Abstract
Human immunodeficiency virus (HIV) capsid plays important roles at multiple stages of viral replication. At the initial stages, controlled uncoating (disassembly) of the capsid ensures efficient reverse transcription of the single-stranded RNA genome, into the double-stranded DNA. Whereas at later stages, a proper assembly of capsid ensures the formation of a mature infectious virus particle. Hence, the inhibition of capsid assembly and/or disassembly has been recognized as a potential therapeutic strategy, and several capsid inhibitors have been reported. Of these, PF-3450074 (PF74) has been extensively studied. Recently reported GS-CA inhibitors (GS-CA1 and GS-6207), have shown a strong potential and appear to contain a PF74 scaffold. The location of resistance mutations and the results of structural studies further suggest that GS-CA compounds and PF74 share the same binding pocket, which is located between capsid monomers. Additionally, phenylalanine derivatives containing the PF74 scaffold show slightly enhanced capsid inhibiting activity. A comparison of capsid structures in complex with host factors and PF74, reveals the presence of common chemical entities at topologically equivalent positions. Here we present the status of capsid inhibitors that contain PF74 scaffolds and propose that the PF74 scaffold may be used to develop strong and safe capsid inhibitors.
Keywords:
human immunodeficiency virus; capsid; assembly; small molecule inhibitors; PF74; GS-CA1; GS-6207; disassembly; uncoating 1. Introduction
Currently recommended antiretroviral therapies (cART) effectively suppress HIV and maintain HIV viral load below the detection levels (<50 copies/mL). As a result, HIV/AIDS is now a chronic and manageable disease [1,2,3,4,5,6]. However, HIV diversity, HIV persistence and the emergence of or the transfer of drug resistance mutations (DRMs) challenges the optimal outcome. Hence, new antiviral targets and agents that can inhibit HIV-1 replication through novel mechanisms are constantly sought. One such target is the HIV capsid, a structural protein that plays important roles at multiple stages of viral replication.
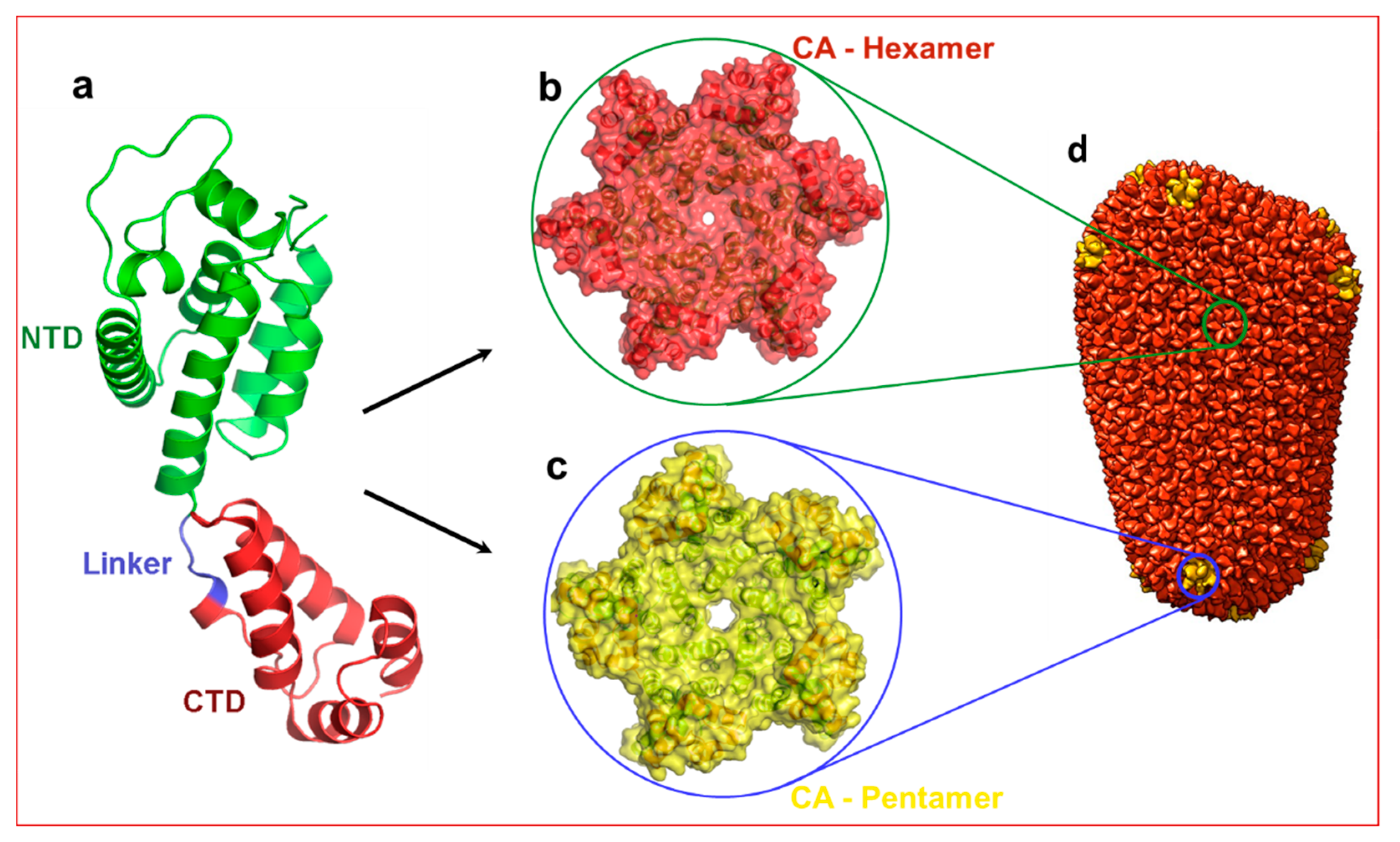
The capsid monomer (CA) is a 24 kDa α-helical protein with two distinct domains: an N-terminal domain (NTD) and a C-terminal domain (CTD) connected by a five residue flexible linker (Figure 1). During late stages of viral replication, the Gag polyprotein localizes at the plasma membrane and buds off as spherical, immature non-infectious virus. Auto-cleavage of the viral protease during maturation and the subsequent processing of the Gag polyprotein generates several structural proteins and small peptides, including capsid monomers (Figure 1a). These CA monomers then assemble into ~250 hexamers (Figure 1b) and 12 pentamers (Figure 1c) to form a mature fullerene-like core structure known as capsid core (hereafter referred to as capsid) (Figure 1d) [7,8].
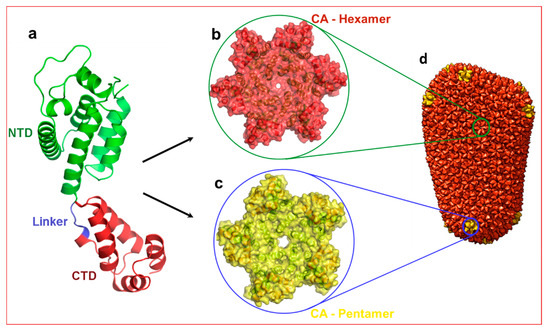
Figure 1.
Structure of HIV-1 capsid. (a) Structure of capsid (CA) monomer. This figure was generated from the X-ray crystal structure of native HIV-1 capsid protein bound to PF74 [9] (PDB entry 4XFZ). NTD: N-terminal domain. CTD: C-terminal domain. Panels (b,c) show hexamer and pentamers formed from the same CA monomer. (d) Approximately 250 hexamers and 12 pentamers form a conical shaped capsid that houses necessary and sufficient components to initiate reverse transcription immediately after infection. Panel d is reproduced from PDB101 with permission.
At the early stages of HIV-1 infection, i.e., after infection and the fusion of the viral envelope with the host cell membrane, capsid is released into the cytosol where it undergoes controlled disassembly (uncoating). While the timing, mechanism, and extent of capsid uncoating is controversial [10,11,12,13,14,15,16,17,18], published reports suggest that capsid uncoating is associated with the initiation or continuation of the reverse transcription [14,19,20,21,22,23,24], and integration of viral DNA into the host chromosome [17,20,25,26,27,28]. Cosnefroy et al. [29] showed that HIV-1 capsid uncoating initiates after the first strand transfer of reverse transcription. Another report demonstrated that HIV-1 core undergoes morphological changes during reverse transcription [30]. These studies suggest that reverse transcription initiates within the virus particle and continues with capsid disassembly in the cytoplasm of the infected cell. The uncoating of capsid also involves the interaction of capsid with viral proteins (e.g., reverse transcriptase and integrase) [12,13], and host proteins including cyclophilin A [17], kinesin [31], dynein [31,32], microtubules [33], nucleoporins NUP358 and NUP153 [34], cleavage and polyadenylation specificity factor 6 (CPSF6) [35,36], transportin 3 (TNPO3) [34], and β-karyopherin Transportin-1 (TRN-1) [37]. The uncoating of capsid is influenced by certain mutations that change stability. Second, interactions between CA protomers alter replication events that may severely affect viral infectivity [21,38]. The role of capsid in the above-mentioned processes highlights capsid as an attractive antiviral target for the development of inhibitors [39,40,41].
2. PF74 and Capsid Inhibitors Containing the PF74-Scaffold
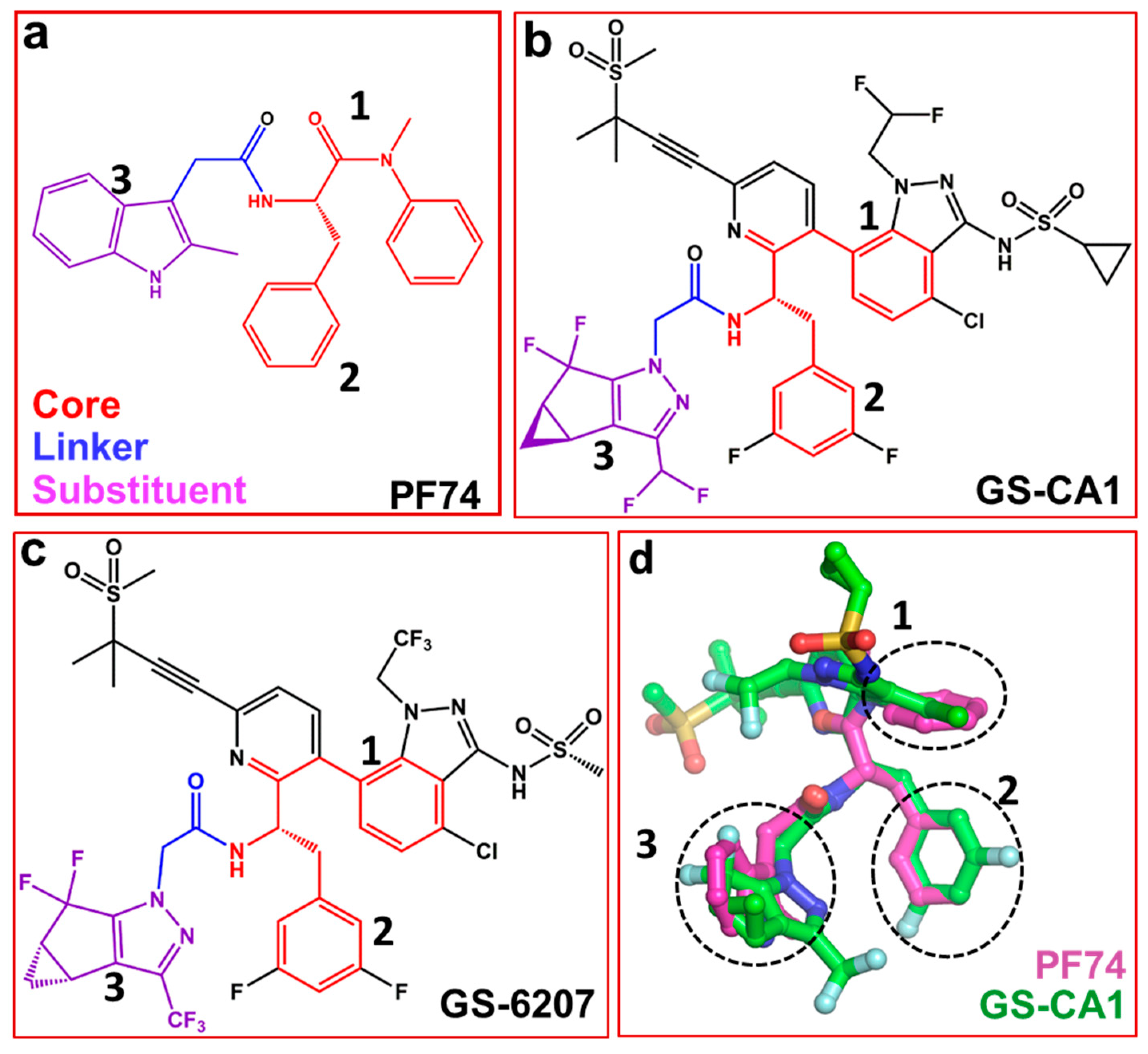
Several capsid-binding small molecules, which inhibit viral replication have been identified [41,42,43,44,45,46,47,48,49,50,51,52]. One of the most studied inhibitors is PF-3450074, also known as PF74 (Figure 2a) [25,53,54,55]. It inhibits infection, blocks reverse transcription [56] and prevents replication in vitro [3]. PF74 binds in a pocket between the NTD and CTD of monomers [45,57,58]. The structure of PF74 can be divided in to three components: a polyphenyl core (red, Figure 2a), a linker (blue, Figure 2a) and an indole substituent (violet, Figure 2a) [59]. In the following sections, we discuss compounds that contain PF74 scaffolds and have potential as future capsid inhibitors.
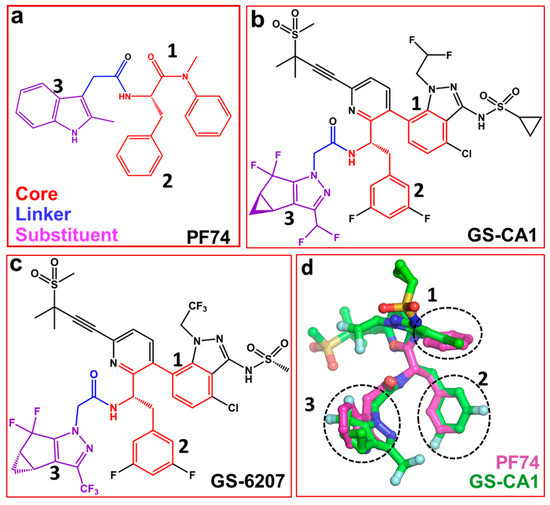
Figure 2.
Structures of PF74 and GS-CA compounds. (a) PF74 can be divided into three components: polyphenyl core (red), linker (blue) and substituent (violet). Numbers 1, 2 and 3 represent three ring structures in PF74. Panels (b) and (c) show the chemical structure of GS-CA1 and GS-6207 compounds, respectively. Chemical moieties colored red, blue and violet correspond to those in PF74 (panel a). (d) Superposition of PF74 and GS-CA1. Dotted circles show the superposition of three ring structures present in PF74, GS-CA1 and GS-6207.
Recently reported two capsid inhibitors, GS-CA1 (Figure 2b) and GS-62072 (an analog of GS-CA1) (Figure 2c) display greater potency than currently approved antivirals in vitro [60,61]. GS-CA1 inhibits HIV-1 replication in T-lymphocytes and peripheral blood mononuclear cells (PBMCs) with EC50s of 240 pM and 140 pM, respectively) [60]. Alternatively, GS-6207 inhibits HIV-1 in MT-4 cells and PBMCs with EC50s of 100 pM and 50 pM, respectively [61]. Both compounds also have a potential as long acting inhibitors [60,62,63]. Analyses of the structures of GS-CA1 and GS-6207 show that both compounds contain a polyphenyl core and the linker region similar to that in PF74 (Figure 2a–c).
The structure of GS-CA1/CA complex has been solved, but it is not available in the public domains such as the Protein Data Bank [64]. However, molecular docking studies shows that both GS-CA1, GS-6207and PF74, share the same binding site [65] which is located between capsid NTD and CTD [57,60,66]. The docking results show the rings of PF74 superpose on the different rings of GS-CA1 (dotted circles 1, 2, and 3 in Figure 2d). More specifically, one of the two phenyl rings of PF74 is at a topologically equivalent position in the indazole ring of GS-CA1 (Figure 2d, circle 1), and the other phenyl ring of PF74 superposes on the difluorobenzene ring of GS-CA1 (Figure 2d, circle 2). The indole ring of PF74 superposes closely on the tetrahydrocyclopenta-pyrazole ring of GS-CA1 (Figure 2d, circle 3). In addition, the polar groups of the two molecules (PF74 and GS-CA1) superpose well [65].
In vitro resistance selection experiments have identified mutations L56I, M66I, Q67H, N74D and A105E under GS-CA1 pressure [67]. Mutations Q67H, N74D, K70N, Q67H/N74S, Q67H/T107N, L56I, Q67H/N74D, M66I and T107N are associated with GS-6207 resistance in vitro [68]. Several of these mutations are either associated with PF74 resistance or they are in the vicinity of PF74 binding pocket [45,55,56,58,69]. These data further support molecular docking studies and implicate that GS-CA1 binds at the PF74 binding pocket as predicted recently [65].
Together with common structural moieties in GS-CA1, GS-6207 and PF74, the GS-CA compounds have additional chemical groups that have extensive interactions with the NTD of adjacent capsid monomer. More specifically, methanesulfonyl moiety of GS-CA1 and GS-6207 is within interacting distance of I37, P38, S41, I135 and N139 [65]. It is possible that these interactions result in a better binding affinity of CA with GS-CA1 than PF74 [65], which results in the enhanced potency of GS-CA compounds.
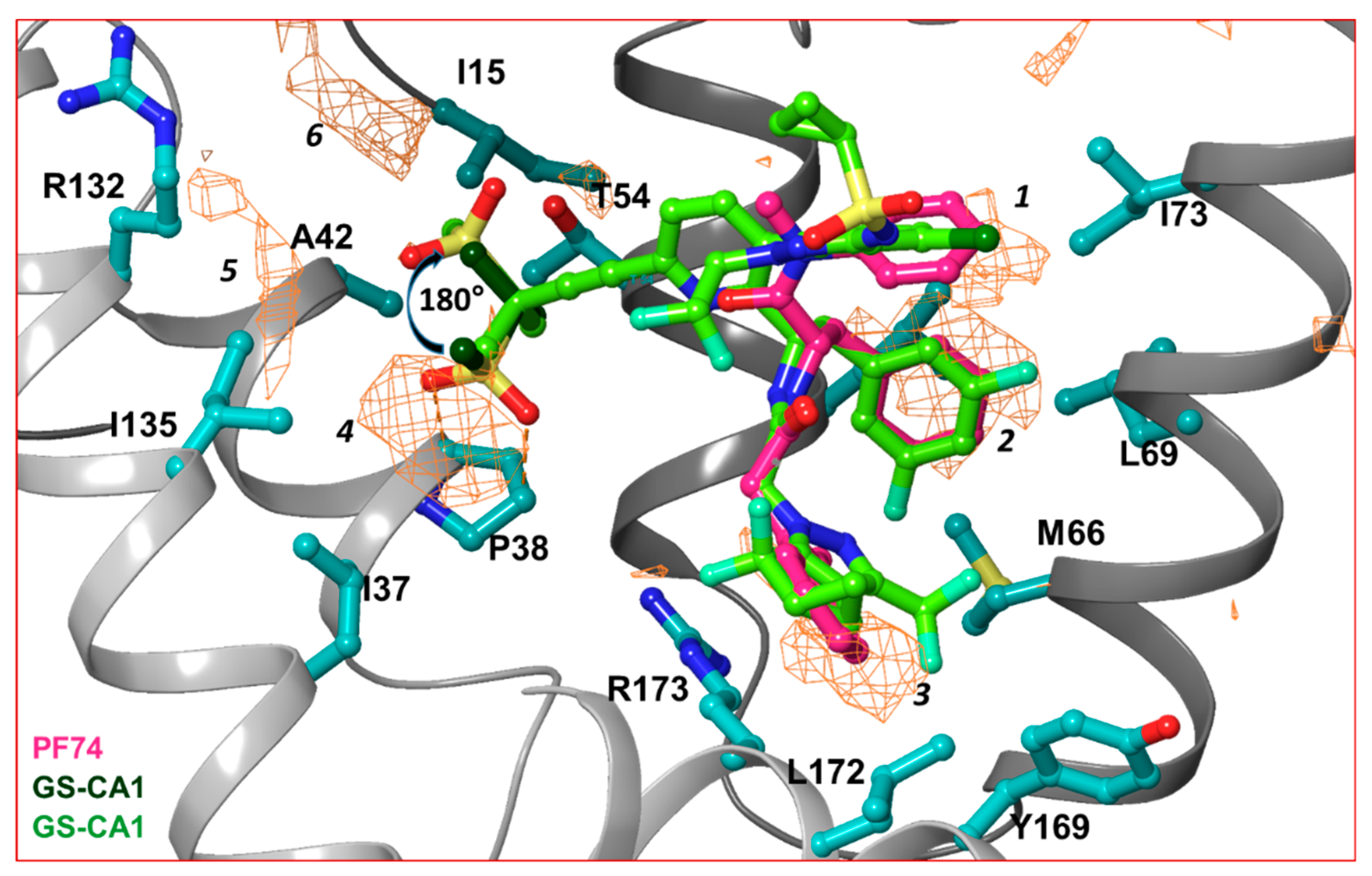
In order to dissect the small molecule binding site between two capsid monomers of an hexamer, we employed the SiteMap program [70] of Schrödinger Suite (Schrödinger Inc., NY) in the crystal structure of the hexamer/PF74 complex (after removing PF74) (PDB file 5HGL [69]). Six hydrophobic pockets between two capsid monomers, as predicted by the SiteMap program, are shown as orange wire-mesh in Figure 3. Three of these hydrophobic pockets (marked 1, 2 and 3 in Figure 3) are present at the topologically equivalent positions of the three ring-structures of PF74/GS-CA compounds; regions 4, 5 and 6 are close to methanesulfonyl moieties of GS-CA compounds (Figure 3). This analysis suggests that methanesulfonyl moiety of GS-CA compounds may adopt a conformation that is rotated by ~180° from the one predicted recently [65]. This analysis also indicates that the methyl groups of methanesulfonyl moiety may have been strategically incorporated in GS-CA compounds to improve binding to the capsid. It is unclear if methanesulfonyl groups contribute to the low solubility of these compounds. In spite of low solubility [61] and violation of theoretical rules of drug-likeliness [61,71,72], GS-CA compounds have a strong potential to be developed as long-acting drugs.
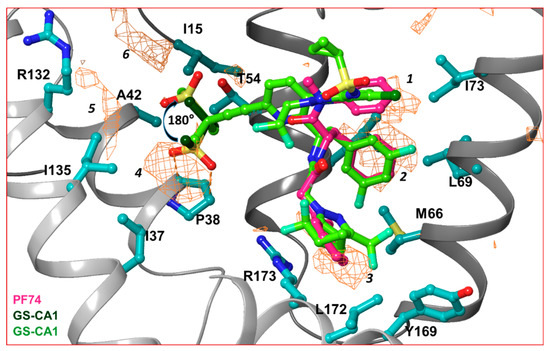
Figure 3.
Hydrophobic pockets between two capsid monomers. This figure shows the hydrophobic pockets (orange wire-mesh) present between two capsid monomers of a hexamer. The amino acid residues that contribute in formation of hydrophobic pockets are displayed in ball-and-stick representation. The presence of hydrophobic pockets 4, 5 and 6 suggests that the conformation of methanesulfonyl moieties of GS-CA1 and GS-6207 can assume a conformation that is 180° rotated from previously predicted [65]. Note that hydrophobic pockets 1, 2 and 3 are at a topologically equivalent position to three ring structures of PF74, GS-CA1 or GS-6207.
The proposal that interactions of compounds with both capsid monomers involved in binding site formation may enhance potency of PF74-based molecules, was recently studied by synthesizing phenylalanine derivatives and testing their inhibitory activity [59]. One of these compounds had a selectivity index (SI) (CC50/EC50) of 13.33 compared to the SI of PF74 (SI = 11.85) [59]. These results suggest that the PF74 scaffold can be used to develop better capsid inhibitors.
Another reported capsid inhibitor BI-2 that contains a partial PF-74 polyphenyl core binds at the PF74 binding site [57]. A superposition of capsid bound to PF74 (PDB files 4XFZ and 5HGL) [9,69] and BI-2 (PDB file 4U0F) [57] demonstrates that two phenyl rings of BI-2 superposed well with the phenyl rings of PF74 (Figure 2b) [65]. BI-2 does not have a chemical moiety equivalent to the indole ring of PF74, which forms some contacts with CTD. It appears that a greater potency of PF74 compared to BI-2 is also the result of interactions of the indole ring with capsid.
3. Similarity among PF74, PF74-Based Compounds and Host Factors
CPSF6 and NUP153 are host factors that have been implicated in nuclear entry of HIV-1 through their interactions with capsid [66,73,74,75,76,77,78,79]. CPSF6 is a pre-mRNA splicing factor and member of the serine/arginine-rich (SR) protein family [80]. It is predominately a nuclear protein but localizes with capsid in the cytoplasm [25,81]. Depletion of CPSF6 marginally increases viral infection [73,81]. NUP153 is a nucleoprotein located at the nucleus side of the nuclear pore complex (NPC) [74]. NUP153 participates in HIV-1 translocation to nucleus through the nuclear pore [16,76]. The role of CPSF6 and NUP153 together with other host factors in HIV-1 infection have been reviewed by Yamashita and Engelman [81].
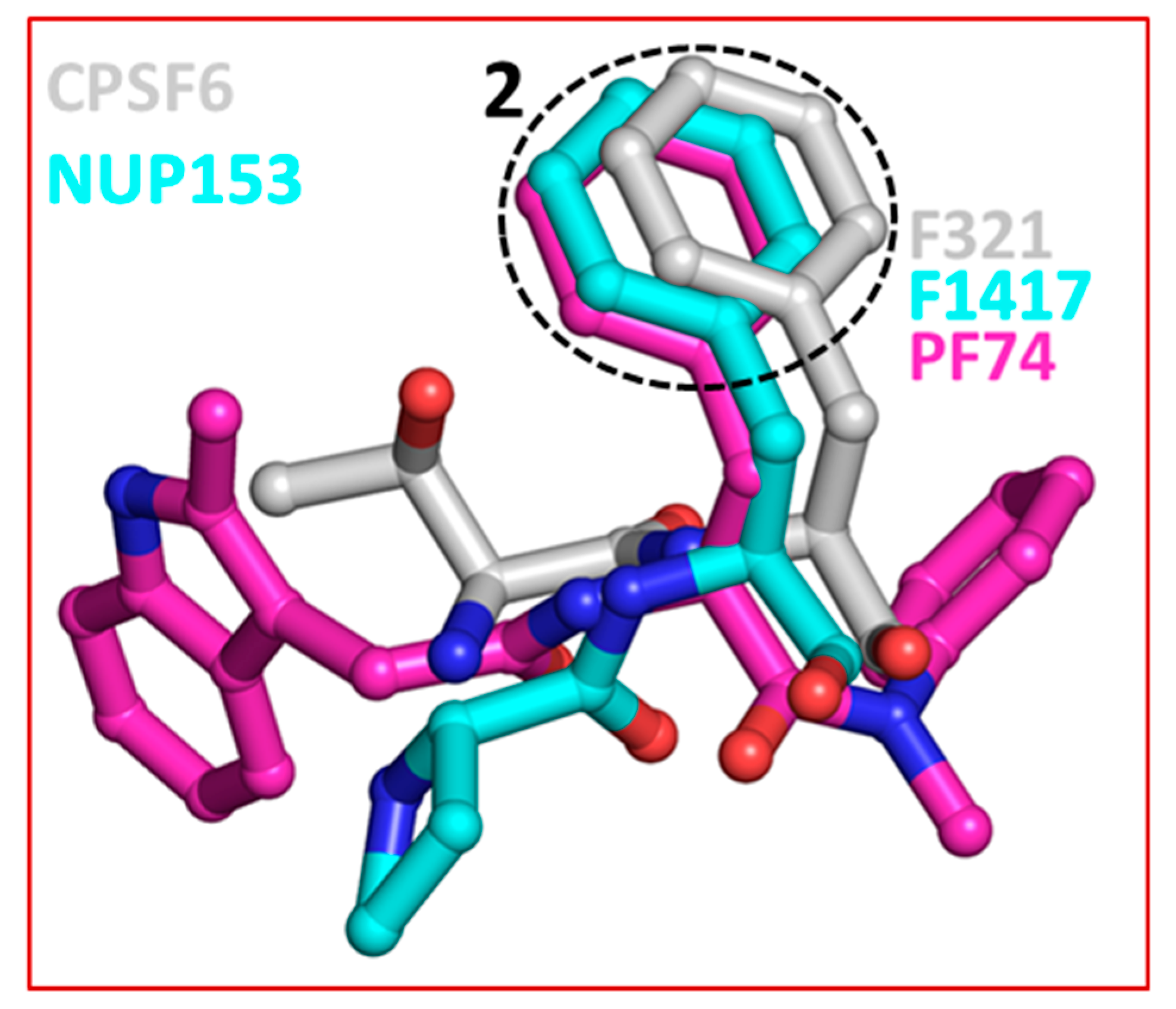
The crystal structures of capsid bound to the peptides from CPSF6 (313–327) and NUP153 (1407–1423) have been reported [57]. Structural comparison shows that both CPSF6 and NUP153 peptides bind at the PF74/BI-2 binding site [52,57]. Therefore, as expected both PF74 and BI-2 interfere with CPSF6 or NUP153 binding to the capsid [57,66]. This comparison also shows that F321 of CPSF6 and F1417 of NUP153 perfectly superpose on the phenyl ring of PF74 (or BI-2) (Figure 4) [65]. As discussed above (Section 2), two most potent capsid inhibitors GS-CA1 and GS-6207 also contain a similar structural moiety (difluorobenzyl) at the topologically equivalent position. This analysis further suggests that chemical features present in host factors can be exploited to develop capsid inhibitors. Indeed, a cyclic peptide (Pep-1) containing chemical features of CPSF6, NUP153, PF74 and BI-2, bind capsid hexamers with nanomolar affinity [65]. Common structural moieties at topologically equivalent positions in CPSF6, NUP154, PF74, BI-2, GS-CA1, GS-6207 and Pep-1 [65] are shown in Table 1.
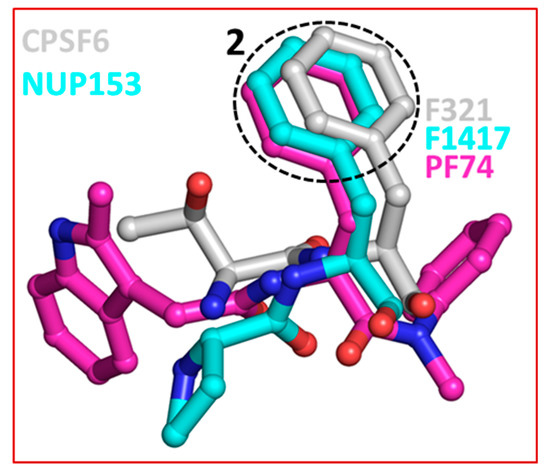
Figure 4.
Superposition of CPSF6, NUP153 and PF74. The dotted circle highlights the equivalent position of phenylalanine residues in CPSF6 and NUP153 and one of the two phenyl rings of PF74. The equivalent ring structure in GS-CA1 and GS-6207 is difluorobenzene, which is not shown in this figure. The structures of capsid inhibitors shown here have either been solved or in complex with capsid.

Table 1.
Structural similarity among PF74, PF74-based compounds and host factors.
4. Capsid Inhibitors without PF74 Scaffolds
Other reported capsid inhibitors include benzodiazepine (BD) and benzimidazole (BM) compounds [82,83,84], CAP-1 [43,46], or peptide inhibitors, such as NYAD-1. These compounds do not bind at the PF74 binding site and they inhibit capsid and mature virus by different mechanisms. For example, NYAD-1 disrupts the formation of both immature- and mature-like virus particles [85] and it inhibits viral replication [44,49,52].
5. Conclusions
In conclusion, here we present the current predicted mechanisms of capsid inhibitors containing PF74 scaffold. PF74, although used extensively in experimental settings, has poor metabolic properties suggesting that improved capsid inhibitors can be developed using PF74 as the starting point.
Author Contributions
Conceptualization, C.M. and K.S.; methodology, K.S., F.G., T.P.Q. and C.M., formal analyses, K.S.; writing original draft preparation, K.S. and C.M.; writing—review and editing, C.M., F.G., T.P.Q. and K.S.
Funding
This research was funded by the National Institute of Health R01 Grants GM118012 (subcontract to KS). The study was also supported by the University of Missouri Mizzou Advantage (KS and CM). In addition, K.S. acknowledges support from the Bond Life Sciences Center grant (DU108). CM acknowledges the support from the National Institutes of Health (NIH) Clinical Translational Science Award (CTSA).
Acknowledgments
The authors acknowledge Molecular Interactions Core, University of Missouri for the use of Schrödinger Suite.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: A collaborative analysis of cohort studies. Lancet HIV 2017, 4, e349–e356. [Google Scholar] [CrossRef]
- Harries, A.D.; Suthar, A.B.; Takarinda, K.C.; Tweya, H.; Kyaw, N.T.; Tayler-Smith, K.; Zachariah, R. Ending the HIV/AIDS epidemic in low- and middle-income countries by 2030: Is it possible? F1000Res 2016, 5, 2328. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.C. HIV epidemiology and the effects of antiviral therapy on long-term consequences. AIDS 2008, 22, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Teeraananchai, S.; Kerr, S.J.; Amin, J.; Ruxrungtham, K.; Law, M.G. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: A meta-analysis. HIV Med. 2017, 18, 256–266. [Google Scholar] [CrossRef] [PubMed]
- May, M.T.; Gompels, M.; Delpech, V.; Porter, K.; Orkin, C.; Kegg, S.; Hay, P.; Johnson, M.; Palfreeman, A.; Gilson, R.; et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS 2014, 28, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Sabin, C.A. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med. 2013, 11, 251. [Google Scholar] [CrossRef]
- Pornillos, O.; Ganser-Pornillos, B.K.; Kelly, B.N.; Hua, Y.; Whitby, F.G.; Stout, C.D.; Sundquist, W.I.; Hill, C.P.; Yeager, M. X-ray structures of the hexameric building block of the HIV capsid. Cell 2009, 137, 1282–1292. [Google Scholar] [CrossRef]
- Pornillos, O.; Ganser-Pornillos, B.K.; Yeager, M. Atomic-level modelling of the HIV capsid. Nature 2011, 469, 424–427. [Google Scholar] [CrossRef]
- Gres, A.T.; Kirby, K.A.; KewalRamani, V.N.; Tanner, J.J.; Pornillos, O.; Sarafianos, S.G. X-ray crystal structures of native HIV-1 capsid protein reveal conformational variability. Science 2015, 349, 99–103. [Google Scholar] [CrossRef]
- Arhel, N. Revisiting HIV-1 uncoating. Retrovirology 2010, 7, 96. [Google Scholar] [CrossRef]
- Mamede, J.I.; Cianci, G.C.; Anderson, M.R.; Hope, T.J. Early cytoplasmic uncoating is associated with infectivity of HIV-1. Proc. Natl. Acad. Sci. USA 2017, 114, E7169–E7178. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Farnet, C.M.; Bushman, F.D. Human immunodeficiency virus type 1 preintegration complexes: Studies of organization and composition. J. Virol. 1997, 71, 5382–5390. [Google Scholar] [PubMed]
- Fassati, A.; Goff, S.P. Characterization of intracellular reverse transcription complexes of human immunodeficiency virus type 1. J. Virol. 2001, 75, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Hulme, A.E.; Perez, O.; Hope, T.J. Complementary assays reveal a relationship between HIV-1 uncoating and reverse transcription. Proc. Natl. Acad. Sci. USA 2011, 108, 9975–9980. [Google Scholar] [CrossRef]
- Hulme, A.E.; Kelley, Z.; Okocha, E.A.; Hope, T.J. Identification of capsid mutations that alter the rate of HIV-1 uncoating in infected cells. J. Virol. 2015, 89, 643–651. [Google Scholar] [CrossRef]
- Di Nunzio, F.; Danckaert, A.; Fricke, T.; Perez, P.; Fernandez, J.; Perret, E.; Roux, P.; Shorte, S.; Charneau, P.; Diaz-Griffero, F.; et al. Human nucleoporins promote HIV-1 docking at the nuclear pore, nuclear import and integration. PLoS ONE 2012, 7, e46037. [Google Scholar] [CrossRef]
- Schaller, T.; Ocwieja, K.E.; Rasaiyaah, J.; Price, A.J.; Brady, T.L.; Roth, S.L.; Hue, S.; Fletcher, A.J.; Lee, K.; KewalRamani, V.N.; et al. HIV-1 capsid-cyclophilin interactions determine nuclear import pathway, integration targeting and replication efficiency. PLoS Pathog. 2011, 7, e1002439. [Google Scholar] [CrossRef]
- Arhel, N.J.; Souquere-Besse, S.; Munier, S.; Souque, P.; Guadagnini, S.; Rutherford, S.; Prevost, M.C.; Allen, T.D.; Charneau, P. HIV-1 DNA flap formation promotes uncoating of the pre-integration complex at the nuclear pore. EMBO J. 2007, 26, 3025–3037. [Google Scholar] [CrossRef]
- Campbell, E.M.; Hope, T.J. HIV-1 capsid: The multifaceted key player in HIV-1 infection. Nat. Rev. Microbiol. 2015, 13, 471–483. [Google Scholar] [CrossRef]
- Ambrose, Z.; Aiken, C. HIV-1 uncoating: Connection to nuclear entry and regulation by host proteins. Virology 2014, 454–455, 371–379. [Google Scholar] [CrossRef]
- Forshey, B.M.; von Schwedler, U.; Sundquist, W.I.; Aiken, C. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 2002, 76, 5667–5677. [Google Scholar] [CrossRef] [PubMed]
- Rihn, S.J.; Wilson, S.J.; Loman, N.J.; Alim, M.; Bakker, S.E.; Bhella, D.; Gifford, R.J.; Rixon, F.J.; Bieniasz, P.D. Extreme genetic fragility of the HIV-1 capsid. PLoS Pathog. 2013, 9, e1003461. [Google Scholar] [CrossRef] [PubMed]
- von Schwedler, U.K.; Stray, K.M.; Garrus, J.E.; Sundquist, W.I. Functional surfaces of the human immunodeficiency virus type 1 capsid protein. J. Virol. 2003, 77, 5439–5450. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Franks, T.; Gibson, G.; Huber, K.; Rahm, N.; Strambio De Castillia, C.; Luban, J.; Aiken, C.; Watkins, S.; Sluis-Cremer, N.; et al. Evidence for biphasic uncoating during HIV-1 infection from a novel imaging assay. Retrovirology 2013, 10, 70. [Google Scholar] [CrossRef]
- Peng, K.; Muranyi, W.; Glass, B.; Laketa, V.; Yant, S.R.; Tsai, L.; Cihlar, T.; Muller, B.; Krausslich, H.G. Quantitative microscopy of functional HIV post-entry complexes reveals association of replication with the viral capsid. Elife 2014, 3, e04114. [Google Scholar] [CrossRef]
- Chin, C.R.; Perreira, J.M.; Savidis, G.; Portmann, J.M.; Aker, A.M.; Feeley, E.M.; Smith, M.C.; Brass, A.L. Direct visualization of HIV-1 replication intermediates shows that capsid and CPSF6 modulate HIV-1 intra-nuclear invasion and integration. Cell Rep. 2015, 13, 1717–1731. [Google Scholar] [CrossRef]
- Stultz, R.D.; Cenker, J.J.; McDonald, D. Imaging HIV-1 genomic DNA from entry through productive infection. J. Virol. 2017, 91, e00034-17. [Google Scholar] [CrossRef]
- Francis, A.C.; Melikyan, G.B. Single HIV-1 imaging reveals progression of infection through CA-dependent steps of docking at the nuclear pore, uncoating, and nuclear transport. Cell Host Microbe 2018, 23, 536–548. [Google Scholar] [CrossRef]
- Cosnefroy, O.; Murray, P.J.; Bishop, K.N. HIV-1 capsid uncoating initiates after the first strand transfer of reverse transcription. Retrovirology 2016, 13, 58. [Google Scholar] [CrossRef]
- Rankovic, S.; Varadarajan, J.; Ramalho, R.; Aiken, C.; Rousso, I. Reverse transcription mechanically initiates HIV-1 capsid disassembly. J. Virol. 2017, 91, e00289-17. [Google Scholar] [CrossRef]
- Lukic, Z.; Dharan, A.; Fricke, T.; Diaz-Griffero, F.; Campbell, E.M. HIV-1 uncoating is facilitated by dynein and kinesin 1. J. Virol. 2014, 88, 13613–13625. [Google Scholar] [CrossRef] [PubMed]
- Pawlica, P.; Berthoux, L. Cytoplasmic dynein promotes HIV-1 uncoating. Viruses 2014, 6, 4195–4211. [Google Scholar] [CrossRef] [PubMed]
- Sabo, Y.; Walsh, D.; Barry, D.S.; Tinaztepe, S.; de Los Santos, K.; Goff, S.P.; Gundersen, G.G.; Naghavi, M.H. HIV-1 induces the formation of stable microtubules to enhance early infection. Cell Host Microbe 2013, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Valle-Casuso, J.C.; Di Nunzio, F.; Yang, Y.; Reszka, N.; Lienlaf, M.; Arhel, N.; Perez, P.; Brass, A.L.; Diaz-Griffero, F. TNPO3 is required for HIV-1 replication after nuclear import but prior to integration and binds the HIV-1 core. J. Virol. 2012, 86, 5931–5936. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ambrose, Z.; Martin, T.D.; Oztop, I.; Mulky, A.; Julias, J.G.; Vandegraaff, N.; Baumann, J.G.; Wang, R.; Yuen, W.; et al. Flexible use of nuclear import pathways by HIV-1. Cell Host Microbe 2010, 7, 221–233. [Google Scholar] [CrossRef]
- Rasaiyaah, J.; Tan, C.P.; Fletcher, A.J.; Price, A.J.; Blondeau, C.; Hilditch, L.; Jacques, D.A.; Selwood, D.L.; James, L.C.; Noursadeghi, M.; et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature 2013, 503, 402–405. [Google Scholar] [CrossRef]
- Fernandez, J.; Machado, A.K.; Lyonnais, S.; Chamontin, C.; Gärtner, K.; Léger, T.; Henriquet, C.; Garcia, C.; Portilho, D.M.; Pugnière, M.; et al. Transportin-1 binds to the HIV-1 capsid via a nuclear localization signal and triggers uncoating. Nat. Microbiol. 2019, 4, 1840–1850. [Google Scholar] [CrossRef]
- Craveur, P.; Gres, A.T.; Kirby, K.A.; Liu, D.; Hammond, J.A.; Deng, Y.; Forli, S.; Goodsell, D.S.; Williamson, J.R.; Sarafianos, S.G.; et al. Novel intersubunit interaction critical for HIV-1 core assembly defines a potentially targetable inhibitor binding pocket. mBio 2019, 10. [Google Scholar] [CrossRef]
- Li, G.; Verheyen, J.; Rhee, S.Y.; Voet, A.; Vandamme, A.M.; Theys, K. Functional conservation of HIV-1 gag: Implications for rational drug design. Retrovirology 2013, 10, 126. [Google Scholar] [CrossRef]
- Prevelige, P.E., Jr. New approaches for antiviral targeting of HIV assembly. J. Mol. Biol. 2011, 410, 634–640. [Google Scholar] [CrossRef]
- Bocanegra, R.; Rodriguez-Huete, A.; Fuertes, M.A.; Del Alamo, M.; Mateu, M.G. Molecular recognition in the human immunodeficiency virus capsid and antiviral design. Virus Res. 2012, 169, 388–410. [Google Scholar] [CrossRef] [PubMed]
- Ternois, F.; Sticht, J.; Duquerroy, S.; Krausslich, H.G.; Rey, F.A. The HIV-1 capsid protein c-terminal domain in complex with a virus assembly inhibitor. Nat. Struct. Mol. Biol. 2005, 12, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Kelly, B.N.; Kyere, S.; Kinde, I.; Tang, C.; Howard, B.R.; Robinson, H.; Sundquist, W.I.; Summers, M.F.; Hill, C.P. Structure of the antiviral assembly inhibitor CAP-1 complex with the HIV-1 CA protein. J. Mol. Biol. 2007, 373, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Loeliger, E.; Kinde, I.; Kyere, S.; Mayo, K.; Barklis, E.; Sun, Y.; Huang, M.; Summers, M.F. Antiviral inhibition of the HIV-1 capsid protein. J. Mol. Biol. 2003, 327, 1013–1020. [Google Scholar] [CrossRef]
- Blair, W.S.; Pickford, C.; Irving, S.L.; Brown, D.G.; Anderson, M.; Bazin, R.; Cao, J.; Ciaramella, G.; Isaacson, J.; Jackson, L.; et al. HIV capsid is a tractable target for small molecule therapeutic intervention. PLoS Pathog. 2010, 6, e1001220. [Google Scholar] [CrossRef]
- Curreli, F.; Zhang, H.; Zhang, X.; Pyatkin, I.; Victor, Z.; Altieri, A.; Debnath, A.K. Virtual screening based identification of novel small-molecule inhibitors targeted to the HIV-1 capsid. Bioorg. Med. Chem. 2011, 19, 77–90. [Google Scholar] [CrossRef][Green Version]
- Sticht, J.; Humbert, M.; Findlow, S.; Bodem, J.; Muller, B.; Dietrich, U.; Werner, J.; Krausslich, H.G. A peptide inhibitor of HIV-1 assembly in vitro. Nat. Struct. Mol. Biol. 2005, 12, 671–677. [Google Scholar] [CrossRef]
- Xu, J.P.; Francis, A.C.; Meuser, M.E.; Mankowski, M.; Ptak, R.G.; Rashad, A.A.; Melikyan, G.B.; Cocklin, S. Exploring modifications of an HIV-1 capsid inhibitor: Design, synthesis, and mechanism of action. J. Drug Des. Res. 2018, 5, 1070. [Google Scholar]
- Kortagere, S.; Madani, N.; Mankowski, M.K.; Schon, A.; Zentner, I.; Swaminathan, G.; Princiotto, A.; Anthony, K.; Oza, A.; Sierra, L.J.; et al. Inhibiting early-stage events in HIV-1 replication by small-molecule targeting of the HIV-1 capsid. J. Virol. 2012, 86, 8472–8481. [Google Scholar] [CrossRef]
- Kortagere, S.; Xu, J.P.; Mankowski, M.K.; Ptak, R.G.; Cocklin, S. Structure-activity relationships of a novel capsid targeted inhibitor of HIV-1 replication. J. Chem. Inf. Model. 2014, 54, 3080–3090. [Google Scholar] [CrossRef]
- Xu, J.P.; Branson, J.D.; Lawrence, R.; Cocklin, S. Identification of a small molecule HIV-1 inhibitor that targets the capsid hexamer. Bioorg. Med. Chem. Lett. 2016, 26, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Lamorte, L.; Titolo, S.; Lemke, C.T.; Goudreau, N.; Mercier, J.F.; Wardrop, E.; Shah, V.B.; von Schwedler, U.K.; Langelier, C.; Banik, S.S.; et al. Discovery of novel small-molecule HIV-1 replication inhibitors that stabilize capsid complexes. Antimicrob. Agents Chemother. 2013, 57, 4622–4631. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Saito, A.; Halambage, U.D.; Ferhadian, D.; Fischer, D.K.; Francis, A.C.; Melikyan, G.B.; Ambrose, Z.; Aiken, C.; Yamashita, M. A novel phenotype links HIV-1 capsid stability to cgas-mediated DNA sensing. J. Virol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, M.; Zhou, J.; Addai, A.; Martinez, P.; Pandhare, J.; Aiken, C.; Dash, C. PF74 inhibits HIV-1 integration by altering the composition of the preintegration complex. J. Virol. 2019, 93, e01741-18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Price, A.J.; Halambage, U.D.; James, L.C.; Aiken, C. HIV-1 resistance to the capsid-targeting inhibitor PF74 results in altered dependence on host factors required for virus nuclear entry. J. Virol. 2015, 89, 9068–9079. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, J.; Shah, V.B.; Aiken, C.; Whitby, K. Small-molecule inhibition of human immunodeficiency virus type 1 infection by virus capsid destabilization. J. Virol. 2011, 85, 542–549. [Google Scholar] [CrossRef]
- Price, A.J.; Jacques, D.A.; McEwan, W.A.; Fletcher, A.J.; Essig, S.; Chin, J.W.; Halambage, U.D.; Aiken, C.; James, L.C. Host cofactors and pharmacologic ligands share an essential interface in HIV-1 capsid that is lost upon disassembly. PLoS Pathog. 2014, 10, e1004459. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Alam, S.L.; Fricke, T.; Zadrozny, K.; Sedzicki, J.; Taylor, A.B.; Demeler, B.; Pornillos, O.; Ganser-Pornillos, B.K.; Diaz-Griffero, F.; et al. Structural basis of HIV-1 capsid recognition by PF74 and CPSF6. Proc. Natl. Acad. Sci. USA 2014, 111, 18625–18630. [Google Scholar] [CrossRef]
- Wu, G.; Zalloum, W.A.; Meuser, M.E.; Jing, L.; Kang, D.; Chen, C.H.; Tian, Y.; Zhang, F.; Cocklin, S.; Lee, K.H.; et al. Discovery of phenylalanine derivatives as potent HIV-1 capsid inhibitors from click chemistry-based compound library. Eur. J. Med. Chem. 2018, 158, 478–492. [Google Scholar] [CrossRef]
- Tse, W.C.; Link, J.O.; Mulato, A.; Niedziela-Majka, A.; Rowe, W.; Somoza, J.R.; Villasenor, A.G.; Yant, S.R.; Zhang, J.R.; Zheng, J. Discovery of novel potent HIV capsid inhibitors with long-acting potential. In Proceedings of the Conference on Retroviruses and Opportunistic Infections, Abstract No. 38, Seattle, WA, USA, 13–16 February 2017. [Google Scholar]
- Zheng, J.; Yant, S.R.; Ahmadyar, S.; Chan, T.Y.; Chiu, A.; Cihlar, T.; Link, J.O.; Lu, B.; Mwangi, J.; Rowe, W.; et al. 539. Gs-ca2: A novel, potent, and selective first-in-class inhibitor of HIV-1 capsid function displays nonclinical pharmacokinetics supporting long-acting potential in humans. Open Forum Infect. Dis. 2018, 5, S199–S200. [Google Scholar] [CrossRef]
- Carnes, S.K.; Sheehan, J.H.; Aiken, C. Inhibitors of the HIV-1 capsid, a target of opportunity. Curr. Opin. HIV AIDS 2018, 13, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, L.M. Conquering HIV’s capsid. Chem. Eng. News 2017, 95, 23–25. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gallazzi, F.; Hill, K.J.; Burke, D.H.; Lange, M.J.; Quinn, T.P.; Neogi, U.; Sonnerborg, A. Gs-CA compounds: First-in-class HIV-1 capsid inhibitors covering multiple grounds. Front. Microbiol. 2019, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Price, A.J.; Fletcher, A.J.; Schaller, T.; Elliott, T.; Lee, K.; KewalRamani, V.N.; Chin, J.W.; Towers, G.J.; James, L.C. CPSF6 defines a conserved capsid interface that modulates HIV-1 replication. PLoS Pathog. 2012, 8, e1002896. [Google Scholar] [CrossRef] [PubMed]
- Perrier, M.; Bertine, M.; Le Hingrat, Q.; Joly, V.; Visseaux, B.; Collin, G.; Landman, R.; Yazdanpanah, Y.; Descamps, D.; Charpentier, C. Prevalence of gag mutations associated with in vitro resistance to capsid inhibitor gs-ca1 in HIV-1 antiretroviral-naive patients. J. Antimicrob. Chemother. 2017, 72, 2954–2955. [Google Scholar] [CrossRef]
- Yant, S.R.; Mulato, A.; Hansen, D.; Tse, W.C.; Niedziela-Majka, A.; Zhang, J.R.; Stepan, G.J.; Jin, D.; Wong, M.H.; Perreira, J.M.; et al. A highly potent long-acting small-molecule HIV-1 capsid inhibitor with efficacy in a humanized mouse model. Nat. Med. 2019, 25, 1377–1384. [Google Scholar] [CrossRef]
- Jacques, D.A.; McEwan, W.A.; Hilditch, L.; Price, A.J.; Towers, G.J.; James, L.C. HIV-1 uses dynamic capsid pores to import nucleotides and fuel encapsidated DNA synthesis. Nature 2016, 536, 349–353. [Google Scholar] [CrossRef]
- Halgren, T.A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 2009, 49, 377–389. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Sowd, G.A.; Serrao, E.; Wang, H.; Wang, W.; Fadel, H.J.; Poeschla, E.M.; Engelman, A.N. A critical role for alternative polyadenylation factor CPSF6 in targeting HIV-1 integration to transcriptionally active chromatin. Proc. Natl. Acad. Sci. USA 2016, 113, E1054–E1063. [Google Scholar] [CrossRef] [PubMed]
- Buffone, C.; Martinez-Lopez, A.; Fricke, T.; Opp, S.; Severgnini, M.; Cifola, I.; Petiti, L.; Frabetti, S.; Skorupka, K.; Zadrozny, K.K.; et al. Nup153 unlocks the nuclear pore complex for HIV-1 nuclear translocation in nondividing cells. J. Virol. 2018, 92, e00648-18. [Google Scholar] [CrossRef] [PubMed]
- Bejarano, D.A.; Peng, K.; Laketa, V.; Borner, K.; Jost, K.L.; Lucic, B.; Glass, B.; Lusic, M.; Muller, B.; Krausslich, H.G. HIV-1 nuclear import in macrophages is regulated by CPSF6-capsid interactions at the nuclear pore complex. Elife 2019, 8, e41800. [Google Scholar] [CrossRef]
- Di Nunzio, F.; Fricke, T.; Miccio, A.; Valle-Casuso, J.C.; Perez, P.; Souque, P.; Rizzi, E.; Severgnini, M.; Mavilio, F.; Charneau, P.; et al. Nup153 and nup98 bind the HIV-1 core and contribute to the early steps of HIV-1 replication. Virology 2013, 440, 8–18. [Google Scholar] [CrossRef]
- Matreyek, K.A.; Engelman, A. The requirement for nucleoporin NUP153 during human immunodeficiency virus type 1 infection is determined by the viral capsid. J. Virol. 2011, 85, 7818–7827. [Google Scholar] [CrossRef]
- Matreyek, K.A.; Yucel, S.S.; Li, X.; Engelman, A. Nucleoporin NUP153 phenylalanine-glycine motifs engage a common binding pocket within the HIV-1 capsid protein to mediate lentiviral infectivity. PLoS Pathog. 2013, 9, e1003693. [Google Scholar] [CrossRef]
- Achuthan, V.; Perreira, J.M.; Sowd, G.A.; Puray-Chavez, M.; McDougall, W.M.; Paulucci-Holthauzen, A.; Wu, X.; Fadel, H.J.; Poeschla, E.M.; Multani, A.S.; et al. Capsid-CPSF6 interaction licenses nuclear HIV-1 trafficking to sites of viral DNA integration. Cell Host Microbe 2018, 24, 392–404. [Google Scholar] [CrossRef]
- Rasheedi, S.; Shun, M.C.; Serrao, E.; Sowd, G.A.; Qian, J.; Hao, C.; Dasgupta, T.; Engelman, A.N.; Skowronski, J. The cleavage and polyadenylation specificity factor 6 (CPSF6) subunit of the capsid-recruited pre-messenger RNA cleavage factor i (cfim) complex mediates HIV-1 integration into genes. J. Biol. Chem. 2016, 291, 11809–11819. [Google Scholar] [CrossRef]
- Yamashita, M.; Engelman, A.N. Capsid-dependent host factors in HIV-1 infection. Trends Microbiol 2017, 25, 741–755. [Google Scholar] [CrossRef]
- Fader, L.D.; Bethell, R.; Bonneau, P.; Bos, M.; Bousquet, Y.; Cordingley, M.G.; Coulombe, R.; Deroy, P.; Faucher, A.M.; Gagnon, A.; et al. Discovery of a 1,5-dihydrobenzo[b][1,4]diazepine-2,4-dione series of inhibitors of HIV-1 capsid assembly. Bioorg. Med. Chem. Lett. 2011, 21, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Lemke, C.T.; Titolo, S.; von Schwedler, U.; Goudreau, N.; Mercier, J.F.; Wardrop, E.; Faucher, A.M.; Coulombe, R.; Banik, S.S.; Fader, L.; et al. Distinct effects of two HIV-1 capsid assembly inhibitor families that bind the same site within the n-terminal domain of the viral CA protein. J. Virol. 2012, 86, 6643–6655. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.; Bonneau, P.; Bousquet, Y.; DeRoy, P.; Duan, J.; Duplessis, M.; Gagnon, A.; Garneau, M.; Goudreau, N.; Guse, I.; et al. Inhibition of HIV-1 capsid assembly: Optimization of the antiviral potency by site selective modifications at N1, C2 and C16 of a 5-(5-furan-2-yl-pyrazol-1-yl)-1h-benzimidazole scaffold. Bioorg. Med. Chem. Lett. 2012, 22, 7512–7517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Curreli, F.; Waheed, A.A.; Mercredi, P.Y.; Mehta, M.; Bhargava, P.; Scacalossi, D.; Tong, X.; Lee, S.; Cooper, A.; et al. Dual-acting stapled peptides target both HIV-1 entry and assembly. Retrovirology 2013, 10, 136. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).