. 1999, 39, 355–366. [Google Scholar] [CrossRef]
- Terzolo, M.; Revelli, A.; Guidetti, D.; Piovesan, A.; Cassoni, P.; Paccotti, P.; Angeli, A.; Massobrio, M. Evening administration of melatonin enhances the pulsatile secretion of prolactin but not of LH and TSH in normally cycling women. Clin. Endocrinol. 1993, 39, 185–191. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Shen-Orr, Z.; Nave, R.; Lavi, S.; Lavie, P. Melatonin administration alters semen quality in healthy men. J. Androl. 2002, 23, 572–578. [Google Scholar]
- Hardeland, R. Melatonin in aging and disease—Multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 2012, 3, 194–225. [Google Scholar]
- Hardeland, R. Agomelatine and the risk of hepatotoxicity. J. Symptoms Signs 2014, 3, 341–346. [Google Scholar]
- Pandi-Perumal, S.R.; Srinivasan, V.; Poeggeler, B.; Hardeland, R.; Cardinali, D.P. Drug insight: The use of melatonergic agonists for the treatment of insomnia – focus on ramelteon. Nat. Clin. Pract. Neurol. 2007, 3, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Pagano, E.S.; Scacchi Bernasconi, P.A.; Reynoso, R.; Scacchi, P. Disrupted chronobiology of sleep and cytoprotection in obesity: Possible therapeutic value of melatonin. Neuro Endocrinol. Lett. 2011, 32, 588–606. [Google Scholar] [PubMed]
- Dowling, G.A.; Mastick, J.; Colling, E.; Carter, J.H.; Singer, C.M.; Aminoff, M.J. Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med. 2005, 6, 459–466. [Google Scholar] [CrossRef]
- Weishaupt, J.H.; Bartels, C.; Pölking, E.; Dietrich, J.; Rohde, G.; Poeggeler, B.; Mertens, N.; Sperling, S.; Bohn, M.; Hüther, G.; et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J. Pineal Res. 2006, 41, 313–323. [Google Scholar] [CrossRef]
- Tan, D.-X.; Hardeland, R. Estimated doses of melatonin for treating deadly virus infections: Focus on COVID-19. Melatonin Res. 2020, 3, 276–296. [Google Scholar] [CrossRef]
- De Boer, A.G.; Moolenaar, F.; de Leede, L.G.; Breimer, D.D. Rectal drug administration: Clinical pharmacokinetic considerations. Clin. Pharmacokinet. 1982, 7, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Nordlund, J.J.; Lerner, A.B. The effects of oral melatonin on skin color and on the release of pituitary hormones. J. Clin. Endocrinol. Metab. 1977, 45, 768–774. [Google Scholar] [CrossRef]
- Reiter, R.J.; Abreu-Gonzalez, P.; Marik, P.E.; Dominguez-Rodriguez, A. Therapeutic algorithm for use of melatonin in patients with COVID-19. Front. Med. (Lausanne) 2020, 7, 226. [Google Scholar] [CrossRef]
- Kleszczyński, K.; Slominski, A.T.; Steinbrink, K.; Reiter, R.J. Clinical trials for use of melatonin to fight against COVID-19 are urgently needed. Nutrients 2020, 12, 2561. [Google Scholar] [CrossRef] [PubMed]
- Nickkholgh, A.; Schneider, H.; Sobirey, M.; Venetz, W.P.; Hinz, U.; Pelzl, L.H.; Gotthardt, D.N.; Cekauskas, A.; Manikas, M.; Mikalauskas, S.; et al. The use of high-dose melatonin in liver resection is safe: First clinical experience. J. Pineal Res. 2011, 50, 381–388. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; BaHammam, A.S.; Brown, G.M.; Spence, D.W.; Bharti, V.K.; Kaur, C.; Hardeland, R.; Cardinali, D.P. Melatonin antioxidative defense: Therapeutical implications for aging and neurodegenerative processes. Neurotox. Res. 2013, 23, 267–300. [Google Scholar] [CrossRef]
- Borbély, A.A.; Daan, S.; Wirz-Justice, A.; Deboer, T. The two-process model of sleep regulation: A reappraisal. J. Sleep Res. 2016, 25, 131–143. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and inflammation: Partners in sickness and in health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Haack, M. The sleep-immune crosstalk in health and disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef]
- Hardeland, R. Noncoding RNAs: Bridging regulation of circadian rhythms and inflammation. Adv. Neuroimmune Biol. 2019, 7, 155–177. [Google Scholar] [CrossRef]
- Hergenhan, S.; Holtkamp, S.; Scheiermann, C. Molecular interactions between components of the circadian clock and the immune system. J. Mol. Biol. 2020, 432, 3700–3713. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and inflammation—Story of a double-edged blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Hardeland, R. Aging, melatonin and the pro- and anti-inflammatory networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; McIntyre, R.S.; Rosenblat, J.; Hardeland, R. Depressive disorders: Processes leading to neurogeneration and potential novel treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 80 Pt C, 189–204. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62, e12377. [Google Scholar] [CrossRef] [PubMed]
- Reinberg, A.E.; Ashkenazi, I.; Smolensky, M.H. Euchronism, allochronism, and dyschronism: Is internal desynchronization of human circadian rhythms a sign of illness? Chronobiol. Int. 2007, 24, 553–588. [Google Scholar] [CrossRef]
- Baron, K.G.; Reid, K.J. Circadian misalignment and health. Int. Rev. Psychiatry 2014, 26, 139–154. [Google Scholar] [CrossRef]
- Arendt, J.; Skene, D.J.; Middleton, B.; Lockley, S.W.; Deacon, S. Efficacy of melatonin treatment in jet lag, shift work, and blindness. J. Biol. Rhythms 1997, 12, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Skene, D.J.; Lockley, S.W.; Arendt, J. Use of melatonin in the treatment of phase shift and sleep disorders. Adv. Exp. Med. Biol. 1999, 467, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.M.; Pandi-Perumal, S.R.; Trakht, I.; Cardinali, D.P. Melatonin and its relevance to jet lag. Travel Med. Infect. Dis. 2009, 7, 69–81. [Google Scholar] [CrossRef]
- Aschoff, J.; Wever, R. Human circadian rhythms: A multioscillatory system. Fed. Proc. 1976, 35, 2326–2332. [Google Scholar]
- Brzezinski, A.; Vangel, M.G.; Wurtman, R.J.; Norrie, G.; Zhdanova, I.; Ben-Shushan, A.; Ford, I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS ONE 2013, 8, e63773. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and synthetic melatoninergic agonists in psychiatric and age-associated disorders: Successful and unsuccessful approaches. Curr. Pharm. Des. 2016, 22, 1086–1101. [Google Scholar] [CrossRef]
- León, J.; Macías, M.; Escames, G.; Camacho, E.; Khaldy, H.; Martín, M.; Espinosa, A.; Gallo, M.A.; Acuña-Castroviejo, D. Structure-related inhibition of calmodulin-dependent neuronal nitric-oxide synthase activity by melatonin and synthetic kynurenines. Mol. Pharmacol. 2000, 58, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Entrena, A.; Camacho, M.E.; Carrión, M.D.; López-Cara, L.C.; Velasco, G.; León, J.; Escames, G.; Acuña-Castroviejo, D.; Tapias, V.; Gallo, M.A.; et al. Kynurenamines as neural nitric oxide synthase inhibitors. J. Med. Chem. 2005, 48, 8174–8181. [Google Scholar] [CrossRef]
- León, J.; Escames, G.; Rodríguez, M.I.; López, L.C.; Tapias, V.; Entrena, A.; Camacho, E.; Carrión, M.D.; Gallo, M.A.; Espinosa, A.; et al. Inhibition of neuronal nitric oxide synthase activity by N1-acetyl-5-methoxykynuramine, a brain metabolite of melatonin. J. Neurochem. 2006, 98, 2023–2033. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a potent agent in antioxidative defense: Actions as a natural food constituent, gastrointestinal factor, drug and prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Burkhardt, S.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Shohami, E.; Huo, Y.S.; Hardeland, R.; Reiter, R.J. N1-acetyl-N2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. FASEB J. 2001, 15, 2294–2296. [Google Scholar] [CrossRef]
- Rosen, J.; Than, N.N.; Koch, D.; Poeggeler, B.; Laatsch, H.; Hardeland, R. Interactions of melatonin and its metabolites with the ABTS cation radical: Extension of the radical scavenger cascade and formation of a novel class of oxidation products, C2-substituted 3-indolinones. J. Pineal Res. 2006, 41, 374–381. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, N.; Srinivasan, V.; Trakht, I.; Pandi-Perumal, S.R.; Cardinali, D.P. Melatonergic drugs in clinical practice. Arzneimittelforschung 2008, 58, 1–10. [Google Scholar] [CrossRef]
- Sinha, K.; Degaonkar, M.N.; Jagannathan, N.R.; Gupta, Y.K. Effect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur. J. Pharmacol. 2001, 428, 185–192. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Sainz, R.M.; Mayo, J.C. Melatonin protects the heart against both ischemia/reperfusion injury and chemotherapeutic drugs. Cardiovasc. Drugs Ther. 2002, 16, 5–6. [Google Scholar] [CrossRef]
- Pei, Z.; Pang, S.F.; Cheung, R.T. Administration of melatonin after onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke 2003, 34, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, M.Y.; Chen, H.Y.; Hsu, Y.S.; Wu, T.S.; Chen, S.T.; Chang, G.L. Melatonin attenuates gray and white matter damage in a mouse model of transient focal cerebral ischemia. J. Pineal Res. 2005, 38, 42–52. [Google Scholar] [CrossRef]
- Escames, G.; Acuña-Castroviejo, D.; López, L.C.; Tan, D.-X.; Maldonado, M.D.; Sánchez-Hidalgo, M.; León, J.; Reiter, R.J. Pharmacological utility of melatonin in the treatment of septic shock: Experimental and clinical evidence. J. Pharm. Pharmacol. 2006, 58, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Ortíz, F.; Ros, E.; Acuña-Castroviejo, D. Age-dependent lipopolysaccharide-induced iNOS expression and multiorgan failure in rats: Effects of melatonin treatment. Exp. Gerontol. 2006, 41, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Tapias, V.; Utrilla, P.; Reiter, R.J.; Hitos, A.B.; León, J.; Rodríguez, M.I.; Acuña-Castroviejo, D. Melatonin counteracts inducible mitochondrial nitric oxide synthase-dependent mitochondrial dysfunction in skeletal muscle of septic mice. J. Pineal Res. 2006, 40, 71–78. [Google Scholar] [CrossRef]
- López, L.C.; Escames, G.; Tapias, V.; Utrilla, P.; León, J.; Acuña-Castroviejo, D. Identification of an inducible nitric oxide synthase in diaphragm mitochondria from septic mice: Its relation with mitochondrial dysfunction and prevention by melatonin. Int. J. Biochem. Cell Biol. 2006, 38, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Escames, G.; López, L.C.; Ortíz, F.; López, A.; García, J.A.; Ros, E.; Acuña-Castroviejo, D. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J. 2007, 274, 2135–2147. [Google Scholar] [CrossRef]
- Koh, P.O. Melatonin prevents ischemic brain injury through activation of the mTOR/p70S6 kinase signaling pathway. Neurosci. Lett. 2008, 444, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Tan, D.-X. Protection by melatonin in respiratory diseases: Valuable information for the treatment of COVID-19. Melatonin Res. 2020, 3, 264–275. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardeland, R. Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies. Diseases 2021, 9, 18. https://doi.org/10.3390/diseases9010018
Hardeland R. Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies. Diseases. 2021; 9(1):18. https://doi.org/10.3390/diseases9010018
Chicago/Turabian StyleHardeland, Rüdiger. 2021. "Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies" Diseases 9, no. 1: 18. https://doi.org/10.3390/diseases9010018
APA StyleHardeland, R. (2021). Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies. Diseases, 9(1), 18. https://doi.org/10.3390/diseases9010018





