Abstract
Background and Aim: Although constitutional and respiratory symptoms such as cough and fever are the most common symptoms in patients infected with COVID-19, gastrointestinal (GI) tract involvement has been observed by endoscopic biopsies. Multiple GI symptoms, including diarrhea, nausea or vomiting and abdominal pain, have also been reported. This review aims to present the currently available data regarding the GI symptoms of COVID-19 patients, and to compare the frequency of GI symptoms in early stage (Eastern) mostly Chinese data to the current stage (Western) non-Chinese data. Methods: We performed a systematic literature search to identify both published studies by using PubMed, Google Scholar, and CNKI (Chinese medical search engine), and yet unpublished studies through medRxiv and bioRxiv. We also reviewed the cross references of the detected articles. We conducted a Medical Subject Headings (MeSH) search up until 20 September 2020. We pooled the prevalence of symptoms of diarrhea, anorexia, nausea, vomiting, and abdominal pain by using the Freeman–Tukey’s transforming random effect model. Results: A total of 118 studies were included in the systematic review and 44 of them were included in the meta-analysis. There was a significant heterogeneity between the studies; therefore, the random effects model was used. The pooled prevalence estimate of any GI symptoms reported was found to be 0.21 (95%CI, 0.16–0.27). Anorexia was the most commonly reported GI symptom at 18% (95%CI, 0.10–0.27) followed by diarrhea at 15% (95%CI, 0.12–0.19). Diarrhea, abdominal pain, nausea/vomiting, and respiratory symptoms were more common in non-Chinese studies. The prevalence of abdominal pain was lower in the “inpatient-only” studies when compared with studies that included outpatients only and those including both inpatients and outpatients. Conclusions: In this comprehensive systematic review and meta-analysis study, we observed higher rates of diarrhea, nausea/vomiting, and abdominal pain in COVID-19 infected patients among non-Chinese studies compared to Chinese studies. We also observed a higher prevalence of GI symptoms in Chinese studies than was reported previously. Non-respiratory symptoms, including GI tract symptoms, should be more thoroughly and carefully evaluated and reported in future studies.
Keywords:
COVID-19; gastrointestinal symptom; systematic review; meta-analysis; diarrhea; nausea; vomit; abdominal pain 1. Introduction
Coronavirus disease 2019 (COVID-19) emerged in December 2019 in the Wuhan region of China and spread across the world [1]. The causative virus was subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. The infection is spread by droplets, aerosols, and direct contact with contaminated surfaces. As of 2 October 2020, there have been over 33,842,281 confirmed cases of COVID-19, including 1,010,634 deaths, reported to WHO [3]. Although respiratory symptoms such as fever and cough are the most common findings in patients with COVID-19, GI tract involvement has been identified by endoscopic biopsies. In addition, the presence of several GI symptoms, including diarrhea, nausea/vomiting, and abdominal pain, has been reported [4]. COVID-19 uses an ACE2 protein to gain entry into cells [5]. ACE2 receptors are widely distributed in the human body, including in the lung, liver, stomach, ileum, colon, and kidney, allowing COVID-19 to infect many different organs.
Human coronaviruses are known to cause respiratory and enteric symptoms. During the SARS outbreak in 2002–2003, 16–73% of patients with SARS had diarrhea [6]. Early reports of COVID-19 may not represent the actual rate of GI symptoms, because in the early times of the outbreak the most notable symptoms were severe respiratory symptoms. Viral RNA has been detected in GI epithelial cells and stool of COVID-19 patients, pointing to a possible fecal–oral transmission of the SARS-CoV-2 infection [7]. This review aims to present the currently available data regarding GI symptoms of COVID-19 patients and to compare the frequency of GI symptoms in early stage (Eastern) to current stage (Western) data.
2. Methods
Literature search strategies: This study was designed to be a systematic review and meta-analysis. Literature for this study was identified by searching the following online databases in the English and Chinese languages: PubMed, Google Scholar, and CNKI (Chinese medical search engine); and MedRvix and BioRxiv for the yet unpublished manuscripts. Additionally, the cross references of the detected articles were screened. We did a Medical Subject Headings (MeSH) search for two terms:
- (1)
- Term 1: COVID-19(supplementary concept).
- (2)
- Term 2: Severe acute respiratory syndrome Coronavirus 2 (supplementary concept).
Besides those two MeSH terms, we added the terms gastrointestinal, digestive, diarrhea, nausea, vomiting, anorexia, abdominal pain, and abdominal discomfort symptoms to our search.
The inclusion criteria were:
- (1)
- Any study including data of “COVID-19 documented patients” which was defined as patients with relevant clinical manifestations and radiologic findings of COVID-19 infection, plus PCR and/or antigen test for COVID-19 infection positivity.
- (2)
- Any study reporting respiratory and GI symptoms of “documented COVID-19 infection” (as defined above) was included.
- (3)
- Authors that clearly reported the absence of GI symptoms were also included in this study.
The exclusion criteria were:
- (1)
- If the index study included non-documented or suspected COVID-19 infection data.
- (2)
- If the presence or absence of GI symptoms was not reported.
- (3)
- Non-scientific commentaries and reports, reviews, meta-analysis studies, and scientific news.
All studies between 01 December 2019 and 19 September 2020 were screened through the relevant literature and then detection of possibly related studies through a search of manuscript titles and abstracts was done. After selection of possibly concordant studies, a full text article evaluation was conducted. During this full text evaluation phase, we assessed whether the manuscript was fulfilling the inclusion and exclusion criteria. That phase was done by each of the three researchers (H.A., A.S. and R.K.) independently. The three sets of study selections were then compared. The disagreements between selected reports were resolved through discussion with the three other authors (F.T., R.O. and V.T.). After the articles were selected for systematic review, data were extracted by (H.A., A.S., R.K., F.T. and R.O.) to an Excel data sheet. During data extraction, for the parameters that included more than one subgroup, we counted patients who had more than one of the subgroups only once in that parameter. For instance, if the parameter was a symptom—which could have included multiple subgroups, such as respiratory symptoms, any GI symptom, nausea or vomiting, and abdominal pain or discomfort, we counted a patient who had nausea and abdominal pain only once in the symptoms parameter. In studies where the number of patients having symptoms was given for each symptom, but did not indicate how many patients had multiple symptoms, we chose the highest numbered symptom as the minimum total number of that parameter.
The type and definitions of data extracted to the Excel spread sheet are given below:
- Authors’ names;
- Title of the manuscript;
- Study language: no language restriction was done;
- Place of study: where the study population’s data were collected and not where they were published;
- Study date: beginning and end period of study period;
- Number of patients included: study population;
- Age (mean or median (± 1 SD or IQR); range);
- Age related type of the study: we separated the studies according to age groups since age data were significantly heterogeneous;
- Pediatric studies: 0–14 years;
- Mixed studies: studies including both children and adults, adult studies of 15 years or higher;
- Male: number and percentage of male subjects included in the study
Severe disease* (n, %): We modified and used the Chinese definition of COVID-19 severity from “Diagnosis and Treatment Plan for Pneumonia Infected by New Coronavirus (Trial Sixth Edition)” National Health Commission of the People’s Republic of China [8]. We combined the severe plus critically ill group plus deaths then recoded as “severe COVID-19 disease.” The other severity definition was a modified definition (mild and moderate cases) recoded as “non-severe COVID-19 disease.” The Chinese definition of severe disease and critical disease is as follows [8];
Severe cases: Adult cases meeting any of the following criteria: (1) Respiratory distress (≥ 30 breaths/min). (2) Oxygen saturation ≤93% at rest. (3) Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mmHg (l mmHg = 0.133 kPa). In high-altitude areas (at an altitude of over 1000 m above the sea level), PaO2/FiO2 shall be corrected by the following formula: PaO2/FiO2x (Atmospheric pressure (mmHg)/760). Cases with chest imaging that show obvious lesion progression within 24–48 h >50% shall be managed as severe cases. Child cases meeting any of the following criteria: (1) Tachypnea (RR ≥60 breaths/min for infants aged below 2 months; RR ≥ 50 BPM for infants aged 2–12 months; RR ≥40 BPM for children aged 1–5 years; and RR ≥ 30 BPM for children above 5 years old) independent of fever and crying. (2) Oxygen saturation ≤92% on finger pulse oximeter taken at rest. (3) Labored breathing (moaning, nasal fluttering, and infrasternal, supraclavicular, and intercostal retraction), cyanosis, and intermittent apnea. (4) Lethargy and convulsion. (5) Difficulty feeding and signs of dehydration.
Critical cases: Cases meeting any of the following criteria: 4.1 Respiratory failure and requiring mechanical ventilation; 4.2 Shock; 4.3 with other organ failure that requires ICU care.
Fever (n, %): number and percentage of patients having clinical fever.
Respiratory symptoms (n, %): Number and percentage of patients having any type of respiratory symptoms, including cough, dyspnea, expectoration, and any other reported respiratory symptoms. Here we combined the respiratory symptoms and got one highest patient numbered symptom item as minimum number of “respiratory symptom” parameter.
Presence of any GI symptoms (n, %): number and percentage of patients having any type of GI symptoms, including anorexia, nausea/vomiting, diarrhea, and abdominal pain/discomfort. As some patients may have more than one symptom at the same time, we counted each as one even if the patient had multiple symptoms. On the other hand, we combined the symptoms and made the highest number of patient symptoms as the minimum number of “any GI symptom” parameter.
Anorexia (n, %): number and percentage of patients having anorexia symptom.
Nausea/vomiting (n, %): Number and percentage of patients having nausea and/or vomiting symptom. Here we combined the numbers and got the highest number of patient symptoms as the minimum number of “nausea/vomiting” parameter.
Diarrhea (n, %): number and percentage of patients having any type of diarrhea symptom.
Abdominal pain/discomfort (n, %): Number and percentage of patients having abdominal pain and/or abdominal discomfort symptoms. Here we combined the numbers and made the highest number of patient symptoms as the minimum number of the “abdominal pain/discomfort” parameter.
After the Excel data sheet was fulfilled, data were then transformed to the statistical program by the researchers H.A., A.S., R.K., F.T. and R.O.
3. Statistical Analysis
The prevalence and 95% confidence interval (CI)values of diarrhea, nausea/vomiting, abdominal pain, and anorexia symptoms were estimated using the meta-analysis package of the R program (4.14-0 Meta package, http://cran.r-project.org/web/packages/meta/meta.pdf). The Freeman–Tukey’s transforming random effect model was used for the analyses. I2 values were used to measure heterogeneity between the studies. Chinese studies were compared with non-Chinese studies, and “inpatient-only” studies were compared with studies including any outpatients, i.e., “mixed studies” reporting the data of inpatients together with outpatients as clinical setting plus outpatient-only. Using sub-group analysis and random effects, the differences between the groups were assessed.
4. Results
A total of 1050 full text studies were screened and 205 potential studies were evaluated in detail. Among these, 118 studies were included in the systematic review and 44 were included in the meta-analysis. The flow chart of the study is shown in Figure 1.
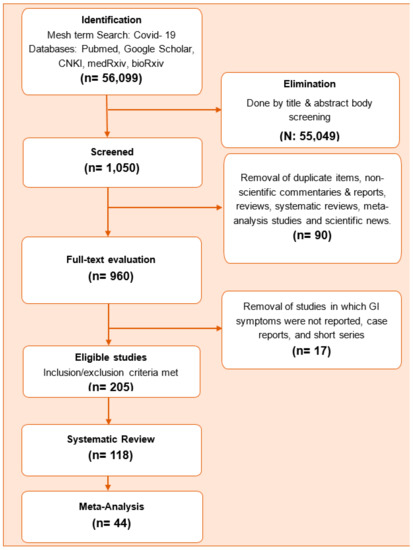
Figure 1.
Study flow chart.
Table 1 summarizes the characteristics of the studies included in the systematic review. Most of the studies reported the rate of diarrhea (105/118), while they less commonly reported the other GI symptoms (nausea 74/118, abdominal pain 39/118, and anorexia 38/118).

Table 1.
Characteristics of the studies included in the systematic review and meta-analysis.
Among the studies, 92 took place in China and the remaining 26 took place in other countries. The pooled prevalence of diarrhea, abdominal pain, nausea/vomiting, and respiratory symptoms was significantly more common in non-Chinese studies when compared with Chinese studies (Table 2). The occurrences of other study variables in Chinese and non-Chinese studies were similar.

Table 2.
Summary of the pooled prevalence estimates of gastrointestinal symptoms with respect to whether the study took place in China or out of China.
While most of the studies included “inpatient-only” (n = 81), 34 of them included both inpatients and outpatients, and two comprised “outpatient-only.” One study did not report inpatient or outpatient status of the included patients. The pooled prevalence of abdominal pain was significantly lower in “inpatient-only” studies. We could only compare these “inpatient-only” studies with those called mixed studies, i.e., inpatients together with outpatients (Table 3).

Table 3.
Summary of the pooled prevalence estimates of gastrointestinal symptoms with respect to the inpatient/outpatient status.
In this study’s evaluation phase, we detected four “outpatient-only” studies out of 205 eligible studies. Since there were only 951 patients from the “outpatient-only” studies, it was not suitable to compare those with “inpatient-only” studies. Thus, we performed a separate meta-analysis for the four “outpatient-only” studies. The prevalence of GI symptoms was as follows in the “outpatient-only” studies: diarrhea 0.10 (95% CI, 0.01, 0.27), any GI symptoms 0.13 (95% CI, 0.01, 0.41), respiratory symptoms 0.19 (95% CI, 0.07, 0.36), fever 0.63 (95% CI, 0.27, 0.93), and severe COVID-19 disease 0.08 (95% 0.01, 0.18).
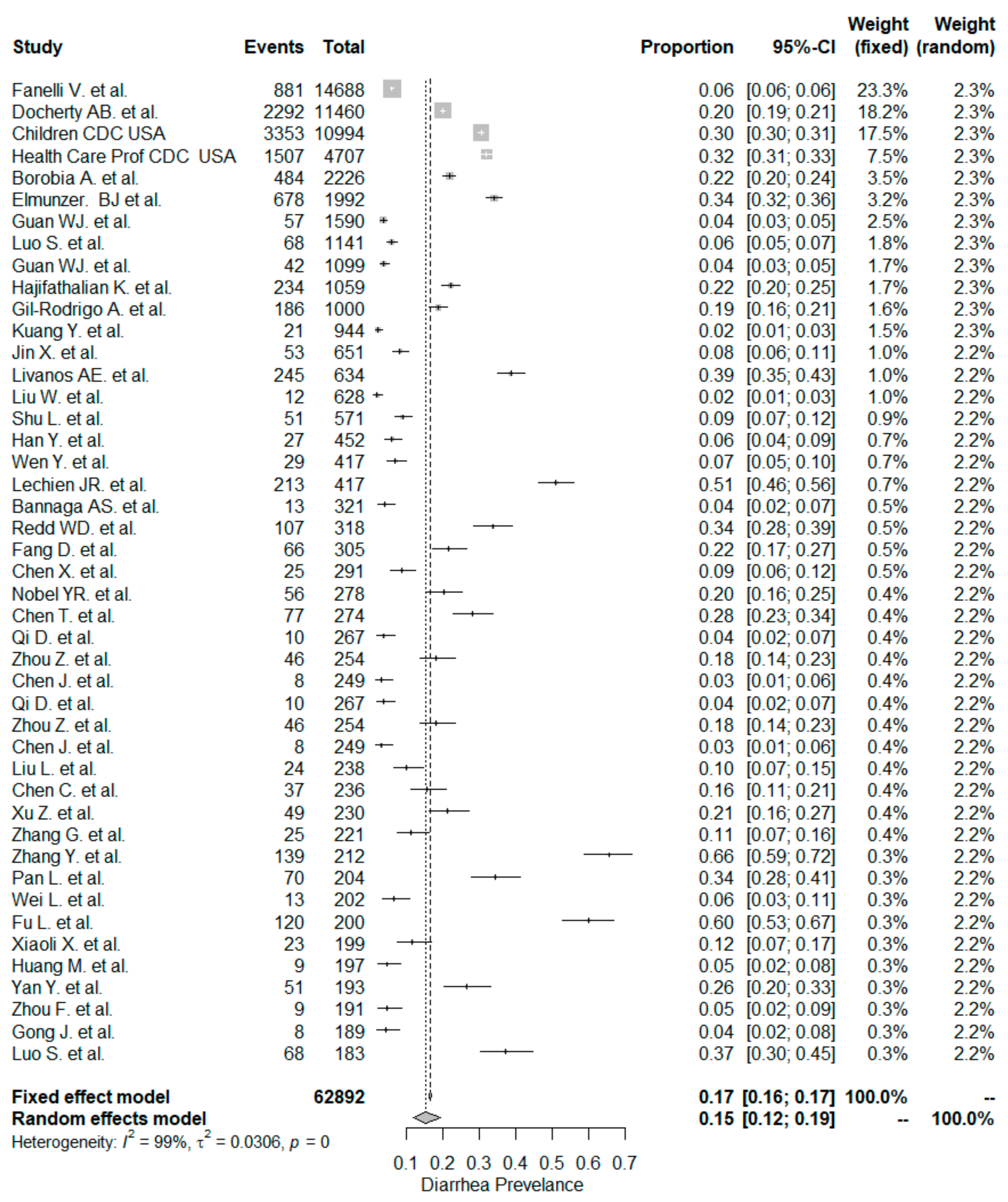
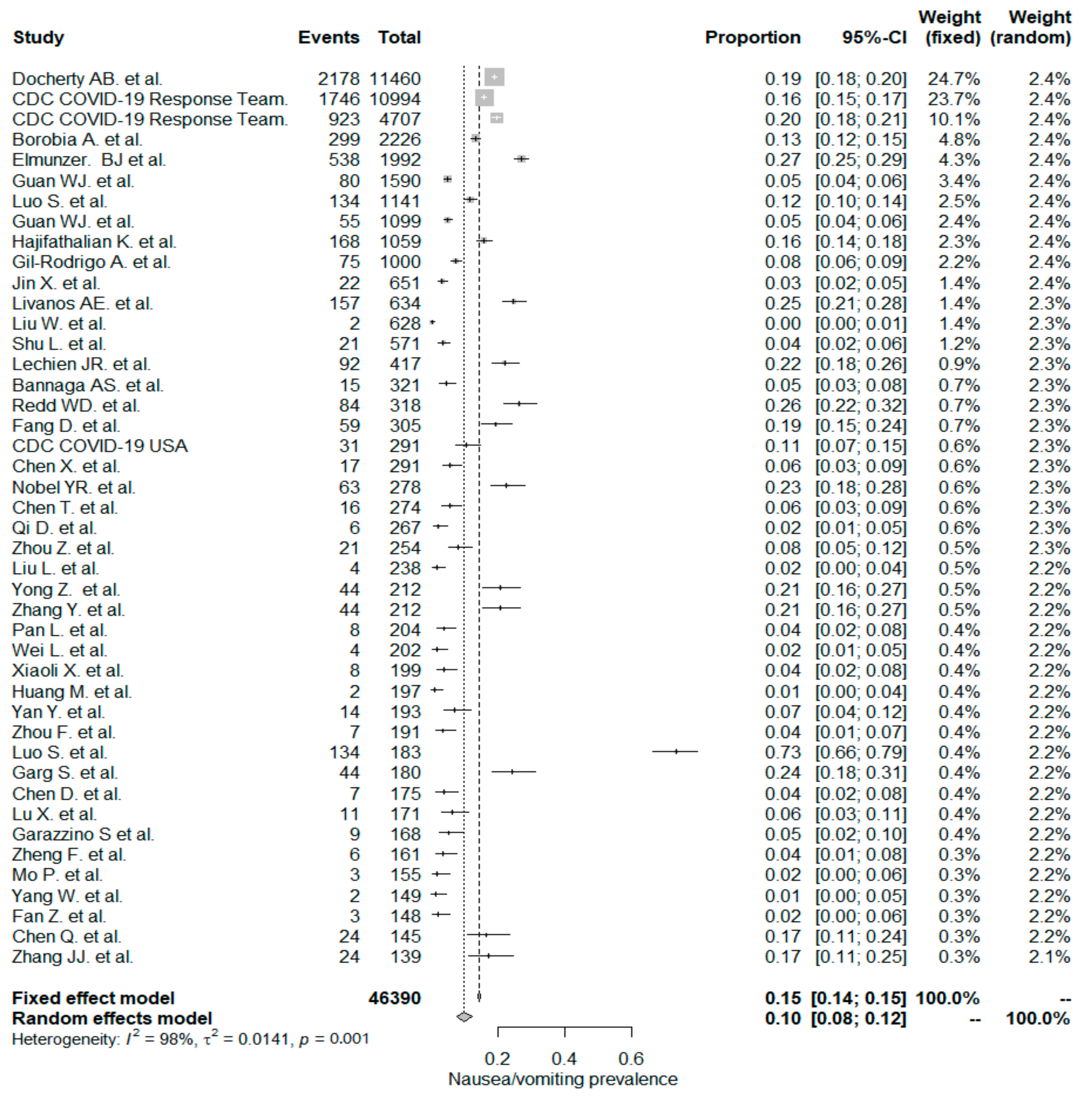
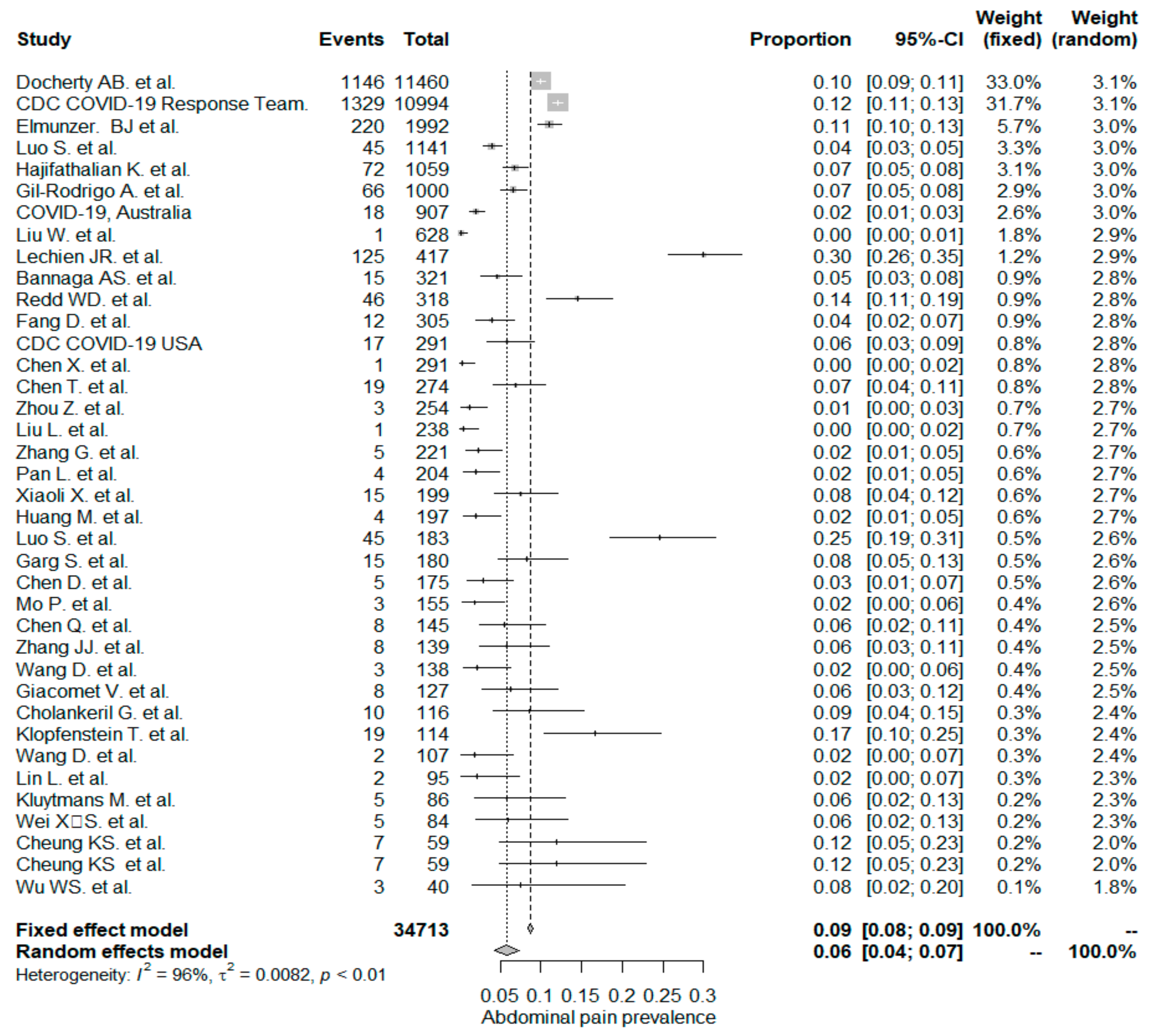
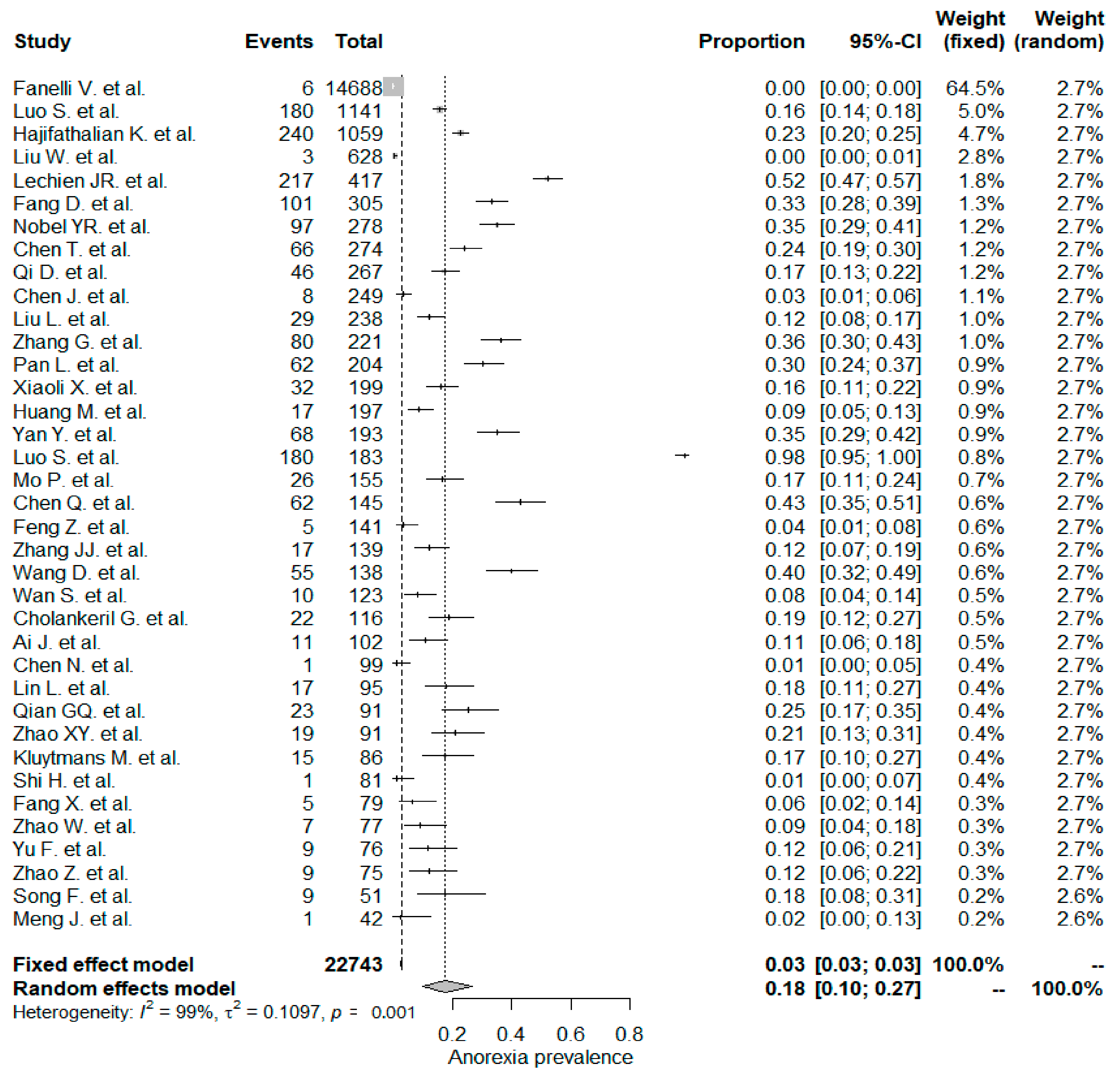
The studies included in the meta-analysis had significant heterogeneity (I2 = 99%, p< 0.001); therefore, the random effects model was taken into consideration. The meta-analysis revealed that the most commonly reported GI symptoms were anorexia followed by diarrhea, whereas the least common symptom was abdominal pain. Forest plot graphics for diarrhea, nausea/vomiting, abdominal pain, and anorexia are shown in Figure 2, Figure 3, Figure 4 and Figure 5, respectively.
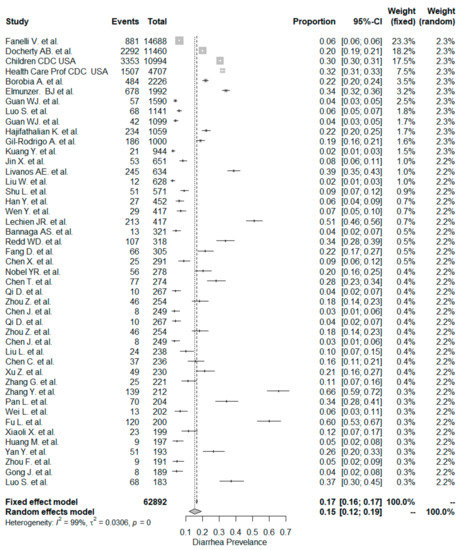
Figure 2.
All studies, diarrhea forest plot and meta-analysis results.
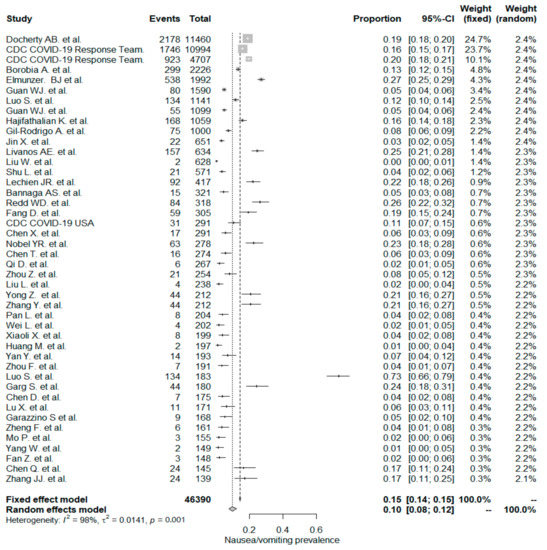
Figure 3.
All studies, nausea/vomiting forest plot and meta-analysis results.
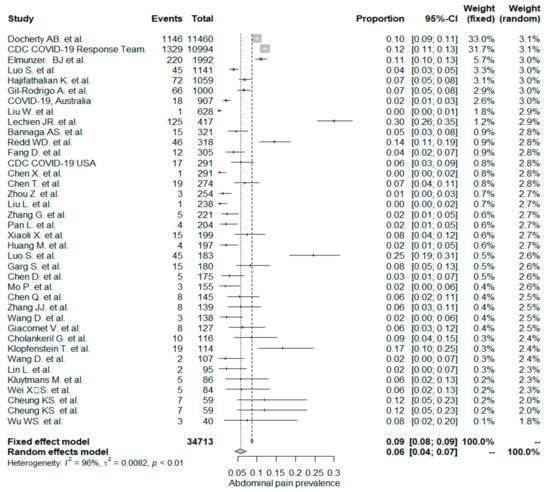
Figure 4.
All studies, abdominal pain/discomfort forest plot and meta-analysis results.
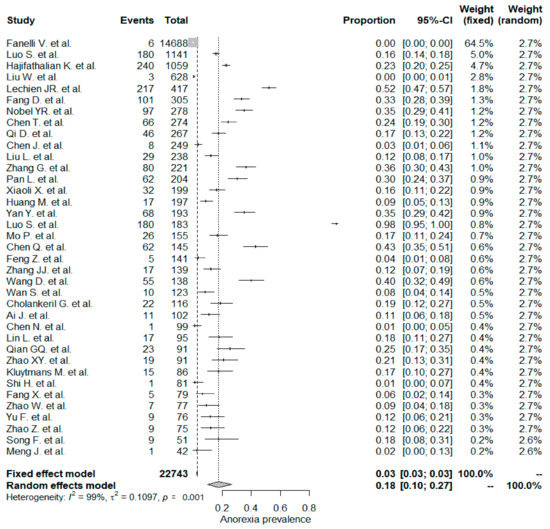
Figure 5.
All studies, anorexia forest plot and meta-analysis results.
5. Discussion
The novel SARS-CoV-2 is currently causing a major pandemic that constitutes a world health crisis. COVID-19 patients commonly have fever and respiratory illness. However, some patients also complain of GI symptoms such as diarrhea, nausea/vomiting, and abdominal pain. Fecal–oral transmission of COVID-19 infection has been confirmed by the fact that the virus can replicate in both the respiratory and digestive tracts [123]. The first step of viral entry into enterocytes occurs via angiotensin-converting enzyme 2 (ACE2) binding to ACE2 receptors on the surfaces of enterocytes, similarly to SARS-CoV [4]. After entry into the host cell, viral RNA and proteins are produced by ribosomes. Viral capsids, RNA, and proteins combine to form multiple new copies of COVID-19. These viral particles lead to cytokine release (interleukin (IL)-2, IL-7, tumor necrosis factor (ΤΝF)-α, and macrophage and monocyte products), which mediate various effects on organs. The virus can then spread to other digestive organs, such as the liver using the same ACE2 enzyme [124].
Patients with metabolic conditions such as obesity, diabetes, cardio-metabolic problems, and liver diseases were repeatedly reported to have higher rates of COVID-19 related morbidity in various studies [17,19,125].Gut microbiota can influence the immune response via affecting disease progression. Not only over-active, but also a hypo-active immune response possibly mediated by gut microbiota may lead to severe clinically adverse events. The colon includes a large density of bacteria in the families of Bacteroidaceae, Prevotellaceae, Rikenellaceae, Lachnospiraceae, and Ruminococcaceae [126], while Bacteroidetes, Firmicutes, and Proteobacteria are more preponderate in the lung [127]. The gut microbiota may affect pulmonary health through interactions between the gut microbiota and the lungs, named the “gut–lung axis” [128]. The gut–lung axis is reciprocal, so endotoxins, microbial metabolites, can affect the lung through the blood and inflammation of the lungs can affect the gut microbiota as well [129]. Several studies have shown that respiratory infections are associated with a change in the composition of the gut microbiota [130]. Multiple data suggest that the gut microbiota play a key role in the pathogenesis of sepsis and Adult Respiratory Distress Syndrome (ARDS). Loss of gut bacterial diversity may lead to dysbiosis that can be associated with many diseases [131]. As many elderly and immune-compromised patients progress to serious adverse clinical consequences in Covid-19, possible cross-talk may be occurring between the lung and intestinal microbiota, which may affect the outcome of the disease’s course.
To the best of our knowledge, this systematic review and meta-analysis presents the largest patient population involving COVID–19 infection and GI symptoms. We used prepublication repositories medRxiv and bioRxiv that enabled us to search and include unpublished manuscripts from gray literature and enlarge our study population drastically. Most of the studies outside of China began reporting in May 2020, especially those from Western countries. We included 31 Chinese studies with a total of 12,798 patients and 13 non-Chinese studies with a total of 50,094 patients. Therefore, we were able to effectively compare Chinese and non-Chinese COVID-19 infection studies according to their GI manifestations. Currently, the majority of original studies, systemic reviews, and meta-analysis studies are from China with a few exceptions and are mostly limited to the “inpatient-only” clinical setting [132]. We included clinical settings of both mixed studies (“inpatient together with outpatient” plus “outpatient-only”) and “inpatient-only” studies.
5.1. Diarrhea
In our meta-analysis the overall prevalence of diarrhea was 15%. The rate of diarrhea was significantly higher in non-Chinese studies (24%) compared to Chinese studies (12% and p < 0.001). Similar but lower prevalence rates have been reported in a recent meta-analysis [132]. The pooled prevalence estimate (PPE) of diarrhea was 7.7% in the overall population, 18.3% in non-Chinese studies, and 5.8% in Chinese studies.
In this study, the occurrence of diarrhea was similar for the “inpatient-only” study group (14%) and mixed patient study group (16% and p = 0.795). The meta-analysis by Sultan et al. [132] included 39 “inpatient-only” studies with a total of 8521 patients, and the PPE for diarrhea was 10.4%, which was lower than the corresponding PPE of 14.4% in this study.
We detected a total of four studies (three Chinese and one USA study), which tailored the clinical setting as “outpatient-only.” Since there was a low number of patients (n = 951) we could not compare the “outpatient-only” study group with the “inpatient-only” study group. However, a meta-analysis for these four “outpatient-only” studies was conducted. The PPE of diarrhea was 10%. On the other hand, the meta-analysis by Sultan et al. [132] analyzed three “outpatient-only” studies, including 1701 patients. In that meta-analysis, the PPE value for diarrhea in “outpatient-only” studies was 4%.
In a recent and large comprehensive meta-analysis about general evaluations of patients with COVID-19 infection included 59,254 patients mostly from China but also including 10 other countries, reported a PPE of 9% for any type of GI symptoms [125]. However, subgroups of GI symptoms were not given in that meta-analysis.
The inception studies from China mostly reported low incidence of diarrhea and other GI symptoms [120]. In an earlier meta-analysis including 6686 patients from 35 Chinese studies, which compromised “inpatient-only” studies, a PPE value of 9% for diarrhea was reported [133]. In contrast with low prevalence reports, in a study from Taizhou, China and another one from Shanghai, China in which 212 mild COVID-19 patients were included, the rates of any GI symptoms were reported as high as 42.8% and 43.8%, respectively [40,63]. More recent reports from China claimed even higher rates of diarrhea (49.5%) and any GI symptoms (79%) [35]. Interestingly, a Chinese study including 232 hospitalized patients from Wuhan compared the rate of diarrhea between a group of patients who were admitted from January 19 to February 11, 2020 with that of another non-overlapping group of patients who were admitted from February 12 to March 2020 at the same hospital setting [43]. They concluded that as the COVID-19 infection outbreak progressed, the rate of diarrhea increased from 19% up to 43% (p = 0.022) in these two distinct groups of hospitalized patients.
The increase in the reported rates of GI symptoms from Chinese studies needs further explanation. Possible explanations may include increased awareness of non-respiratory symptoms, increased documentation, and re-infection as the outbreak progressed. Re-infection is common for “seasonal” coronaviruses 229E, OC43, NL63, and HKU1 [134]. COVID-19 can also reoccur after the first infection. It was confirmatively reported that one patient had a re-infection instead of persistent viral shedding from the first infection, by the epidemiological, clinical, serological, and genomic analyses [135]. These results showed that SARS-CoV-2 may continue to circulate among human populations.
5.2. Nausea/Vomiting
The PPE of nausea/vomiting for the whole study population was 10%, which included 44 studies with a total of 46,390 patients. The PPE of nausea/vomiting was significantly higher in non-Chinese studies (17%) than in Chinese studies (7% and p < 0.001).
In a recent meta-analysis by Sultan et al. [132], the overall PPE of nausea/vomiting was reported to be 7.8%. It was also noted that nausea/vomiting PPE in non-Chinese studies had a higher value (14.9%) than Chinese studies (5.2%). These results are in line with the current study results but have lower rates.
In this study, we reported the PPE for of nausea/vomiting for 22 “inpatient-only” studies was (12%). The PPE was (6%) for nausea/vomiting in an earlier Chinese meta-analysis that included 6686 hospitalized patients from 35 studies [133]. In this study, PPE of nausea/vomiting in the 22 mixed studies was (8%). Still, the difference was not statistically significant between “inpatient-only” and mixed-study groups.
5.3. Abdominal Pain/Discomfort
In the whole study population, the overall PPE of the abdominal pain/discomfort symptom was 6%. The abdominal pain/discomfort symptom was significantly higher in non-Chinese studies (9%), compared to Chinese studies (4% and p < 0.001). In a recent meta-analysis by Sultan et al. (133), the overall PPE of the abdominal pain/discomfort symptom was reported as 3.6%. Sultan et al. reported that non-Chinese studies had a PPE value of 5.3%, which is higher than that of Chinese studies (2.7%) and is in line with our results, but still has lower rates than what we have seen. The occurrence of the abdominal pain or discomfort symptom was significantly higher in the mixed-patient study group (7%) when compared to the “inpatient-only” study group (4% and p= 0.032).
5.4. Anorexia
The overall PPE of anorexia was 18% in the whole study population and was the most prevalent GI symptom. The anorexia symptom was higher in Chinese studies (21%) when compared to non-Chinese studies (17%), but the difference was not statistically significant (p: 0.783). In a Chinese meta-analysis including 6686 patients, the reported PPE of the anorexia symptom was the same as that in this study, 21% [133]. Usage of experimental drugs and herbal medicine against COVID–19 infections might be a possible explanation for the higher rate of anorexia symptom PPE values [136].
5.5. Limitations
In the aftermath of this pandemic, there was a skyrocketing of COVID-19 infection-related publications, which certainly led to a compromised adherence to some scientific publication criteria. Though at a time of disaster, these issues were criticized, and concerns about the quality of publications were raised [137]. The nature of such a big disaster would probably be associated with potential bias. In the related literature, we observed that the patient cohorts included very heterogeneous groups. We tried our best to prevent doubling of patients and we excluded some manuscripts to prevent overlapping. In most of the studies, the authors did not clearly state whether they did a full GI system evaluation upon admission. For most of the studies, the time periods of the GI symptoms and latency of respiratory symptoms and fever was not stated. Most of the studies did not state the underlying systemic diseases, including GI diseases that may have affected the assessment of GI symptoms. Additionally, for most of the studies, treatment strategies, including drug and herbal medicines in Chinese studies, were not well documented. The severity of COVID-19 disease was not well defined with different studies using different definitions. Still, the presence of GI symptoms and the degree of severity of COVID-19 infection were not well documented in most of the studies. Since liver function tests were not mentioned in most of the studies, we did not scan laboratory liver abnormalities. Another limitation is the fact that most of the included studies did not report the rate of GI symptoms in subgroups of severe or non-severe patients or between other subgroups. Therefore, we could not perform a head-to-head comparison in this regard. Lastly, there were very few “outpatient-only” studies and we could not compare them specifically with “inpatient-only” studies.
6. Conclusions
In this highly populated and comprehensive systematic review and meta-analysis study, we reported high PPE rates of anorexia, diarrhea, nausea/vomiting, and abdominal pain. Although healthcare providers and patients are well aware of the common symptoms of COVID-19 such as fever, cough, and shortness of breath, there is need to raise the awareness about the fact that not all individuals present with these symptoms, as gastrointestinal symptoms are relatively common with this disease. The PPE rates of diarrhea, nausea/vomiting, and abdominal pain were significantly higher in non-Chinese studies compared to Chinese studies. We also observed a higher prevalence for GI symptoms in the Chinese studies than what was reported previously. Non-respiratory symptoms, including those related to the GI tract, should be more carefully evaluated and reported in future studies.
Author Contributions
G.H., V.T. and H.A.: Conception, design, critical revision of the manuscript for important intellectual content; H.A., R.K., A.S. and F.T.: Manuscript drafting; H.A., R.K., R.O. and F.T.: Entry of data into the statistical program, statistical analysis, H.A., R.K. and A.S.: database searching, identification and elimination of manuscripts, data extraction. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are very thankful to Mehmet Karadag (Orcid ID number: 0000-0001-9539-4193) for conducting our statistical analysis, especially for meta-analysis and Forest plots.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- WHO. COVID-19 Update (25 September 2020). Wkly. Epidemiol. Rec. 2020, 95, 461–476. [Google Scholar]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Krüger, N.; Müller, M.; Drosten, C.; Pöhlmann, S. The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020. [Google Scholar] [CrossRef]
- WHO. WHO issues consensus document on the epidemiology of SARS. Wkly Epidemiol. Rec. Relev. Épidémiol. Hebd. 2003, 78, 373–375. [Google Scholar]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Diagnosis and Treatment Plan for Pneumonia Infected by New Coronavirus (TrialSixthEdition)[EB/OL]. Available online: http://www.nhc.gov.cn (accessed on 2 August 2020).
- Docherty, A.B.; Green, C.A. Featuresof 16, 749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterization Protocol. MedRvix 2020. [Google Scholar] [CrossRef]
- Fanelli, V.; Fiorentino, M.; Cantaluppi, V.; Gesualdo, L.; Stallone, G.; Ronco, C.; Castellano, G. Acute kidney injury in SARS-CoV-2 infected patients. Crit. Care 2020, 24, 155. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children-UnitedStates, February12-April2, 2020. Mmwr. Morb. Mortal. Wkly Rep. 2020, 69, 422–426. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team. Characteristics of Health Care Personnel with COVID-19-UnitedStates, February12-April9, 2020. Mmwr. Morb. Mortal. Wkly Rep. 2020, 69, 477–481. [Google Scholar] [CrossRef]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020, 10, 1016. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Arnalich, F.; Álvarez-Sala, R.; Monserrat-Villatoro, J.; Quintana, M.; Figueira, J.C.; Torres Santos-Olmo, R.M.; García-Rodríguez, J.; Martín-Vega, A.; et al. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. J. Clin. Med. 2020, 9, 1733. [Google Scholar] [CrossRef] [PubMed]
- Elmunzer, B.J.; Spitzer, R.L.; Foster, L.D.; Merchant, A.A.; Howard, E.F.; Patel, V.A.; West, M.K.; Qayad, E.; Nustas, R.; Zakaria, A.; et al. Digestive Manifestations in Patients Hospitalized with COVID-19. MedRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Liang, W.H.; Zhao, Y.; Liang, H.R.; Chen, Z.S.; Li, Y.M.; Liu, X.Q.; Chen, R.C.; Tang, C.L.; Wang, T.; et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: A nationwide analysis. Eur. Respir. J. 2020, 55, 547–557. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, X.; Xu, H. Don’t Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19). Clin. Gastroenterol. Hepatol. 2020, 18, 1636–1637. [Google Scholar] [CrossRef]
- Cheng, J.L.; Huang, C.; Zhang, G.J.; Liu, D.W.; Li, P.; Lu, C.Y.; Li, J. Epidemiological characteristics of novel coronavirus pneumonia in Henan. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, 327–331. [Google Scholar] [CrossRef]
- Hajifathalian, K.; Krisko, T.; Mehta, A.; Kumar, S.; Schwartz, R.; Fortune, B.; Sharaiha, R.Z. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology 2020, 159, 1137–1140. [Google Scholar] [CrossRef]
- Gil-Rodrigo, A.; Miro, O.; Pinera, P.; Burillo-Putze, G.; Jimenez, S.; Martin, A.; Martin-Sanchez, F.J.; Jacob, J.; Guardiola, J.M.; Garcia-Lamberechts, E.J.; et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias 2020, 32, 233–241. [Google Scholar]
- Kuang, Y.; Zhang, H.; Zhou, R.; Lin, S.; Lin, M.; Wang, J.; Pang, P.; Ma, L.; Ji, W. Epidemiological and clinical characteristics of 944 cases of 2019 novel Coronavirus infection of non-COVID-19 exporting city, Zhejiang, China. Available online: https://ssrn.com/abstract=3543604 (accessed on 20 February 2020).
- COVID-19 National Incident Room Surveillance Team. COVID-19, Australia: Epidemiology Report 13 (Reporting week to 23: 59 AEST 26 April 2020). Commun. Dis. Intell. (2018) 2020, 44, 1–27. [Google Scholar]
- Jin, X.; Xu, K.; Jiang, P.; Lian, J.; Hao, S.; Yao, H.; Jia, H.; Zhang, Y.; Zheng, L.; Zheng, N. Virus strain from a mild COVID-19 patient in Hangzhou represents a new trend in SARS-CoV-2 evolution potentially related to Furin cleavage site. Emerg. Microbes Infect. 2020, 9, 1474–1488. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Lian, J.-S.; Hu, J.-H.; Gao, J.; Zheng, L.; Zhang, Y.-M.; Hao, S.-R.; Jia, H.-Y.; Cai, H.; Zhang, X.-L. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020, 69, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Livanos, A.E.; Jha, D.; Cossarini, F.; Gonzalez-Reiche, A.S.; Tokuyama, M.; Aydillo, T.; Parigi, T.L.; Ramos, I.; Dunleavy, K.; Lee, B. Gastrointestinal involvement attenuates COVID-19 severity and mortality. MedRxiv 2020. [Google Scholar] [CrossRef]
- Liu, W.; Wang, F.; Li, G.; Wei, Y.; Li, X.; He, L.; Yue, H.; Zhang, F.; Hu, Q.; Chu, J. Analysis of 2019 Novel Coronavirus Infection and Clinical Characteristics of Outpatients: An Epidemiological Study from the Fever Clinic in Wuhan, China. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3539646 (accessed on 20 February 2020).
- Shu, L.; Wang, X.; Li, M.; Chen, X.; Shi, L.; Wu, M.; Deng, K.; Wei, J.; Wang, X.; Cao, Y. Clinical Characteristics of 545 Cases Confirmed COVID-19 in Wuhan Stadium Cabin Hospital. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3552844 (accessed on 20 February 2020).
- Han, Y.; Liu, Y.; Zhou, L.; Chen, E.; Liu, P.; Pan, X.; Lu, Y. Epidemiological Assessment of Imported Coronavirus Disease 2019 (COVID-19) Cases in the Most Affected City Outside of Hubei Province, Wenzhou, China. Jama Netw. Open 2020, 3, e206785. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Wei, L.; Li, Y.; Tang, X.; Feng, S.; Leung, K.; Wu, X.; Pan, X.-F.; Chen, C.; Xia, J. Epidemiological and clinical characteristics of COVID-19 in Shenzhen, the largest migrant city of China. MedRvix 2020. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Bannaga, A.S.; Tabuso, M.; Farrugia, A.; Chandrapalan, S.; Somal, K.; Lim, V.K.; Mohamed, S.; Nia, G.J.; Mannath, J.; Wong, J.L. C-reactive protein and albumin association with mortality of hospitalised SARS-CoV-2 patients: A tertiary hospital experience. Clin. Med. 2020, 20, 463–467. [Google Scholar] [CrossRef]
- Reddm, W.D.; Zhou, J.C.; Hathorn, K.E.; McCarty, T.R.; Bazarbashi, A.N.; Thompson, C.C.; Shen, L.; Chan, W.W. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: A multicenter cohort study. Gastroenterology 2020, 159, 765–767. [Google Scholar] [CrossRef]
- Fang, D.; Ma, J.; Guan, J.; Wang, M.; Song, Y.; Tian, D. Manifestations of digestive system in hospitalized patients with novel coronavirus pneumonia in Wuhan, China: A single-center, descriptive study. Chin. J. Dig. 2020, 40, 110–115. [Google Scholar]
- Chen, X.; Zheng, F.; Qing, Y.; Ding, S.; Yang, D.; Lei, C.; Yin, Z.; Zhou, X.; Jiang, D.; Zuo, Q. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: A double-center observational study. MedRvix 2020. [Google Scholar] [CrossRef]
- Covid, C.; COVID, C.; COVID, C.; Bialek, S.; Gierke, R.; Hughes, M.; McNamara, L.A.; Pilishvili, T.; Skoff, T. Coronavirus Disease 2019 in Children—United States, 12 February–2 April 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 422. [Google Scholar]
- Nobel, Y.; Phipps, M.; Zucker, J. Gastrointestinal Symptoms and COVID-19: Case-Control Study from the United States. Gastroenterology 2020, 159, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. Bmj 2020, 368, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Yan, X.; Tang, X.; Peng, J.; Yu, Q.; Feng, L.; Yuan, G.; Zhang, A.; Chen, Y.; Yuan, J. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: A retrospective, descriptive, multiple-center study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, N.; Shu, Y.; Han, S.; Chen, B.; Shu, X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology 2020, 158, 2294. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qi, T.; Liu, L.; Ling, Y.; Qian, Z.; Li, T.; Li, F.; Xu, Q.; Zhang, Y.; Xu, S. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020, 80, 1–6. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; Zheng, Y.; Jiang, X.; Kou, G.; Ding, J.; Wang, Q.; Huang, Q.; Ding, Y.; Ni, W. A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients. Microbes Infect. 2020, 22, 206–211. [Google Scholar] [CrossRef]
- Chen, C.; Huang, J.; Cheng, Z.; Wu, J.; Chen, S.; Zhang, Y.; Chen, B.; Lu, M.; Luo, Y.; Zhang, J. Favipiravir versus arbidol for COVID-19: A randomized clinical trial. MedRxiv 2020. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Shen, L.; Zou, Y.; Hou, L.; Zhu, L.; Faden, H.S.; Tang, Z.; Shi, M.; Jiao, N. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020, 5, 534–535. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, C.; Luo, L.; Fang, F.; Chen, Y.; Li, J.; Peng, Z.; Pan, H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020, 127, 104364. [Google Scholar] [CrossRef]
- Zhang, Y. Gastrointestinal tract symptoms in coronavirus disease 2019: Analysis of clinical symptoms in adult patients. MedRxiv 2020. [Google Scholar] [CrossRef]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; JIN, D.; Zhang, J.; Wang, B.; Sun, M.; Li, X.; Zhang, Y.; Lian, F.; Tong, X. Clinical Findings of 100 Mild Cases of COVID-19 in Wuhan: A Descriptive Study. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3551332 (accessed on 20 February 2020).
- Fu, L.; Fei, J.; Xiang, H.-X.; Xiang, Y.; Tan, Z.-X.; Li, M.-D.; Liu, F.-F.; Liu, H.-Y.; Zheng, L.; Li, Y. Influence factors of death risk among COVID-19 patients in Wuhan, China: A hospital-based case-cohort study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Xiong, X.; Wong, K.K.-Y.; Chi, S.; Zhou, A.; Tang, J.; Zhou, L.; Chung, P.H.-y.; Chua, G.; Tung, K.T.; Wong, I.C. Are COVID-19 infected children with gastrointestinal symptoms different from those without symptoms? A comparative study of the clinical characteristics and epidemiological trend of 244 pediatric cases from Wuhan. MedRxiv 2020. [Google Scholar] [CrossRef]
- Huang, M.; Zhan, F.; Wang, J.; Yi, Q.; Zhu, F.; Yang, H.; Xiang, Q.; Xiang, J.; Fan, S.; Zhang, X. Epidemiological and Clinical Features of 197 Patients Infected with 2019 Novel Coronavirus in Chongqing, China: A Single Center Descriptive Study. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3539687 (accessed on 20 February 2020).
- Yan, Y.; Yang, Y.; Wang, F.; Ren, H.; Zhang, S.; Shi, X.; Yu, X.; Dong, K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. Bmj Open Diabetes Res. Care 2020, 8, e001343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Gong, J.; Ou, J.; Qiu, X.; Jie, Y.; Chen, Y.; Yuan, L.; Cao, J.; Tan, M.; Xu, W.; Zheng, F. A tool to early predict severe 2019-novel coronavirus pneumonia (COVID-19): A multicenter study using the risk nomogram in Wuhan and Guangdong, China. MedRxiv 2020. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-NET: COVID-19 associated hospitalization surveillance network. Access: April 2020, 8, 6. [Google Scholar]
- Li, X.; Hu, C.; Su, F.; Dai, J. Hypokalemia and clinical implications in patients with coronavirus disease 2019 (COVID-19). MedRxiv 2020. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- Garazzino, S.; Montagnani, C.; Donà, D.; Meini, A.; Felici, E.; Vergine, G.; Bernardi, S.; Giacchero, R.; Vecchio, A.L.; Marchisio, P. Multicentre Italian study of SARS-CoV-2 infection in children and adolescents, preliminary data as at 10 April 2020. Eurosurveillance 2020, 25, 2000600. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Tang, W.; Li, H.; Huang, Y.; Xie, Y.; Zhou, Z. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur. Rev. Med. Pharm. Sci. 2020, 24, 3404–3410. [Google Scholar]
- Mo, P.; Xing, Y.; Xiao, Y.; Deng, L.; Zhao, Q.; Wang, H.; Xiong, Y.; Cheng, Z.; Gao, S.; Liang, K. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin. Infect. Dis. 2020. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cao, Q.; Qin, L.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, L.; Li, J.; Cheng, X.; Yang, J.; Tian, C.; Zhang, Y.; Huang, S.; Liu, Z.; Cheng, J. Clinical features of COVID-19-related liver damage. Clin. Gastroenterol. Hepatol. 2020, 18, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, Z.; Zhang, C.; Zhang, X.; Wu, H.; Wang, J.; Wang, S.; Zheng, C. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection 2020, 48, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yu, Q.; Yao, S.; Luo, L.; Duan, J.; Yan, Z.; Yang, M.; Tan, H.; Ma, M.; Li, T. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics. MedRxiv 2020. [Google Scholar] [CrossRef]
- Zhang, J.-j.; Dong, X.; Cao, Y.-y.; Yuan, Y.-d.; Yang, Y.-b.; Yan, Y.-q.; Akdis, C.A.; Gao, Y.-d. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Liu, K.; Fang, Y.-Y.; Deng, Y.; Liu, W.; Wang, M.-F.; Ma, J.-P.; Xiao, W.; Wang, Y.-N.; Zhong, M.-H.; Li, C.-H. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020, 133, 1025–1031. [Google Scholar] [CrossRef]
- Li, X.; Zeng, W.; Li, X.; Chen, H.; Shi, L.; Li, X.; Xiang, H.; Cao, Y.; Chen, H.; Liu, C. CT imaging changes of corona virus disease 2019 (COVID-19): A multi-center study in Southwest China. J. Transl. Med. 2020, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Giacomet, V.; Barcellini, L.; Stracuzzi, M.; Longoni, E.; Folgori, L.; Leone, A.; Zuccotti, G.V. Gastrointestinal Symptoms in Severe COVID-19 Children. Pediatric Infect. Dis. J. 2020, 39, e317–e320. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.; Tu, S.; Wei, Y.; Xiao, L.; Jin, Y.; Zhang, L.; Song, J.; Liu, W.; Zhu, Q.; Yang, L. Clinical and Laboratory Factors Predicting the Prognosis of Patients with COVID-19: An Analysis of 127 Patients in Wuhan, China. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3546118 (accessed on 20 February 2020).
- Wan, S.; Yi, Q.; Fan, S.; Lv, J.; Zhang, X.; Guo, L.; Lang, C.; Xiao, Q.; Xiao, K.; Yi, Z. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020, 189, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Cholankeril, G.; Podboy, A.; Aivaliotis, V.I.; Tarlow, B.; Pham, E.A.; Spencer, S.P.; Kim, D.; Hsing, A.; Ahmed, A. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology 2020, 159, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-L.; Xia, S.-Y.; Wang, M.; Zhang, S.-M.; WH, D.; Chen, Q. Clinical features of children with SARS-CoV-2 infection: An analysis of 115 cases. Zhongguo Dang Dai Er Ke Za Zhi= Chin. J. Contemp. Pediatrics 2020, 22, 290–293. [Google Scholar]
- Wang, K.; Kang, S.; Tian, R.; Zhang, X.; Wang, Y. Imaging manifestations and diagnostic value of chest CT of corona virus disease 2019 (COVID-19) in the Xiaogan area. Clin. Radiol. 2020, 75, 341–347. [Google Scholar] [CrossRef]
- Klopfenstein, T.; N’driJuliette Kadiane-Oussou, P.; Royer, Y.; Toko, L.; Gendrin, V.; Zayet, S. Diarrhea: An under estimated symptom in Corona virus disease 2019. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 282–283. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, K.; Guan, H.; Leng, L.; Zhu, R.; Wang, B.; He, M.; Cheng, L.; Huang, K.; Zeng, Q. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019 -n CoV. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, E004. [Google Scholar]
- Liu, Y.; Sun, W.; Li, J.; Chen, L.; Wang, Y.; Zhang, L.; Yu, L. Clinical features and progression of acute respiratory distress syndrome in corona virus disease 2019. MedRxiv 2020. [Google Scholar] [CrossRef]
- Han, R.; Huang, L.; Jiang, H.; Dong, J.; Peng, H.; Zhang, D. Early clinical and CT manifestations of corona virus disease 2019 (COVID-19)pneumonia. Am. J. Roentgenol. 2020, 215, 338–343. [Google Scholar] [CrossRef]
- Wang, D.; Yin, Y.; Hu, C.; Liu, X.; Zhang, X.; Zhou, S.; Jian, M.; Xu, H.; Prowle, J.; Hu, B. Clinical course and outcome of 107 patients infected with the novel corona virus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit. Care 2020, 24, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-Y.; Wang, L.-W.; Chen, Y.-Y.; Shen, X.-K.; Wang, Q.; Yan, Y.-Q.; Yu, Y.; Wu, Q.; Wang, X.; Zhong, Y.-H. A multi centre study of 2019 novel corona virus disease outcomes of cancer patients in Wuhan, China. MedRxiv 2020. [Google Scholar] [CrossRef]
- Tabata, S.; Imai, K.; Kawano, S.; Ikeda, M.; Kodama, T.; Miyoshi, K.; Obinata, H.; Mimura, S.; Kodera, T.; Kitagaki, M. The clinical characteristics of COVID-19: A retrospective analysis of 104 patients from the outbreak on board the Diamond Princess cruiseship in Japan. MedRxiv 2020. [Google Scholar] [CrossRef]
- Ai, J.; Chen, J.; Wang, Y.; Liu, X.; Fan, W.; Qu, G.; Zhang, M.; Pei, S.P.; Tang, B.; Yuan, S. The cross-sectional study of hospitalized corona virus disease 2019 patients in Xiangyang, Hubei province. MedRxiv 2020. [Google Scholar] [CrossRef]
- Zhao, W.; Zhong, Z.; Xie, X.; Yu, Q.; Liu, J. Relation between chest CT findings and clinical conditions of corona virus disease (COVID-19) pneumonia: A multi center study. Am. J. Roentgenol. 2020, 214, 1072–1077. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel corona virus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, X.; Zhang, Z.; Huang, S.; Zhang, Z.; Fang, Z.; Gu, Z.; Gao, L.; Shi, H.; Mai, L. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020, 69, 997–1001. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Shi, Y.; Wang, H.; Zhao, R.; Sheng, J. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef]
- Qian, G.-Q.; Yang, N.-B.; Ding, F.; Ma, A.H.Y.; Wang, Z.-Y.; Shen, Y.-F.; Shi, C.-W.; Lian, X.; Chu, J.-G.; Chen, L. Epidemiologic and Clinical Characteristics of 91 Hospitalized Patients with COVID-19 in Zhejiang, China: A retrospective, multi-centre case series. Qjm: Int. J. Med. 2020, 113, 474–481. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Xu, X.-X.; Yin, H.-S.; Hu, Q.-M.; Xiong, T.; Tang, Y.-Y.; Yang, A.-Y.; Yu, B.-P.; Huang, Z.-P. Clinical characteristics of patients with 2019 corona virus disease in a non-Wuhan area of Hubei Province, China: A retrospective study. Bmc Infect. Dis. 2020, 20, 1–8. [Google Scholar] [CrossRef]
- Xu, X.; Yu, C.; Qu, J.; Zhang, L.; Jiang, S.; Huang, D.; Chen, B.; Zhang, Z.; Guan, W.; Ling, Z. Imaging and clinical features of patients with 2019 novel corona virus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1275–1280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Wang, T.; Shi, Z.; Zhang, Z. Caution: Clinical Characteristics of COVID-19 Patients Are Changing at Admission. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3546044 (accessed on 20 February 2020).
- Xu, W.; Qu, S.; Xing, M.; Zhang, M.; Lu, G.; Liao, Z.; Griffin, K.; Wang, J.; Zen, K.; Yao, B. Epidemiologic Features and Clinical Findings of COVID-19 Infected Patients in Suzhou. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3551352 (accessed on 20 February 2020).
- Kluytmans, M.; Buiting, A.; Pas, S.; Bentvelsen, R.; vanden Bijllaardt, W.; van Oudheusden, A.; van Rijen, M.; Verweij, J.; Koopmans, M.; Kluytmans, J. SARS-CoV-2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. MedRxiv 2020. [Google Scholar] [CrossRef]
- Wei, X.-S.; Wang, X.; Niu, Y.-R.; Ye, L.-L.; Peng, W.-B.; Wang, Z.-H.; Yang, W.-B.; Yang, B.-H.; Zhang, J.-C.; Ma, W.-L. Diarrhea is associated with prolonged symptoms and viral carriage in COVID-19. Clin. Gastroenterol. Hepatol. 2020, 18, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wu, J.; Wu, F.; Guo, D.; Chen, L.; Fang, Z.; Li, C. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Investig. Radiol. 2020, 55, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-1 9 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering 2020, Article in press. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, J.; Zhao, X.; Liu, C.; Wang, W.; Wang, D.; Xu, W.; Zhang, C.; Yu, J.; Jiang, B. Clinical characteristics of imported cases of COVID-19 in Jiangs uprovince: A multicenter descriptive study. Clin Infect Dis 2020, 10, 1–7. [Google Scholar]
- Wu, J.; Wu, X.; Zeng, W.; Guo, D.; Fang, Z.; Chen, L.; Huang, H.; Li, C. Chest CT findings in patients with corona virus disease 2019 and its relationship with clinical features. Investig. Radiol. 2020, 55, 257. [Google Scholar] [CrossRef]
- Fang, X.; Mei, Q.; Yang, T.; Zhang, L.; Yang, Y.; Wang, Y. Clinical characteristics and treatment strategies of 79 patients with COVID-19. Chin. Pharmacol. Bull. 2020, 36, 11–18. [Google Scholar]
- Zhao, W.; Yu, S.; Zha, X.; Wang, N.; Pang, Q.; Li, T.; Li, A. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: A retrospective cohortstudy. MedRxiv 2020. [Google Scholar] [CrossRef]
- Yu, F.; Yan, L.; Wang, N.; Yang, S.; Wang, L.; Tang, Y.; Gao, G.; Wang, S.; Ma, C.; Xie, R. Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. 2020, 71, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Xie, J.; Yin, M.; Yang, Y.; He, H.; Jin, T.; Li, W.; Zhu, X.; Xu, J.; Zhao, C. Clinical and laboratory profiles of 75 hospitalized patients with novel corona virus disease 2019 in Hefei, China. MedRxiv 2020. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Tang, X.; Du, R.; Wang, R.; Cao, T.; Guan, L.; Yang, C.; Zhu, Q.; Hu, M.; Li, X.; Li, Y. Comparison of hospitalized patients with acute respiratory distress syndrome caused by Covid-19 and H1N1. Chest 2020, 158, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Y.; Zhu, T.; Xia, L. CT features of corona virus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. Am. J. Roentgenol. 2020, 214, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-W.; Wu, X.-X.; Jiang, X.-G.; Xu, K.-J.; Ying, L.-J.; Ma, C.-L.; Li, S.-B.; Wang, H.-Y.; Zhang, S.; Gao, H.-N. Clinical findings in a group of patients infected with the 2019 novel corona virus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. Bmj 2020, 368, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhuang, J.; Jin, M.; Xiong, H.; Huang, P.; Zhao, Q.; Miao, L.; Du, J.; Yang, X.; Huang, P. A comparative multi-centre study on the clinical and imaging features of comfirmed and uncomfirmed patients with COVID-19. MedRxiv 2020. [Google Scholar] [CrossRef]
- Cheung, K.S.; Hung, I.F.; Chan, P.P.; Lung, K.; Tso, E.; Liu, R.; Ng, Y.; Chu, M.Y.; Chung, T.W.; Tam, A.R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from the Hong Kong cohort and systematic review and meta-analysis. Gastroenterology 2020, 159, 81–95. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.-X. Compassionate use of remdesivir for patients with severe Covid-19. New Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xu, H.; Zhang, N.; Xu, H.; Li, Z.; Chen, H.; Xu, R.; Sun, R.; Wen, L.; Xie, L. Association between Clinical, Laboratory and CT Characteristics and RT-PCR Results in the Follow-up of COVID-19 patients. MedRxiv 2020. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Song, F.; Shi, N.; Shan, F.; Zhang, Z.; Shen, J.; Lu, H.; Ling, Y.; Jiang, Y.; Shi, Y. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020, 295, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, C.; Zhu, Z.; Cui, M.; Chen, C.; Dai, H.; Xue, Y. Clinical features and dynamics of viral load in imported and non-imported patients with COVID-19. Int. J. Infect. Dis. 2020, 94, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; Kersten, A.; Bickenbach, J.; Balfanz, P.; Hartmann, B.; Cornelissen, C.; Daher, A.; Stöhr, R.; Kleines, M.; Lemmen, S. Charakteristik von 50 hospitalisierten COVID-19-Patienten mit un dohne ARDS. Dtsch Arztebl Int 2020, 117, 271–278. [Google Scholar]
- Xu, Y.-H.; Dong, J.-H.; An, W.-M.; Lv, X.-Y.; Yin, X.-P.; Zhang, J.-Z.; Dong, L.; Ma, X.; Zhang, H.-J.; Gao, B.-L. Clinical and computed tomographic imaging features of novel corona virus pneumonia caused by SARS-CoV-2. J. Infect. 2020, 80, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Colaneri, M.; Sacchi, P.; Zuccaro, V.; Biscarini, S.; Sachs, M.; Roda, S.; Pieri, T.C.; Valsecchi, P.; Piralla, A.; Seminari, E. Clinical characteristics of corona virus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Eurosurveillance 2020, 25, 2000460. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-M.; Bai, P.; He, W.; Wu, F.; Liu, X.-F.; Han, D.-M.; Liu, S.; Yang, J.-K. Gender differences in patients with COVID-19: Focus on severity and mortality. Front. Public Health 2020, 8, 152. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, D.; Liu, Y.; Fan, Y.; Zhao, L.; Li, X.; Zhu, W. Clinical and high-resolution CT features of the COVID-19 infection: Comparison of the initial and follow-upchanges. Investig. Radiol. 2020, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Xiao, G.; Zhang, J.; He, X.; Ou, M.; Bi, J.; Yang, R.; Di, W.; Wang, Z.; Li, Z. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020, 9, 757–760. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel corona virus in Wuhan, China. Lancet. 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Rodríguez-Lago, I.; delaPiscina, P.R.; Elorza, A.; Merino, O.; deZárate, J.O.; Cabriada, J.L. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain). Gastroenterology 2020, 159, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, Y.; Wei, Z.; Zhou, P.; Lyu, L.; Zhang, G.; Zhao, Y.; He, H.; Li, X.; Gao, L. Investigation and analysis on characteristics of a cluster of COVID-19 associated with exposure in a department store in Tianjin. Zhonghua Liu Xing Bing Xue Za Zhi = Zhonghua Liuxingbingxue Zazhi 2020, 41, 489–493. [Google Scholar] [PubMed]
- Yao, N.; Wang, S.; Lian, J.; Sun, Y.; Zhang, G.; Kang, W.; Kang, W. Clinical characteristics and influencing factors of patients with novel corona virus pneumonia combined with liver injury in Shaanxi region. Zhonghua Gan Zang Bing Za Zhi = Zhonghua Ganzangbing Zazhi = Chin. J. Hepatol. 2020, 28, E003. [Google Scholar]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A. First case of 2019 novel corona virus in the United States. New Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.; Pochapin, M.; El-Serag, H.; Vargo, J. COVID-19 Clinical Insights for Our Community of Gastroenterologists and Gastroenterology Care Providers. 2020. Available online: https://gastro.org/press-releases/joint-gi-society-message-covid-19 (accessed on 20 February 2020).
- Borgesdo Nascimento, I.J.; Cacic, N.; Abdulazeem, H.M.; vonGroote, T.C.; Jayarajah, U.; Weerasekara, I.; Esfahani, M.A.; Civile, V.T.; Marusic, A.; Jeroncic, A. Novel corona virus infection (COVID-19) in humans: A scoping review and meta-analysis. J. Clin. Med. 2020, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Tolonen, A.C.; Xavier, R.J. Human genetic variation and the gut microbiome in disease. Nat. Rev. Genet. 2017, 18, 690–699. [Google Scholar] [CrossRef]
- Zhang, D.; Li, S.; Wang, N.; Tan, H.Y.; Zhang, Z.; Feng, Y. The Cross-Talk Between Gut Microbiota and Lungs in Common Lung Diseases. Front Microbiol 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012, 5, 7–18. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef]
- Groves, H.T.; Higham, S.L.; Moffatt, M.F.; Cox, M.J.; Tregoning, J.S. Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. mBio 2020, 11, 1–17. [Google Scholar] [CrossRef]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; Altayar, O.; Siddique, S.M.; Davitkov, P.; Feuerstein, J.D.; Lim, J.K.; Falck-Ytter, Y.; El-Serag, H.B. AGA Institute Rapid Review of the GI and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology 2020, 159, 320–334. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Qiu, Y.; He, J.-S.; Tan, J.-Y.; Li, X.-H.; Liang, J.; Shen, J.; Zhu, L.-R.; Chen, Y.; Iacucci, M. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- To, K.K.-W.; Hung, I.F.-N.; Ip, J.D.; Chu, A.W.-H.; Chan, W.-M.; Tam, A.R.; Fong, C.H.-Y.; Yuan, S.; Tsoi, H.-W.; Ng, A.C.-K. COVID-19 re-infection by a phylogenetically distinct SARS-coronavirus-2 strain confirmed by whole genome sequencing. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Edridge, A.W.; Kaczorowska, J.M.; Hoste, A.C.; Bakker, M.; Klein, M.; Jebbink, M.F.; Matser, A.; Kinsella, C.; Rueda, P.; Prins, M. Human corona virus reinfection dynamics: Lessons for SARS-CoV-2. MedRxiv 2020. [Google Scholar] [CrossRef]
- Shu, Z.; Zhou, Y.; Chang, K.; Liu, J.; Min, X.; Zhang, Q.; Sun, J.; Xiong, Y.; Zou, Q.; Zheng, Q. Clinical features and the traditional Chinese medicine therapeutic characteristics of 293 COVID-19 in patient cases. Front. Med. 2020. [Google Scholar] [CrossRef]
- Palayew, A.; Norgaard, O.; Safreed-Harmon, K.; Andersen, T.H.; Rasmussen, L.N.; Lazarus, J.V. Pandemic publishing poses a new COVID-19 challenge. Nat. Hum. Behav. 2020, 4, 666–669. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).