Abstract
Background: Diabetic peripheral neuropathy (DPN) is known to predict foot ulceration, lower-extremity amputation and mortality. Patients with diabetes mellitus have a predisposition toward developing chronic inflammatory demyelinating polyneuropathy, and this may also facilitate the formation of diabetic foot and cutaneous impairment, which are considered one of the most serious impairments of diabetes mellitus, with a prevalence of 4–10% in this population. Biomarkers research provides opportunities for the early diagnosis of these complications for specific treatments useful to prevent amputation and, therefore, physical inability and mental disturbance. The recent literature has suggested that glycemic levels may be a novel factor in the pathogenesis of diabetic foot complications and is an important mediator of axonal dysfunction. The aim of this systematic literary review is to determine whether hemoglobin A1c (HbA1c) is a positive predictor for diabetic foot peripheral neuropathy and its complications, such as foot cutaneous impairments. There is a lack of consensus regarding the effect of glycemic variability on diabetic foot peripheral neuropathy, unlike other complications such as retinopathy, nephropathy or micro/macrovascular pathology. Methods: Relevant articles were searched in the Medline database using PubMed and Scopus and relevant keywords. The primary search terms used were “glycated hemoglobin” OR “HbA1c” AND “diabetic neuropathies” AND “Foot”. Results: A number of articles (336) were initially identified while searching the scientific literature regarding this topic, and 32 articles were selected and included in this review. Conclusions: This review highlights the role of HbA1c in diabetic foot peripheral neuropathy. Biomarkers play an important role in the decision-making process, and HbA1c levels are extensively used for diabetic foot clinical outcomes and settings, but biomarker research in diabetic foot peripheral neuropathy is in its infancy and will require careful attention to a number of factors and associations, since the consequences of DPN also include neurological alterations. HbA1c is an accurate and easy-to-administer test and can be an effective biomarker in establishing the diagnosis of diabetes, but future research should focus on standardizing the HbA1c level and selecting which DPN value and its correlated complications, such as foot cutaneous impairments, are the most informative.
1. Introduction
Diabetes mellitus (DM) is considered to be a serious public health problem due to its high prevalence and related complications, among which is diabetic peripheral neuropathy (DPN). DPN is a disease often associated with neuropathic pain, foot ulceration and lower extremity amputation, which can significantly affect the quality of life of patients [1,2]. The most frequent type of neuropathy associated with diabetic foot complications is the distal symmetric sensorimotor polyneuropathy, and, along with peripheric vascular disease, it is a major contributing factor to the formation of foot ulcers. The control of the disease relies both on individual actions for self-care and on medical treatments and surveillance. A healthy, intact diabetic foot is indeed best maintained by a consistent and recurrent preventive treatment strategy accomplished through a multidisciplinary approach that encompasses instruction in glucose assessment, insulin and other diabetes medication administration; diet; daily foot inspection and care; proper footwear and the necessity for prompt treatment of new lesions. Regarding medical surveillance, a common strategy to evaluate the effectiveness of DM treatment is the use of a biomarker. By definition, a biomarker is a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention” [3]. Specifically, for the case of DM, the levels of glycated hemoglobin (HbA1c or hemoglobin A1c) are periodically measured, as glycemic variability has been recognized as the most important risk factor for DPN.
Early detection and good glycemic control be proven to prevent adverse outcomes associated with DPN, but there is a lack of consensus regarding the effect of glycemic variability on diabetic foot peripheral neuropathy alterations such as cutaneous impairment [4,5,6]. Various etiologies have been suggested to describe the pathogenesis of diabetic neuropathy and its relationship with hyperglycemia [7,8,9,10]. One example was provided, among others, by the Banting Memorial Lecture (2004), mentioning how increased cytosolic glucose causes an accelerated transformation of glucose to sorbitol by aldose reductase with the consumption of free cytosolic NADPH and production of NADP+ [11].
HbA1c provides the better measure, as it reflects levels of blood glucose over several weeks, and it is the main method of monitoring glycaemia in diabetes; for this reason, the purpose of our study was to review articles published from 2010 to 2020 in order to analyze the relation between diabetic foot peripheral neuropathy-related and HbA1c genetic markers in the literature.
1.1. Diabetic Peripheral Neuropathy
Patients with diabetes occasionally develop diabetic polyneuropathy, which is characterized by both positive symptoms such as pain and negative symptoms such as numbness/dysesthesia [12]. Diabetic peripheral neuropathy, defined as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes” is the leading cause of polyneuropathy, affecting up to 50% of older type 2 diabetic patients [13,14,15,16,17,18]. In the management of this condition, treatment centers on control of the patient’s blood sugar level as the first step. Chronic and acute sensory neuropathy are usually precipitated by an episode of glycemic instability (such as ketoacidosis), often accompanied by autonomic dysfunction with late sequelae, which includes foot ulcerations [19]. In addition, achieving stable blood glucose control is also extremely important in terms of painful symptoms management [20].
Sensory symptoms associated with the disease are extremely variable and can be divided into positive—burning, tingling, sharp, dull and/or searing feeling—and negative symptoms—numbness, dysesthesia, loss of balance, heaviness in the legs, stiffness or feelings of something bunched up on the ball or sulcus of the foot [12,21].
Neuropathic symptoms are equally variable and include spontaneous sensations (paresthesia), unpleasant sensations (dysesthesias) or hypersensitivity (hyperalgesia) to pressure or touch but, also, numbness, tingling, unsteadiness, prickling or burning pain in the legs and/or feet. Neuropathic signs were defined as reduced or absent ankle reflexes (using an appropriate reflex hammer) and reduced or absent distal sensation, including a vibration perception (using a 128-Hz tuning fork), touch sensation (using a 10-g monofilament), thermal discrimination (using cold and warm objects), pinprick sensation (using a pin) and proprioception. Patients with DPN also suffer from an altered gait strategy and present a fivefold increased risk of falling [22,23,24,25,26,27]. Moreover, DPN causes sleep interferences, mood disorders and, more in general, a poor health-related quality of life [28]. The progression of DPN leads to a loss of the protective sensation, skin ulcerations and chronic wounds. A lack of awareness and inappropriate management of DPN has led to much unnecessary lower limb amputations, despite the fact that the importance of DPN in the etiopathogenesis of foot ulcerations has been confirmed by numerous studies [29].
Neuropathy is assessed by a variety of techniques: signs are determined using a modified neuropathy disability score (NDS) derived from abnormalities of pain sensation using a Neurotip™, Achilles reflex using a tendon hammer, vibration sensation using a 128-Hz tuning fork and dorsal temperature sensation using warm and cool rods. Cutaneous perception can be detected with a simple neurologic examination of the lower extremities involving the use of a 10-g Semmes Weinstein monofilament, to test sensation, or a composite score such as a modified neuropathy disability score [30]. The Michigan Neuropathy Screening Instrument (MNSI) is a simple, sensitive and specific tool for the screening of DPN validated in many cohort and clinical trial studies [31,32,33].
Thus, all patients with a neuropathic deficit must be considered as being at risk of foot ulceration and require regular podiatric assessments.
Various options are then used to treat the painful symptoms.
A large number of relationships exist between a new diabetic foot ulcer and its potential predictors; some have the strongest evidence, such as a history of foot ulcers or history of amputation, and others will have to be investigated [34].
1.2. Cutaneous Foot Impairment
The frequency of cutaneous impairment in diabetic patients has been reported to range from 30.0% to 91.2%, but its pathogenesis has yet to be elucidated [35,36,37]. Although diabetic foot cutaneous symptoms may not be life-threatening, they may seriously affect the quality of life and serve as external markers for extracutaneous complications, which are strongly associated with DPN [38,39,40]. Carrington et al. (2002) observed how the motor nerve conduction velocity, frequently assessed in clinical trials of diabetic peripheral neuropathy, can predict foot ulcerations [41].
On the other hand, hyperglycemia affects keratinocytes and fibroblast activities, and the combination with diabetic neuropathy may play an important role in the pathogenesis of diabetic foot complications and amputation [42,43].
These two aspects are closely related, as the pathogenesis initially involves unrecognized trauma within skin areas of neuropathy, while hyperglycemia affects chemotaxis, resulting in a badly disturbed cell proliferation and migration healing process [42].
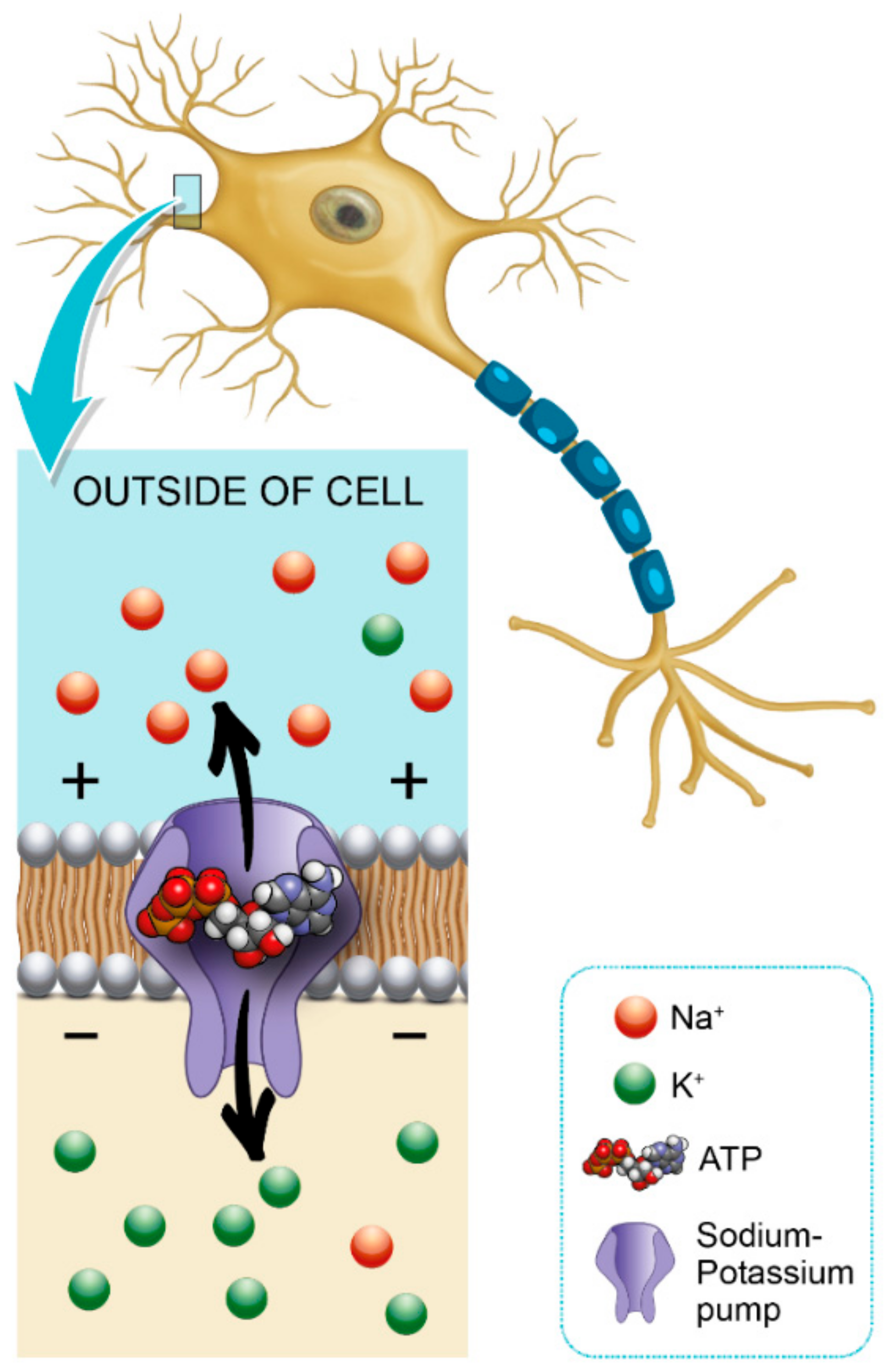
Glycemic variability is an important factor that contributes to axonal ion channel dysfunction, a key mediator in axonal degeneration in diabetes mellitus type 1 [44]. Patients with diabetes mellitus have a predisposition to develop chronic inflammatory demyelinating polyneuropathy, and this may also facilitate the formation of a diabetic foot [45]. Hyperglycemia leads to a shunting of excessive glucose through an activated polyol pathway that disrupts neural Na+ /K+ -ATPase, causing intra-axonal Na+ accumulation (Figure 1). This pattern of change is consistent with axonal depolarization, an abnormality that may occur in the context of dysfunction of the energy-dependent axonal Na+/K+ pump [46].
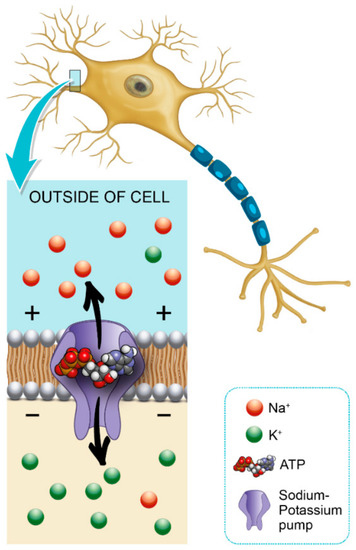
Figure 1.
Role of hemoglobin A1c (HbA1c) in Na+/K+ pump dysfunction and diabetic peripheral neuropathy (DPN) pathogenesis.
Increased glucose may also affect the skin, a phenomenon that occurs in about 30% of people with diabetes that may be a precursor of the disease [47,48]. Hemoglobin A1c (HbA1c) reflects glycemia over two to three months, and according to the guidelines set forth by the American Diabetes Association, the goal of type 2 diabetes therapy is to reduce glycated hemoglobin A1c (HbA1c) to 7% or 6.5% [49].
Elevated HbA1c levels would mostly be associated with poor wound healing, and HbA1c is a good biomarker for foot ulcer outcomes (wound healing time) in diabetic patients [50].
2. Methods
2.1. Search Strategy and Selection Criteria
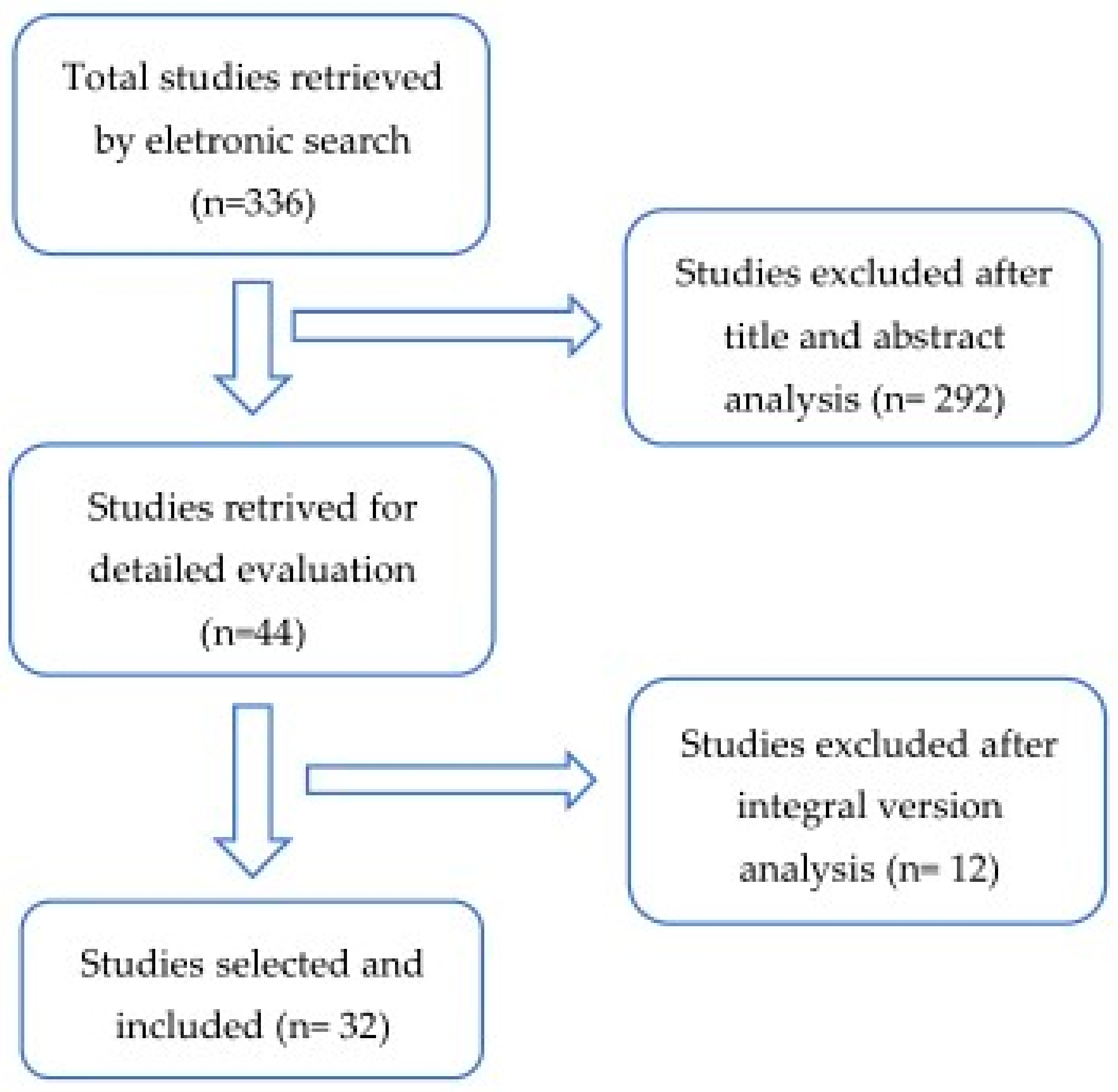
Databases and Literature Search: An electronic search was performed in PubMed for all relevant literature published up to 25 November 2020. The search terms were the following: (“glycated hemoglobin a” (MeSH Terms) OR “glycated hemoglobin a” (All Fields) OR “hba1c” (All Fields) OR “hba1cs” (All Fields)) AND (“diabetic neuropathies” (MeSH Terms) OR (“diabetic” (All Fields) AND “neuropathies” (All Fields)) OR “diabetic neuropathies” (All Fields) OR (“neuropathy” (All Fields) AND “diabetic” (All Fields)) OR “neuropathy diabetic” (All Fields)) AND (“foot” (MeSH Terms) OR “foot” (All Fields)). We supplemented our search by manually reviewing the references of all eligible studies (Figure 2).
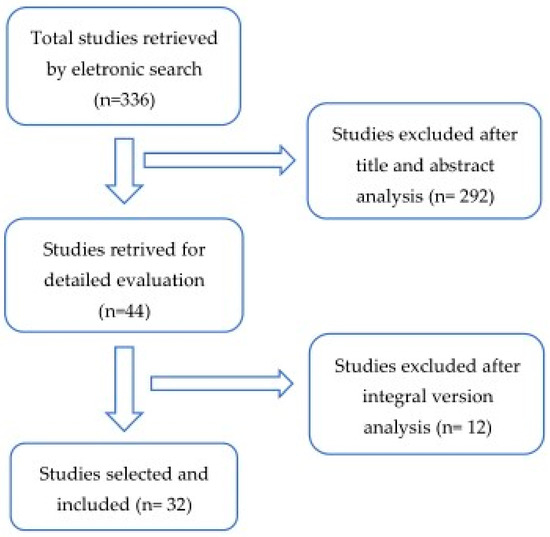
Figure 2.
Flow diagram of the articles selection process through a systematic review.
2.2. Eligibility Criteria
The following inclusion criteria were fulfilled: (a) observational studies (cross-sectional, case–control or cohort study) that allowed for the assessment of a causal association between HbA1c, DPN and diabetic foot complications; (b) the definition of DPN given in the studies included only sensory neuropathy, not Charcot neuroarthropathy and (c) the studies compared HbA1c levels between groups with and without DPN and diabetic foot complications. We excluded conference proceedings and articles reporting results from less than 10 patients and that did not assess diabetic foot peripheral neuropathy complications related to HbA1c values.
3. Results
3.1. Characteristics of Included Studies
We identified 32 studies that met our inclusion criteria for a systematic review (Table 1 and Table 2). Two-thirds of the studies included were prospective or retrospective cross-sectional cohort studies, and all of the 32 studies investigated the relationship between HbA1c and DPN. Type 2 diabetes was assessed in 25 studies, type 1 in four studies and type 1 and type 2 in four studies.

Table 1.
Studies examining the usefulness of hemoglobin A1c (HbA1c) as a biomarker for the early detection of diabetic peripheral neuropathy (DPN) and foot impairment. In this table, we excluded studies that did not report HbA1c values or did not assess the prevalence of a diabetic foot.

Table 2.
Studies examining the usefulness of HbA1c as a biomarker for the early detection of DPN.
Twenty-six studies involved more than 100 participants, but only five studies included healthy adults as a control group.
3.2. Protocols and Characteristics of Studies Examining the Interacting Mechanisms between HbA1c Levels and Diabetic Foot Peripheral Neuropathy
Three studies recorded no significant effect of elevated HbA1c levels or intensive glycaemia therapy on the peripheral neuropathy [51,59,62].
Thirty studies observed that an increase in HbA1c variability is closely associated with DPN in diabetic patients.
Regarding DPN, the clinical features in diabetic patients enrolled, in the majority of studies, were classified using the MNSI staging scale, but, in this review, we did not find homogeneity, since a wide variety of tests and instruments such as electrodiagnostic techniques or nerve conduction studies were reported.
The two studies that tested the effects of HbA1c on diabetic foot peripheral neuropathy showed that the strong relationship between HbA1c values and vibration perception threshold (VPT) could be a predictor for complications in the foot following DPN [56,57].
Kamran M. Hassan et al. in 2016 observed a strong association between HbA1C and neuropathy, leading to a high risk of diabetic foot, since poor footwear, neuropathic foot and ulceration and higher HbA1c levels were interlinked in terms of the pathogenesis (footwear can play a critical role in the pathogenesis of foot complications in diabetic patients with DPN).
In the clinical research community, a consensus on the relationship between hyperglycemic and diabetic foot complications in patients with DPN is still to be reached; this is also true in terms of the cut-off point of HbA1c needed to predict DPN. There is a lack of studies analyzing various items of DPN in light of the foot complications, which would have been interesting in order to statistically compare various impairments and understand which HbA1c values can be predictive and associated with diabetic foot complications. Some of these complications can be investigated in terms of the increased plantar pressures, as done by Mohammed R. Halawa in 2017 [56], and associated foot deformities and risk factors such as ulcers, calluses, dry skin, deformities, footwear condition, dry skin, bunions, fissures, callus and ingrown nails, many of which are already present in the MNSI questionnaire.
The positive effects of intensive glycaemia therapy may add an important benefit to reduce the risk of ulcers, with fewer diabetes-related foot complications.
4. Discussion
Biomarkers play a key role in the diagnosis, prognosis and clinical management of various chronic diseases.
Despite these efforts, we were unable to find any clinical trials successfully investigating the impact of glycemic control and foot complications correlated to DPN. Diabetic foot peripheral neuropathy often cooccurs with other diabetes-induced complications. Different aspects related to diabetic foot syndrome, such as gait alterations, psychological complaints and even disorders, can affect the quality of life of these patients; moreover, there is a general gender-dependent higher prevalence of diabetic foot impairment in men, although this was shown to be dependent on the geographical area [83,84].
We evaluated several foot alterations consisting of the most frequently observed in a diabetic foot according to the Michigan Neuropathy Screening Instrument, but, in this review, we did not find one particular glycated level used to predict diabetic foot peripheral neuropathy alterations. A consensus in this regard in the clinical research community is still to be gained in terms of foot cutaneous impairment or other foot complications associated with DPN and a high level of HbA1c.
The measurement of high levels of HbA1c could be a strategic biomarker to detect diabetic foot peripheral neuropathy. Indeed, intensive glycemic control and lower levels of HbA1c are followed by a reduction in diabetic complications: in HbA1c, <7% is associated a 60% reduction in the incidence of peripheral neuropathy [85].
The use of HbA1c level as an indicator of the severity of polyneuropathy and poor glycemic control (HbA1c level >6.5%) could significantly increase the risk and quantitatively reflect the severity of polyneuropathy in diabetic patients [82]. Evidence suggests that a high level of HbA1c can lead to diabetic peripheral neuropathy, so patients with high levels of HbA1c should be considered to be at the potential risk of diabetic foot complications—foot ulcerations or injuries—that frequently occur in DPN and should receive preventive education from a podiatrist. However, although the majority of studies observed that an increase HbA1c variability is closely associated with DPN in diabetic patients, in contrast, Laura Mayeda, Piotr Dziemidok and Faramarz Ismail-Beigi conversely recorded no significant effects of elevated HbA1c levels or intensive glycemia therapy on peripheral neuropathy. Besides these contrasting results, further studies should investigate the relationship between hyperglycemic and DPN, possibly including a comparison of the different instruments used to assess the clinical features. The crucial issue of defining a relationship between a glycemic control and DPN impairments has not been possible due to the critical bias listed above. Only eight (Table 1) out of 32 studies analyzed the presence of diabetic foot peripheral neuropathy and related problems such as diabetic foot and cutaneous impairment; this lack of data makes a meta-analysis or a statistical investigation impossible to carry out in order to find an association between HbA1c and DPN.
5. Conclusions
The purpose of this literature review was to evaluate the common and interacting mechanisms between HbA1c levels and diabetic foot peripheral neuropathy.
According to high-quality evidence, enhanced glucose control significantly prevents the development of clinical neuropathy [86]. The exact role that intensive glycemic control has in treating diabetic foot complications in patients with diabetic peripheral neuropathy requires further investigation. All patients with diabetes and sensory loss require regular podiatric care and should have a thorough foot examination. The significant reductions in the development of peripheral neuropathy, if further sustained, suggest that intensive glycemia therapy could decrease the risk of ulcers and the number of future leg amputations, reducing diabetes-related foot complications and, thus, significantly improving the quality of life of patients.
Many dermatological foot complications are caused by hyperglycemia, and the pathogenesis is also caused by neuropathy. Improved glycemic control has been shown to have a sustained benefit on diabetes and its complications, but evidence of the effects of HbA1c as a biomarker on some of the diabetic foot peripheral neuropathy complications such as hyperkeratosis, pre-ulcerative lesions or xerosis is still lacking.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank Semeh Bejaoui and MariaPia Cumani.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morales, A. A better future for children with type 1 diabetes: Review of the conclusions from the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study. J. Ark. Med. Soc. 2009, 106, 90–93. [Google Scholar]
- Solders, G.; Thalme, B.; Aguirre-Aquino, M.; Brandt, L.; Berg, U.; Persson, A. Nerve conduction and autonomic nerve function in diabetic children. A 10-year follow-up study. Acta Paediatr. 1997, 86, 361–366. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Albers, J.W.; Herman, W.H.; Pop-Busui, R.; Feldman, E.L.; Martin, C.L.; Cleary, P.A.; Waberski, B.H.; Lachin, J.M. For the DCCT/EDIC Research Group Effect of Prior Intensive Insulin Treatment During the Diabetes Control and Complications Trial (DCCT) on Peripheral Neuropathy in Type 1 Diabetes During the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010, 33, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Low, P.A.; Waberski, B.H.; Martin, C.L.; Albers, J.W.; Feldman, E.L.; Sommer, C.; Cleary, P.A.; Lachin, J.M.; Herman, W.H. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: The diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC). Circulation 2009, 119, 2886–2893. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Stevens, L.K.; Stephenson, J.M.; Fuller, J.H.; Plater, M.; Ionescu-Tirgoviste, C.; Nuber, A.; Pozza, G.; Ward, J.D. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: The EURODIAB IDDM Complications Study. Diabetologia 1996, 39, 1377–1384. [Google Scholar] [CrossRef]
- Mistry, K.P.; Beyer-Mears, A.; Diecke, F.P.J. Mechanisms for d-Glucose Inhibition of myo-Inositol Influx into Rat Lens. Diabetes 1993, 42, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Strokov, I.A.; Bursa, T.R.; Drepa, O.I.; Zotova, E.V.; Nosikov, V.V.; Ametov, A.S. Predisposing genetic factors for diabetic polyneuropathy in patients with type 1 diabetes: A population-based case-control study. Acta Diabetol. 2003, 40, s375–s379. [Google Scholar] [CrossRef]
- Vlassara, H.; Brownlee, M.; Cerami, A. Recognition and Uptake of Human Diabetic Peripheral Nerve Myelin by Macrophages. Diabetes 1985, 34, 553–557. [Google Scholar] [CrossRef]
- Cashman, C.R.; Höke, A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci. Lett. 2015, 596, 33–50. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef]
- Dyck, P.J.; Kratz, K.M.; Karnes, J.L.; Litchy, W.J.; Klein, R.; Pach, J.M.; Wilson, D.M.; O’Brien, P.C.; Melton, L.J. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology 1993, 43, 817. [Google Scholar] [CrossRef]
- Young, M.J.; Boulton, A.J.M.; MacLeod, A.F.; Williams, D.R.R.; Sonksen, P.H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ashe, H.; Parnell, L.; Fernando, D.; Tsigos, C.; Young, R.; Ward, J.; Boulton, A. The Prevalence of Foot Ulceration and its Correlates in Type 2 Diabetic Patients: A Population-based Study. Diabet. Med. 1994, 11, 480–484. [Google Scholar] [CrossRef]
- Cabezas-Cerrato, J. The prevalence of clinical diabetic polyneuropathy in Spain: A study in primary care and hospital clinic groups. Diabetologia 1998, 41, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.; Malik, R.A.; Arezzo, J.C.; Sosenko, J.M. Diabetic Somatic Neuropathies. Diabetes Care 2004, 27, 1458–1486. [Google Scholar] [CrossRef]
- Boulton, A.J. Guidelines for diagnosis and outpatient management of diabetic peripheral neuropathy. Diabetes Metab. 1998, 24, 55–65. [Google Scholar] [CrossRef]
- Boulton, A.J. Management of Diabetic Peripheral Neuropathy. Clin. Diabetes 2005, 23, 9–15. [Google Scholar] [CrossRef]
- Oyibo, S.O.; Prasad, Y.D.M.; Jackson, N.J.; Jude, E.B.; Boulton, A.J.M. The relationship between blood glucose excursions and painful diabetic peripheral neuropathy: A pilot study. Diabet. Med. 2002, 19, 870–873. [Google Scholar] [CrossRef]
- Inoue, R.; Sumitani, M.; Yasuda, T.; Tsuji, M.; Nakamura, M.; Shimomura, I.; Shibata, M.; Yamada, Y. Independent Risk Factors for Positive and Negative Symptoms in Patients with Diabetic Polyneuropathy. J. Pain Palliat. Care Pharmacother. 2016, 30, 178–183. [Google Scholar] [CrossRef]
- Katoulis, E.C.; Ebdon-Parry, M.; Lanshammar, H.; Vileikyte, L.; Kulkarni, J.; Boulton, A.J.M. Gait abnormalities in diabetic neuropathy. Diabetes Care 1997, 20, 1904–1907. [Google Scholar] [CrossRef]
- Allet, L.; Armand, S.; Golay, A.; Monnin, D.; De Bie, R.A.; De Bruin, E.D. Gait characteristics of diabetic patients: A systematic review. Diabetes/Metab. Res. Rev. 2008, 24, 173–191. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; George, R.S.; Fitzpatrick, R.C. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch. Phys. Med. Rehabil. 2004, 85, 245–252. [Google Scholar] [CrossRef]
- Bonnet, C.T.; Ray, C. Peripheral neuropathy may not be the only fundamental reason explaining increased sway in diabetic individuals. Clin. Biomech. 2011, 26, 699–706. [Google Scholar] [CrossRef]
- Richardson, J.K.; Hurvitz, E.A. Peripheral Neuropathy: A True Risk Factor for Falls. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 1995, 50, M211–M215. [Google Scholar] [CrossRef] [PubMed]
- Tilling, L.M.; Darawil, K.; Britton, M. Falls as a complication of diabetes mellitus in older people. J. Diabetes Its Complicat. 2006, 20, 158–162. [Google Scholar] [CrossRef]
- Zelman, D.C.; Gore, M.; Dukes, E.; Tai, K.-S.; Brandenburg, N. Validation of a modified version of the Brief Pain Inventory for painful diabetic peripheral neuropathy. J. Vasc. Nurs. 2005, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Kirsner, R.S.; Vileikyte, L. Neuropathic Diabetic Foot Ulcers. N. Engl. J. Med. 2004, 351, 48–55. [Google Scholar] [CrossRef]
- Abbott, C.A.; Carrington, A.L.; Ashe, H.; Bath, S.; Every, L.C.; Griffiths, J.; Hann, A.W.; Hussein, A.; Jackson, N.; Johnson, K.E.; et al. The North-West Diabetes Foot Care Study: Incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet. Med. 2002, 19, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Moghtaderi, A.; Bakhshipour, A.; Rashidi, H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin. Neurol. Neurosurg. 2006, 108, 477–481. [Google Scholar] [CrossRef]
- Herman, W.H.; Pop-Busui, R.; Braffett, B.H.; Martin, C.L.; Cleary, P.A.; Albers, J.W.; Feldman, E.L. The DCCT/EDIC Research Group Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: Results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet. Med. 2012, 29, 937–944. [Google Scholar] [CrossRef]
- EFeldman, E.L.; Stevens, M.J.; Thomas, P.K.; Brown, M.B.; Canal, N.; Greene, D.A. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994, 17, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Boyko, E.J.; Ahroni, J.H.; Stensel, V.; Forsberg, R.C.; Davignon, D.R.; Smith, D.G.; Tsuji, I.; Nakamoto, K.; Hasegawa, T.; Hisashige, A.; et al. A prospective study of risk factors for diabetic foot ulcer. The Seattle Diabetic Foot Study. Diabetes Care 1999, 22, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Al Robaee, A.; Al Shobaili, H.A.; Alzolibani, A.A.; Al Marshood, A.A.; Al Moteri, B. Skin Manifestations in Diabetic Patients Attending a Diabetic Clinic in the Qassim Region, Saudi Arabia. Med. Princ. Pract. 2011, 20, 137–141. [Google Scholar] [CrossRef]
- Romano, G.; Moretti, G.; Di Benedetto, A.; Giofrè, C.; Di Cesare, E.; Russo, G.; Califano, L.; Cucinotta, D. Skin lesions in diabetes mellitus: Prevalence and clinical correlations. Diabetes Res. Clin. Pract. 1998, 39, 101–106. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Ehsani, A.H.; Hossein, P.F. The Correlation between Skin Lesions, Microalbuminuria and other Microvascular Complications in Type 2 Diabetic Patients. J. Nephro-Urol. Mon. 2010, 2, 553–560. [Google Scholar]
- Foss, N.T.; Polon, D.P.; Takada, M.H.; Foss-Freitas, M.C.; Foss, M.C. Dermatoses em pacientes com diabetes mellitus. Rev. Saude Publica 2005, 39, 677–682. [Google Scholar] [CrossRef]
- Levy, L.; Zeichner, J.A. Dermatologic manifestation of diabetes. J. Diabetes 2012, 4, 68–76. [Google Scholar] [CrossRef]
- Brognara, L.; Volta, I.; Cassano, V.M.; Navarro-Flores, E.; Cauli, O. The Association between Cognitive Impairment and Diabetic Foot Care: Role of Neuropathy and Glycated Hemoglobin. Pathophysiology 2020, 27, 14–27. [Google Scholar] [CrossRef]
- Carrington, A.L.; Shaw, J.E.; Van Schie, C.H.; Abbott, C.A.; Vileikyte, L.; Boulton, A.J. Can Motor Nerve Conduction Velocity Predict Foot Problems in Diabetic Subjects Over a 6-Year Outcome Period? Diabetes Care 2002, 25, 2010–2015. [Google Scholar] [CrossRef]
- Blakytny, R.; Jude, E.B. Altered Molecular Mechanisms of Diabetic Foot Ulcers. Int. J. Low. Extrem. Wounds 2009, 8, 95–104. [Google Scholar] [CrossRef]
- Adler, A.I.; Erqou, S.; Lima, T.A.S.; Robinson, A.H.N. Association between glycated haemoglobin and the risk of lower extremity amputation in patients with diabetes mellitus—Review and meta-analysis. Diabetologia 2010, 53, 840–849. [Google Scholar] [CrossRef]
- Kwai, N.C.; Arnold, R.; Poynten, A.M.; Krishnan, A.V. Association between glycemic variability and peripheral nerve dysfunction in type 1 diabetes. Muscle Nerve 2016, 54, 967–969. [Google Scholar] [CrossRef]
- Kiziltan, M.E.; Gunduz, A.; Kiziltan, G.; Akalin, M.A.; Uzun, N. Peripheral neuropathy in patients with diabetic foot ulcers: Clinical and nerve conduction study. J. Neurol. Sci. 2007, 258, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain 2000, 123, 2542–2551. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Siegel, K.R.; Gujral, U.P.; Narayan, K.V. Type 2 diabetes: A 21st century epidemic. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.I.; Kohn, S.R. Cutaneous manifestations of diabetes mellitus. J. Am. Acad. Dermatol. 1994, 30, 519–531. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2017 Abridged for Primary Care Providers. Clin. Diabetes 2016, 35, 5–26. [Google Scholar] [CrossRef]
- Vella, L.; Gatt, A.; Formosa, C. Does Baseline Hemoglobin A1c Level Predict Diabetic Foot Ulcer Outcome or Wound Healing Time? J. Am. Podiatr. Med. Assoc. 2017, 107, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Dziemidok, P.; Szcześniak, G.; Kostrzewa-Zabłocka, E.; Paprzycki, P.; Korzon-Burakowska, A. Current glycaemic control has no impact on the advancement of diabetic neuropathy. Ann. Agric. Environ. Med. 2012, 19, 742–745. [Google Scholar]
- Jan, Y.-K.; Liao, F.; Cheing, G.L.; Pu, F.; Ren, W.; Choi, H.M. Differences in skin blood flow oscillations between the plantar and dorsal foot in people with diabetes mellitus and peripheral neuropathy. Microvasc. Res. 2019, 122, 45–51. [Google Scholar] [CrossRef]
- El-Mesallamy, H.O.; Hamdy, N.M.; Ezzat, O.A.; Reda, A.M. Levels of Soluble Advanced Glycation End Product-Receptors and Other Soluble Serum Markers as Indicators of Diabetic Neuropathy in the Foot. J. Investig. Med. 2011, 59, 1233–1238. [Google Scholar] [CrossRef]
- De Souza, R.J.; De Souza, A.; Nagvekar, M.D. Nerve conduction studies in diabetics presymptomatic and symptomatic for diabetic polyneuropathy. J. Diabetes Complicat. 2015, 29, 811–817. [Google Scholar] [CrossRef]
- Salvotelli, L.; Stoico, V.; Perrone, F.; Cacciatori, V.; Negri, C.; Brangani, C.; Pichiri, I.; Targher, G.; Bonora, E.; Zoppini, G. Prevalence of neuropathy in type 2 diabetic patients and its association with other diabetes complications: The Verona Diabetic Foot Screening Program. J. Diabetes Its Complicat. 2015, 29, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Maiya, A.G.; Parameshwar, A.; Hande, M.; Nandalike, V. Relationship Between Glycated Hemoglobin and Vibration Perception Threshold in Diabetic Peripheral Neuropathy. Int. J. Low. Extrem. Wounds 2019, 19, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Halawa, M.R.; Eid, Y.M.; El-Hilaly, R.A.; Abdelsalam, M.M.; Amer, A.H. Relationship of planter pressure and glycemic control in type 2 diabetic patients with and without neuropathy. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.M.; Balaji, R.; Gnaneswaran, S.; Kumar, J.S. To Study the Association of HbA1c with Retinopathy, Neuropathy and High Risk Foot among Diabetic Patients Attending Rural Tertiary Care Hospital of Tamil Nadu, India. Int. J. Ophthalmol. Eye Sci. 2016, 4, 206–211. [Google Scholar] [CrossRef]
- Kovač, B.; Kovač, B.; Marušić-Emedi, S.; Svalina, S.; Demarin, V. Clinical and electrophysiological signs of diabetic polyneuropathy—Effect of glycemia and duration of diabetes mellitus. Acta Clin. Croat. 2011, 50, 149–157. [Google Scholar]
- Azam, M.; Marwood, L.; Ismail, K.; Evans, T.; Sivaprasad, S.; Winkley, K.; Amiel, S.A. Diabetes Complications at Presentation and One Year by Glycated Haemoglobin at Diagnosis in a Multiethnic and Diverse Socioeconomic Population: Results from the South London Diabetes Study. J. Diabetes Res. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Ángeles Lazo, M.D.L.; Bernabe-Ortiz, A.; Pinto, M.E.; Ticse, R.; Málaga, G.; Sacksteder, K.; Miranda, J.J.; Gilman, R.H. Diabetic Peripheral Neuropathy in Ambulatory Patients with Type 2 Diabetes in a General Hospital in a Middle Income Country: A Cross-Sectional Study. PLoS ONE 2014, 9, e95403. [Google Scholar] [CrossRef]
- Ismail-Beigi, F.; Craven, T.; Banerji, M.A.; Basile, J.; Calles, J.; Cohen, R.M.; Cuddihy, R.; Cushman, W.C.; Genuth, S.; Grimm, R.H.; et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: An analysis of the ACCORD randomised trial. Lancet 2010, 376, 419–430. [Google Scholar] [CrossRef]
- Türkyilmaz, H.; Güzel, O.; Edizer, S.; Ünalp, A. Evaluation of polyneuropathy and associated risk factors inchildren with type 1 diabetes mellitus. Turk. J. Med. Sci. 2017, 47, 942–946. [Google Scholar] [CrossRef]
- Unmar, Y.; Zafar, M.I.; Gao, F. Factors associated with peripheral neuropathy in type 2 diabetes: Subclinical versus confirmed neuropathy. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 337–342. [Google Scholar] [CrossRef]
- Mayeda, L.; Katz, R.; Ahmad, I.; Bansal, N.; Batacchi, Z.; Hirsch, I.B.; Robinson, N.; Trence, D.L.; Zelnick, L.; De Boer, I.H. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res. Care 2020, 8, e000991. [Google Scholar] [CrossRef] [PubMed]
- Su, J.-B.; Zhao, L.-H.; Zhang, X.-L.; Cai, H.-L.; Huang, H.-Y.; Xu, F.; Chen, T.; Wang, X.-Q. HbA1c variability and diabetic peripheral neuropathy in type 2 diabetic patients. Cardiovasc. Diabetol. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Lai, Y.-R.; Chiu, W.-C.; Huang, C.-C.; Tsai, N.-W.; Wang, H.-C.; Lin, W.-C.; Cheng, B.-C.; Su, Y.-J.; Su, C.-M.; Hsiao, S.-Y.; et al. HbA1C Variability Is Strongly Associated With the Severity of Peripheral Neuropathy in Patients With Type 2 Diabetes. Front. Neurosci. 2019, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, F.; Taniguchi, M.; Kosaka, A.; Uetake, H.; Tavakoli, M. Improvement in Neuropathy Outcomes With Normalizing HbA1c in Patients With Type 2 Diabetes. Diabetes Care 2019, 42, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Barnett, C.; Katzberg, H.D.; Lovblom, L.E.; Perkins, B.A.; Bril, V. Nerve function varies with hemoglobin A1c in controls and type 2 diabetes. J. Diabetes Its Complicat. 2018, 32, 424–428. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Hager, K.K.; Ramulu, P.Y. Physical activity, glycemic control, and diabetic peripheral neuropathy: A national sample. J. Diabetes Complicat. 2014, 28, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Bakhotmah, B.A.; Hu, F.B.; Alzahrani, H.A. Prevalence and Correlates of Diabetic Peripheral Neuropathy in a Saudi Arabic Population: A Cross-Sectional Study. PLoS ONE 2014, 9, e106935. [Google Scholar] [CrossRef]
- Li, L.; Chen, J.; Wang, J.; Cai, D. Prevalence and risk factors of diabetic peripheral neuropathy in Type 2 diabetes mellitus patients with overweight/obese in Guangdong province, China. Prim. Care Diabetes 2015, 9, 191–195. [Google Scholar] [CrossRef]
- Jaiswal, M.; Divers, J.; Dabelea, D.; Isom, S.; Bell, R.A.; Martin, C.L.; Pettitt, D.J.; Saydah, S.; Pihoker, C.; Standiford, D.A.; et al. Prevalence of and Risk Factors for Diabetic Peripheral Neuropathy in Youth With Type 1 and Type 2 Diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017, 40, 1226–1232. [Google Scholar] [CrossRef]
- Peterson, M.; Pingel, R.; Lagali, N.; Dahlin, L.B.; Rolandsson, O. Association between HbA 1c and peripheral neuropathy in a 10-year follow-up study of people with normal glucose tolerance, impaired glucose tolerance and Type 2 diabetes. Diabet. Med. 2017, 34, 1756–1764. [Google Scholar] [CrossRef]
- Yang, C.-P.; Lin, C.-C.; Li, C.-I.; Liu, C.-S.; Lin, W.-Y.; Hwang, K.-L.; Yang, S.-Y.; Chen, H.-J.; Li, T.-C. Cardiovascular Risk Factors Increase the Risks of Diabetic Peripheral Neuropathy in Patients With Type 2 Diabetes Mellitus. Medicine 2015, 94, e1783. [Google Scholar] [CrossRef]
- Braffett, B.H.; Gubitosi-Klug, R.A.; Albers, J.W.; Feldman, E.L.; Martin, C.L.; White, N.H.; Orchard, T.J.; Lopes-Virella, M.; Lachin, J.M.; Pop-Busui, R.; et al. Risk Factors for Diabetic Peripheral Neuropathy and Cardiovascular Autonomic Neuropathy in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes 2020, 69, 1000–1010. [Google Scholar] [CrossRef]
- Adams, O.P.; Herbert, J.R.; Howitt, C.; Unwin, N. The prevalence of peripheral neuropathy severe enough to cause a loss of protective sensation in a population-based sample of people with known and newly detected diabetes in Barbados: A cross-sectional study. Diabet. Med. 2019, 36, 1629–1636. [Google Scholar] [CrossRef]
- Xu, F.; Zhao, L.-H.; Su, J.-B.; Chen, T.; Wang, X.-Q.; Chen, J.-F.; Wu, G.; Jin, Y.; Wang, X.-H. The relationship between glycemic variability and diabetic peripheral neuropathy in type 2 diabetes with well-controlled HbA1c. Diabetol. Metab. Syndr. 2014, 6, 1–7. [Google Scholar] [CrossRef]
- Gibbons, C.H. Treatment induced neuropathy of diabetes—Long term implications in type 1 diabetes. J. Diabetes Its Complicat. 2017, 31, 715–720. [Google Scholar] [CrossRef]
- Pai, Y.-W.; Lin, C.-H.; Lee, I.-T.; Chang, M.-H. Variability of fasting plasma glucose and the risk of painful diabetic peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab. 2018, 44, 129–134. [Google Scholar] [CrossRef]
- Li, S.; Nemeth, I.; Donnelly, L.; Hapca, S.; Zhou, K.; Pearson, E.R. Visit-to-Visit HbA1c Variability Is Associated With Cardiovascular Disease and Microvascular Complications in Patients With Newly Diagnosed Type 2 Diabetes. Diabetes Care 2019, 43, 426–432. [Google Scholar] [CrossRef]
- Lee, W.-J.; Jang, S.; Lee, S.-H.; Lee, H.-S. Correlation Between the Severity of Diabetic Peripheral Polyneuropathy and Glycosylated Hemoglobin Levels: A Quantitative Study. Ann. Rehabil. Med. 2016, 40, 263–270. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, Y.; Wei, L.; Xiao, Y.; Li, L.; Sun, L. Identification of two novel subgroups in patients with diabetes mellitus and their association with clinical outcomes: A two-step cluster analysis. J. Diabetes Investig. 2021. [Google Scholar] [CrossRef]
- Navarro-Flores, E. Quality of Life in Individuals with Diabetic Foot Syndrome. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1365–1372. [Google Scholar] [CrossRef]
- Nathan, D.M. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care 2013, 37, 9–16. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Little, A.A.; Feldman, E.L.; Hughes, R.A. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst. Rev. 2012, 6, CD007543. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).