Kid-Short Marfan Score (Kid-SMS) Is a Useful Diagnostic Tool for Stratifying the Pre-Test Probability of Marfan Syndrome in Childhood
Abstract
:1. Introduction
| Dilatation or Dissection of Aorta | |
|---|---|
| Ectopia Lentis | |
| Systemic Involvement (Positive if at Least 7/20 Points) | Score |
| Pectus carinatum | 2 |
| Pectus excavatum or chest asymmetry | 1 |
| Reduced upper segment/lower segment AND increased armspan/height AND no scoliosis | 1 |
| Characteristic face (3 of 5 facial features—dolichocephaly, enophthalmus, downslanting palpebral fissures, malar hypoplasia, retrognathia) | 1 |
| Wrist AND thumb sign | 3 |
| Wrist OR thumb sign | 1 |
| Scoliosis or thoracolumbar kyphosis | 1 |
| Reduced elbow extension (<170°) | 1 |
| Plain pes planus | 1 |
| Hindfoot deformity | 2 |
| Protusio acetabulae | 2 |
| Myopia (>3diopters) | 1 |
| Mitral valve prolaps | 1 |
| Spontaneous pneumothorax | 2 |
| Striae atrophicae | 1 |
| Lumbosacral dural ectasia | 2 |
| FBN1 mutation | |
| Confirmed MFS: | |
| -Dilatation/Dissection of aorta + ectopia lentis OR systemic manifestation OR FBN1 mutation | |
| -Family history of MFS + Dilatation/Dissection of aorta OR ectopia lentis OR systemic involvement | |
| Required Manifestations | Risk Category for Likelihood of MFS |
|---|---|
| SV + EL | Very high risk (Diagnosis of MFS according Ghent-2) |
| SV + MVP + TVP SV + PA SV + 3 Skeletal Features | High risk (Patient is at high risk of MFS. Complete examination of all symptoms of the revised Ghent Criteria is strictly recommended as soon as possible. Patient should see MFS specialists) |
| EL + MVP + TVP EL + PA | |
| Family history SV | Moderate risk (Patient needs to be verified or excluded with further diagnostic procedures other than or echocardiography and clinical examination) |
2. Experimental Section
3. Results and Discussion
3.1. Results
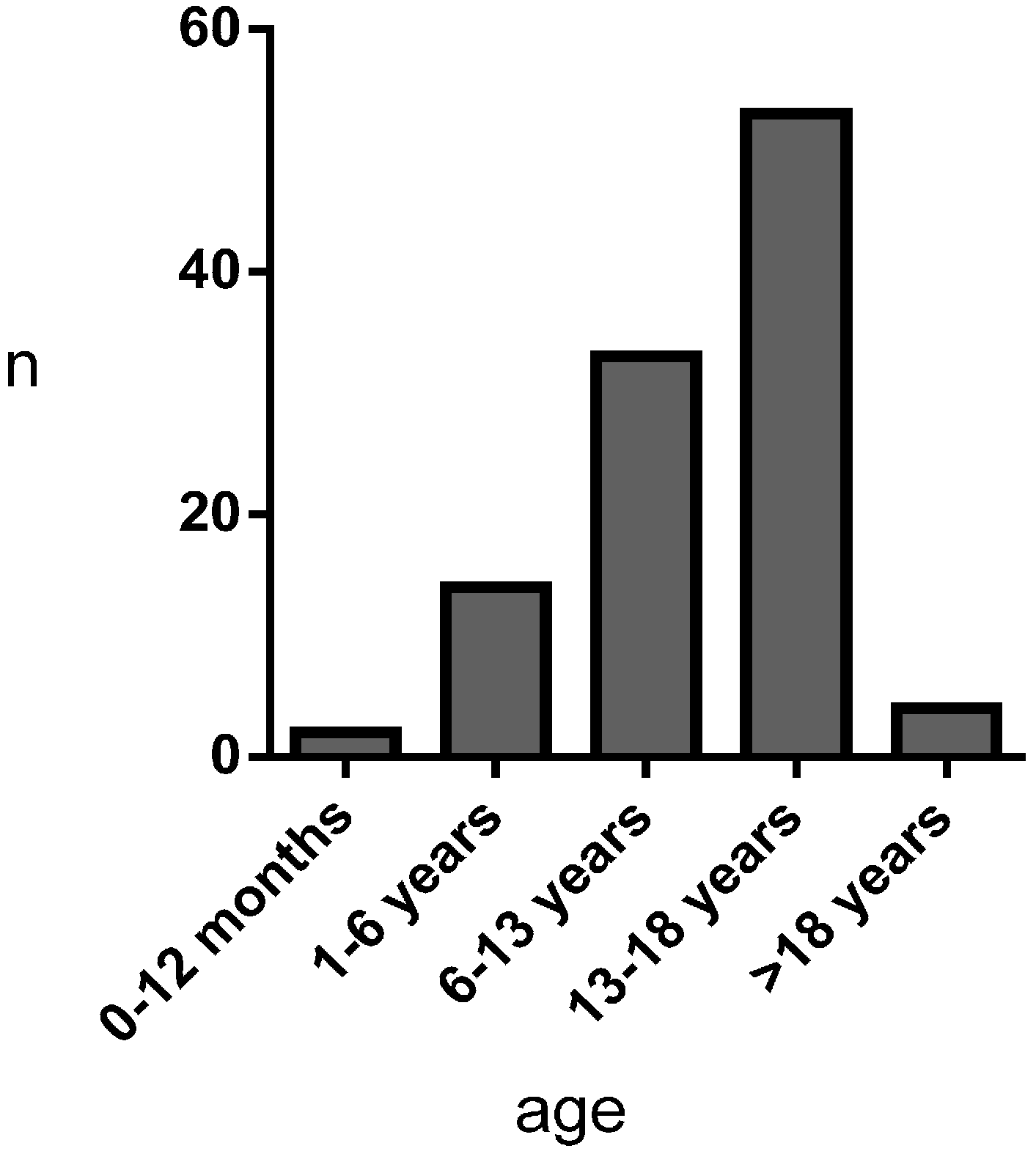
| n = 106 | Ghent-2 Pos | Ghent-2 Neg |
|---|---|---|
| Kid-SMS Pos | 21 | 22 |
| Kid-SMS Neg | 0 | 63 |
| n = 106 | Ghent-2 Pos | Ghent-2 Neg |
|---|---|---|
| Kid-SMS Pos | 24 | 19 |
| Kid-SMS Neg | 0 | 63 |
| Patients Ghent-2 Pos (Ghent-2), n = 27 | Patients Ghent-2 Neg, n = 79 | ||
|---|---|---|---|
| Patients FBN1 Pos, n = 5 | Patients FBN1 Neg, n = 74 | ||
| Very high risk SV + EL | 2 | 0 | 0 |
| High risk SV + 3Skel | 8 | 0 | 2 |
| High risk SV + MVP + TVP | 7 | 0 | 0 |
| High risk SV + PA | 3 | 0 | 0 |
| High risk EL + MVP + TVP | 0 | 0 | 0 |
| High risk EL + PA | 2 | 0 | 0 |
| Moderate risk (FH) | 2 | 2 | 7 |
| Moderate risk (SV) | 3 | 0 | 5 |
| Negative | 0 | 3 | 60 |
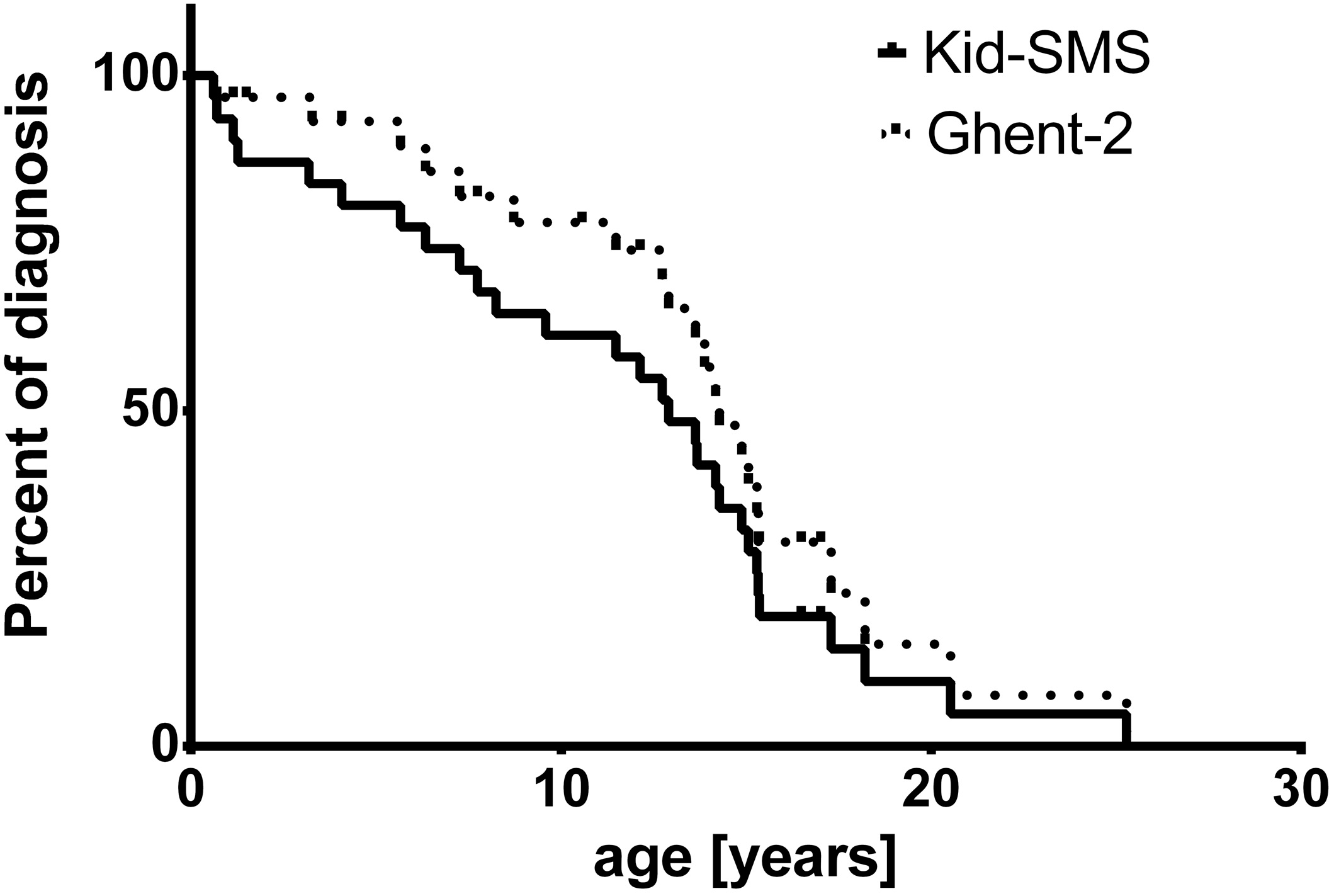
3.2. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Judge, D.P.; Dietz, H.C. Marfan’s syndrome. Lancet 2005, 366, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Rybczynski, M.; Bernhardt, A.M.; Rehder, U.; Fuisting, B.; Meiss, L.; Voss, U.; Habermann, C.; Detter, C.; Robinson, P.N.; Arslan-Kirchner, M.; et al. The spectrum of syndromes and manifestations in individuals screened for suspected Marfan syndrome. Am. J. Med. Genet. A 2008, 146, 3157–3166. [Google Scholar] [CrossRef]
- Faivre, L.; Collod-Beroud, G.; Ades, L.; Arbustini, E.; Child, A.; Callewaert, B.L.; Loeys, B.; Binquet, C.; Gautier, E.; Mayer, K.; et al. The new Ghent criteria for Marfan syndrome: What do they change? Clin. Genet. 2012, 81, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, K.J.; Clayton-Smith, J.; Harris, R. Evolving phenotype of Marfan’s syndrome. Arch. Dis. Child. 1997, 76, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Velvin, G.; Bathen, T.; Rand-Hendiksen, S.; Geirdal, A.O. Systemic review of psychosocial aspects of living with Marfan syndrome. Clin. Genet. 2014, 87, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, K.; Pulkkinen, L.; Savolainen, A.; Kaitila, I.; Peltonen, L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N. Engl. J. Med. 1990, 323, 935–939. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, A.; Devereux, R.B.; Dietz, H.C.; Hennekam, R.C.; Pyeritz, R.E. Revised diagnostic criteria for the Marfan syndrome. Am. J. Med. Genet. 1996, 62, 417–426. [Google Scholar]
- Loeys, B.L.; Dietz, H.C.; Bravermann, A.C.; Callewaert, B.L.; de Backer, J.; Devereux, R.B.; Hilhorst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.M.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med. Genet. 2010, 47, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.C.; Stark, V.; Steiner, K.; Weil, J.; von Kodolitsch, Y.; Mir, T.S. The Kid-short marfan score (Kid-SMS)—An easy executable risk score for suspected paediatric patients with Marfan syndrome. Acta Paediatri. 2013, 102, e84–e89. [Google Scholar] [CrossRef]
- Mueller, G.C.; Stark, V.; Steiner, K.; von Kodolitsch, Y.; Rybczynski, M.; Weil, J.; Mir, T.S. Impact of Age and Gender on cardiac pathology in children and adolescents with Marfan syndrome. Pediatr. Cardiol. 2013, 34, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Knirsch, W.; Hillebrand, D.; Horke, A.; Lewin, M.A.; Rein, J.; Uhlemann, F. Aortic aneurysm rupture in infantile Marfan’s syndrome. Pediatr. Cardiol. 2001, 22, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Devereux, R.B.; Kramer-Fox, R.; O’Loughlin, J. Two-Dimensional echocardiographic aortic root dimensions in normal children and adults. Am. J. Cardiol. 1989, 64, 507–512. [Google Scholar] [CrossRef]
- Pettersen, M.D.; Du, W.; Skeens, M.E.; Humes, R.A. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children and adolescens: An echocardiographic study. J. Am. Soc. Echocardiogr. 2008, 21, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.; Meier, P. Nonparametric estimation from incomlete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Gillis, E.; van Laer, L.; Loeys, B.L. Genetics of thoracic aortic aneurysm—At the crossroad of transforming growth factor-β signaling and vascular smoot muscle cell contractility. Circ. Res. 2013, 113, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Faivre, L.; Masurel-Paulet, A.; Collod-Béroud, G.; Callewaert, B.L.; Child, A.H.; Stheneur, C.; Binquet, C.; Gautier, E.; Chevallier, B.; Huet, F.; et al. Clinical and molecular study of 320 children with Marfan syndrome and related type I fibrillinopathies in a series of 1009 probands with pathogenic FBN1 mutations. Pediatrics 2009, 123, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mueller, G.C.; Stierle, L.; Stark, V.; Steiner, K.; von Kodolitsch, Y.; Weil, J.; Mir, T.S. Retrospective analysis of the effect of angiotensin II receptor blocker versus beta-blocker on aortic root growth in paediatric patients with Marfan syndrome. Heart 2014, 100, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Lacro, R.V.; Dietz, H.C.; Sleeper, L.A.; Yetman, A.T.; Bradley, T.J.; Colan, S.D.; Pearson, G.D.; Selamet Tierney, E.S.; Levine, J.C.; Atz, A.M.; et al. Atenolol versus Losartan in children and young adults with Marfan’s Syndrome. N. Engl. J. Med. 2014, 371, 2061–2071. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stark, V.C.; Arndt, F.; Harring, G.; Von Kodolitsch, Y.; Kozlik-Feldmann, R.; Mueller, G.C.; Steiner, K.J.; Mir, T.S. Kid-Short Marfan Score (Kid-SMS) Is a Useful Diagnostic Tool for Stratifying the Pre-Test Probability of Marfan Syndrome in Childhood. Diseases 2015, 3, 24-33. https://doi.org/10.3390/diseases3010024
Stark VC, Arndt F, Harring G, Von Kodolitsch Y, Kozlik-Feldmann R, Mueller GC, Steiner KJ, Mir TS. Kid-Short Marfan Score (Kid-SMS) Is a Useful Diagnostic Tool for Stratifying the Pre-Test Probability of Marfan Syndrome in Childhood. Diseases. 2015; 3(1):24-33. https://doi.org/10.3390/diseases3010024
Chicago/Turabian StyleStark, Veronika C., Florian Arndt, Gesa Harring, Yskert Von Kodolitsch, Rainer Kozlik-Feldmann, Goetz C. Mueller, Kristoffer J. Steiner, and Thomas S. Mir. 2015. "Kid-Short Marfan Score (Kid-SMS) Is a Useful Diagnostic Tool for Stratifying the Pre-Test Probability of Marfan Syndrome in Childhood" Diseases 3, no. 1: 24-33. https://doi.org/10.3390/diseases3010024
APA StyleStark, V. C., Arndt, F., Harring, G., Von Kodolitsch, Y., Kozlik-Feldmann, R., Mueller, G. C., Steiner, K. J., & Mir, T. S. (2015). Kid-Short Marfan Score (Kid-SMS) Is a Useful Diagnostic Tool for Stratifying the Pre-Test Probability of Marfan Syndrome in Childhood. Diseases, 3(1), 24-33. https://doi.org/10.3390/diseases3010024





