Nutritional Management in Liver Cirrhosis: A Combined Systematic Review and Observational Study
Abstract
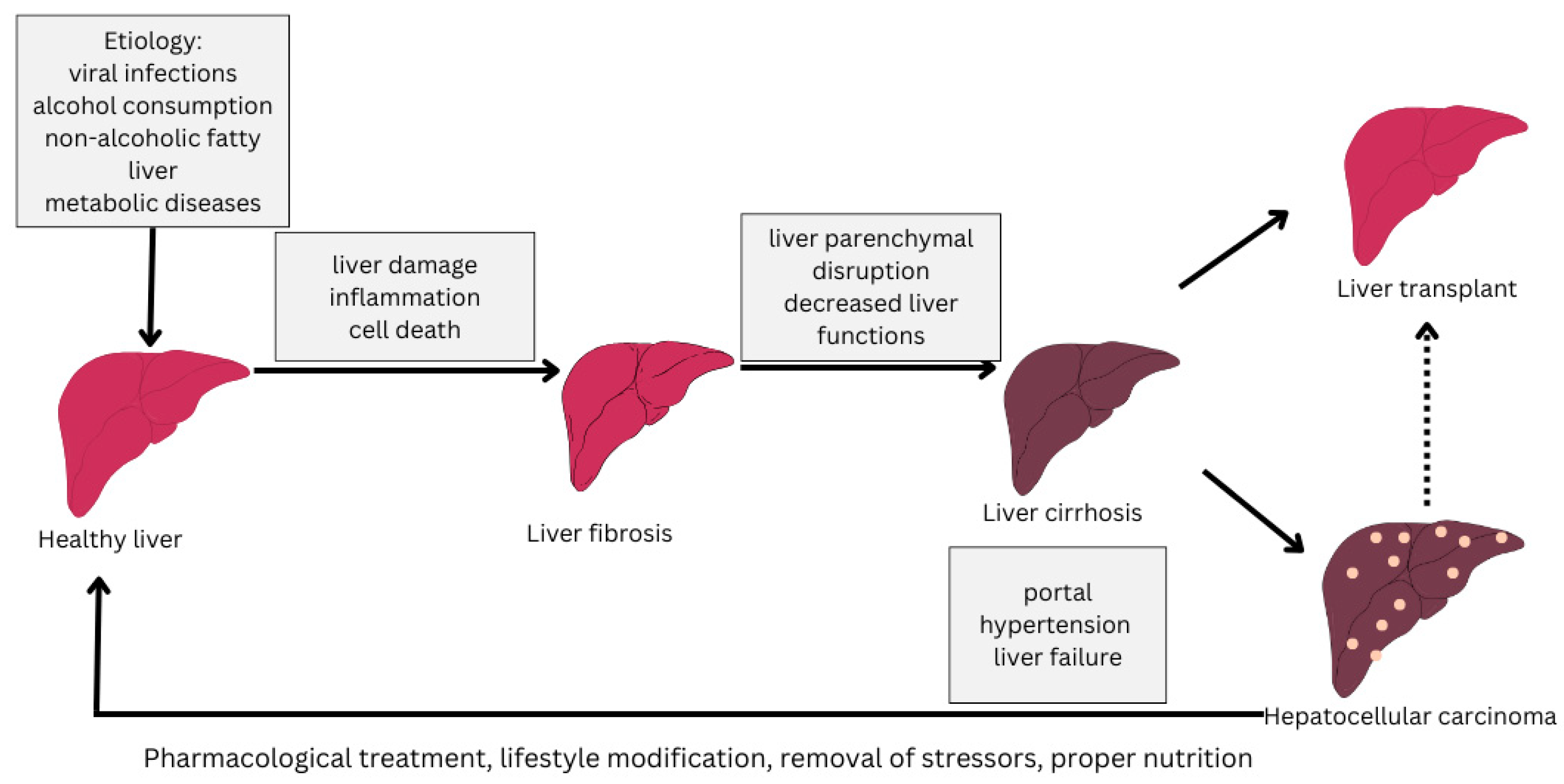
1. Introduction
- Sufficient Energy and Protein: A high-calorie, high-protein diet should be used to prevent or reverse protein–energy malnutrition. Standard goals are 30 to 35 kcal/kg/day of energy and 1.2 to 1.5 g/kg/day of protein (or higher in nutritionally at-risk patients) across multiple meals per day [30]. Protein restriction has also now been discouraged in any patient with hepatic encephalopathy; patients with cirrhosis (including those with encephalopathy) should be given at least 1.2–1.5 g/kg of protein per day as tolerated to maintain nitrogen balance [9]. Protein is vital to the maintenance of muscle mass, and issues related to the exacerbation of encephalopathy due to high protein intake are addressed with drugs (i.e., lactulose), rather than decreasing protein intake, according to current recommendations [31,32].
- Meal Timing and Meal Frequency: Meals should be small and frequent, and prolonged periods of fasting are not recommended [33,34,35,36]. Patients with cirrhosis are advised to receive 3–5 meals with 2–3 snacks in a day, with 1 of them in the late evening consisting of carbohydrates [37]. Moreover, 50 g of complex carbohydrates as a bedtime snack is advised to provide a source of energy while the patient is asleep, as they may not eat food overnight. This approach was demonstrated to ameliorate sarcopenia in cirrhosis, enhance lean body mass, and improve nitrogen status [24]. Generally, frequent feeding (punctuated only by a late-night snack) allows one to minimize fasting-induced muscle catabolism, which makes it an easy yet effective dietary change [9].
- Sodium Control: A mild sodium restriction diet is normally suggested in patients with ascites since it can help control fluid retention. Guidelines frequently recommend the use of a no-added salt diet (approximately 56 g of salt daily [38]). Nevertheless, sodium restriction must be considered together with palatability and overall intake, as overly strict limits can render food unattractive, decreasing food intake and further exacerbating malnutrition [39]. Clinicians are advised to personalize sodium intake limits to reduce ascites without unnecessarily reducing patients’ nutritional intake.
- Oral Nutritional Supplementation: If patients are unable to receive the required proportion of calories or protein via an oral diet, oral nutritional supplements (ONSs) must be introduced. Add-ons (like high-protein, energy-rich oral supplements) have been demonstrated to considerably restore body composition (increase lean mass and BMI) and the serum proteins of nutritionally at-risk cirrhotic individuals [40,41]. A meta-analysis showed that oral supplementation can be used to enhance the clinical outcomes of patients with cirrhosis [42]. Of particular interest is taking supplements in the late evening (nighttime ONSs), which has been found to result in a more beneficial protein status than daytime supplementation, aligning with the prioritization of late evening feeding [43,44]. On the whole, ONSs play a beneficial and supportive role in meeting nutritional outcomes, and they are usually fortified with vitamins and minerals to remedy micronutrient deficits.
- Micronutrient Repletion: Vitamin and mineral deficiencies are commonly found in cirrhotic patients, with levels of vitamin D, zinc, magnesium, and fat-soluble vitamins (A, E, K) often being low as a result of malabsorption and/or the effects of chronic disease [45,46,47,48,49]. Micronutrient deficiencies must be identified and treated. Practice guidelines recommend supplementing any confirmed deficiencies, or those with strong evidence, with the related vitamins or minerals [9]. As an example, vitamin D levels should be assessed, as a majority of the population is deficient, and vitamin D supplementation should be provided in the case of a vitamin deficiency (<20 ng/mL) until sufficient levels are achieved [50]. Another significant micronutrient is zinc. Supplementation is frequently advised for patients with a zinc deficiency or breakthrough hepatic encephalopathy; it is possible that increasing zinc levels can boost both ammonia metabolism and taste performance (and ideally appetite) [50]. In general, although routine mega-dose supplementation is not recommended without evidence of deficiency, supplementation to restore nutritional deficiencies is an important part of comprehensive care [29].
- Branched-Chain Amino Acids (BCAAs): BCAAs (leucine, isoleucine, and valine) are essential amino acids that are usually deficient in people with cirrhosis because of the disturbed metabolism of amino acids and their utilization as alternative sources of energy [51,52]. BCAA supplementation has been shown to have a number of potential benefits in advanced liver disease: BCAA supplementation has the potential to prevent muscle protein breakdown, improve nitrogen balance, enhance muscle mass, limit the development of complications, and improve quality of life [53,54,55]. Clinical studies have also reported that the neuropsychiatric performance of patients with hepatic encephalopathy is improved with BCAA supplementation, probably due to the substitution of an alternative nitrogen source and the detoxification of ammonia [56]. BCAA-enriched nutritional supplements are specifically recommended for patients with decompensated cirrhosis or those who are unable to consume enough protein due to encephalopathy or other problems [57]. This may result in the use of nighttime BCAA drinks or increasing the portion of vegetable protein (which is naturally higher in BCAAs and less likely to produce ammonia) in patients with refractory hepatic encephalopathy [58]. In this way, BCAA supplementation is a scientifically justified intervention to sustain protein intake and muscle anabolism in nutritionally compromised cirrhotic patients.
- Enteral Nutrition Support: Inadequate oral feeding, even after dietary advice and use of ONSs, may necessitate the use of early enteral tube feeding to achieve nutritional goals. Gastrointestinal nutrition (e.g., through a nasogastric or nasoenteric feeding tube) is mostly safe and well-tolerated in patients with cirrhosis; in fact, the absence of non-bleeding esophageal varices, even when a nasogastric feeding tube is to be inserted, is not indicative of contraindication [59]. Elective endoscopic gastrostomy tubes are, however, riskier in advanced disease and should normally be averted [60]. Early enteral nutrition in nutritionally at-risk cirrhotic patients is presumed to stabilize energy and protein intake, prevent further muscle loss, and enhance clinical outcomes. This feeding method is used instead of parenteral nutrition when the gastrointestinal tract is in working order, as it reduces the risk of infection and delivers nutrients in a more physiologic manner. Parenteral nutrition is used only in exceedingly unique cases when enteral nutrition cannot be carried out (such as in intestinal failure or protracted ileus) or when oral/enteral nutrition cannot be withheld longer than 72 h [59,61]. In these cases, close follow-up is advised since infections (e.g., central line IV infections) and metabolic problems can occur in cirrhotic patients. Thus, early use of enteral nutrition in individuals with the inability to receive oral nutrition is an essential measure to avoid starvation and treat malnutrition in this at-risk group [62].
2. Materials and Methods
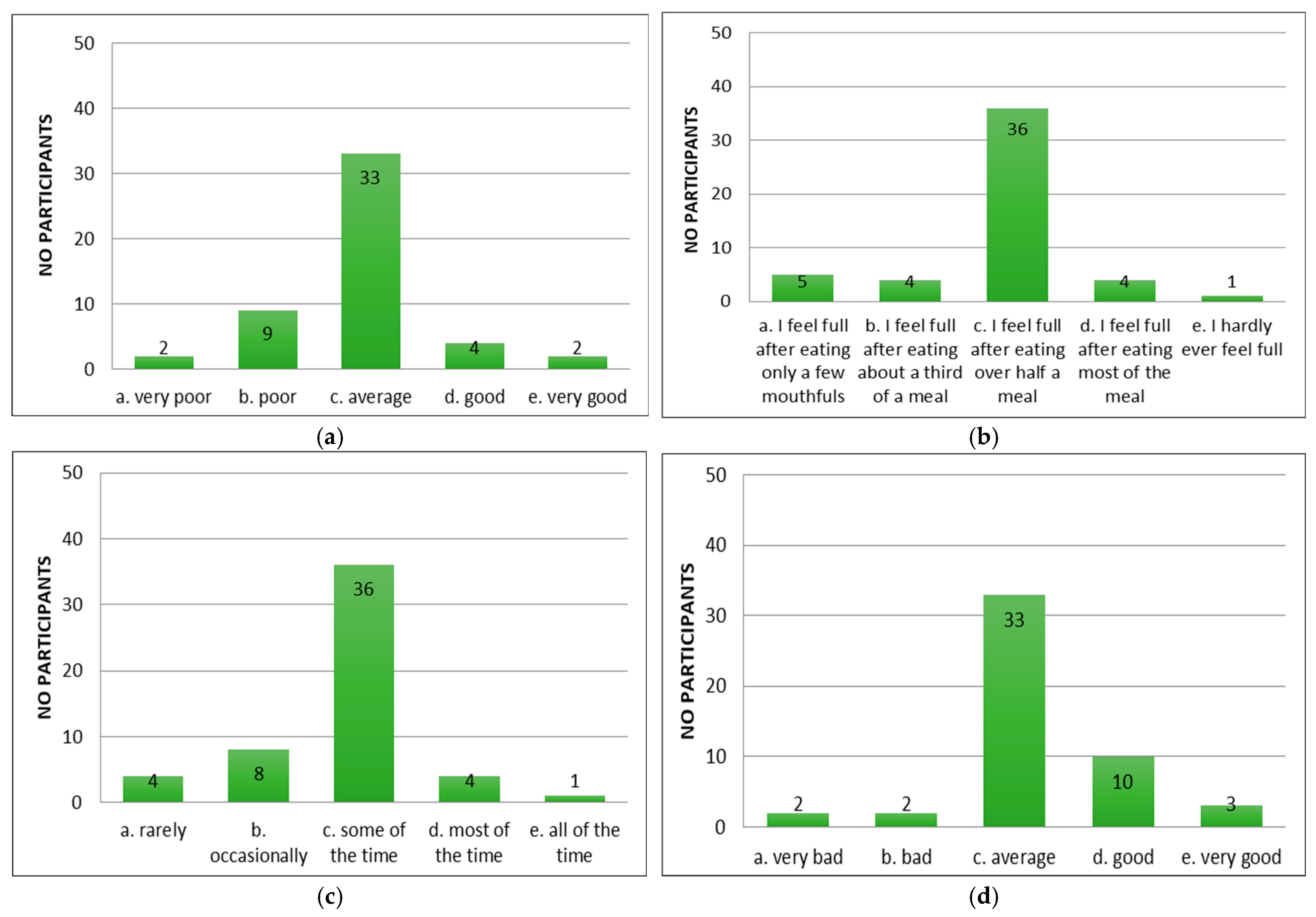
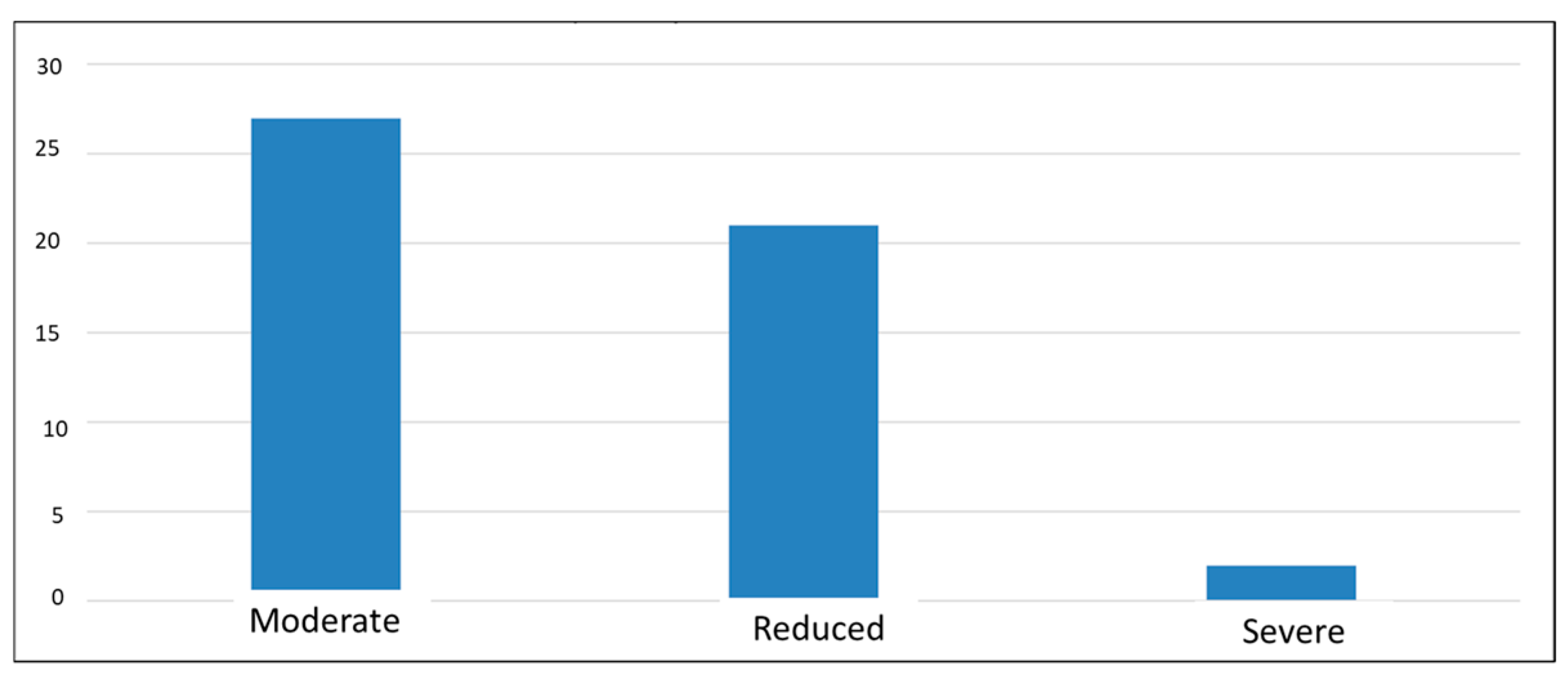
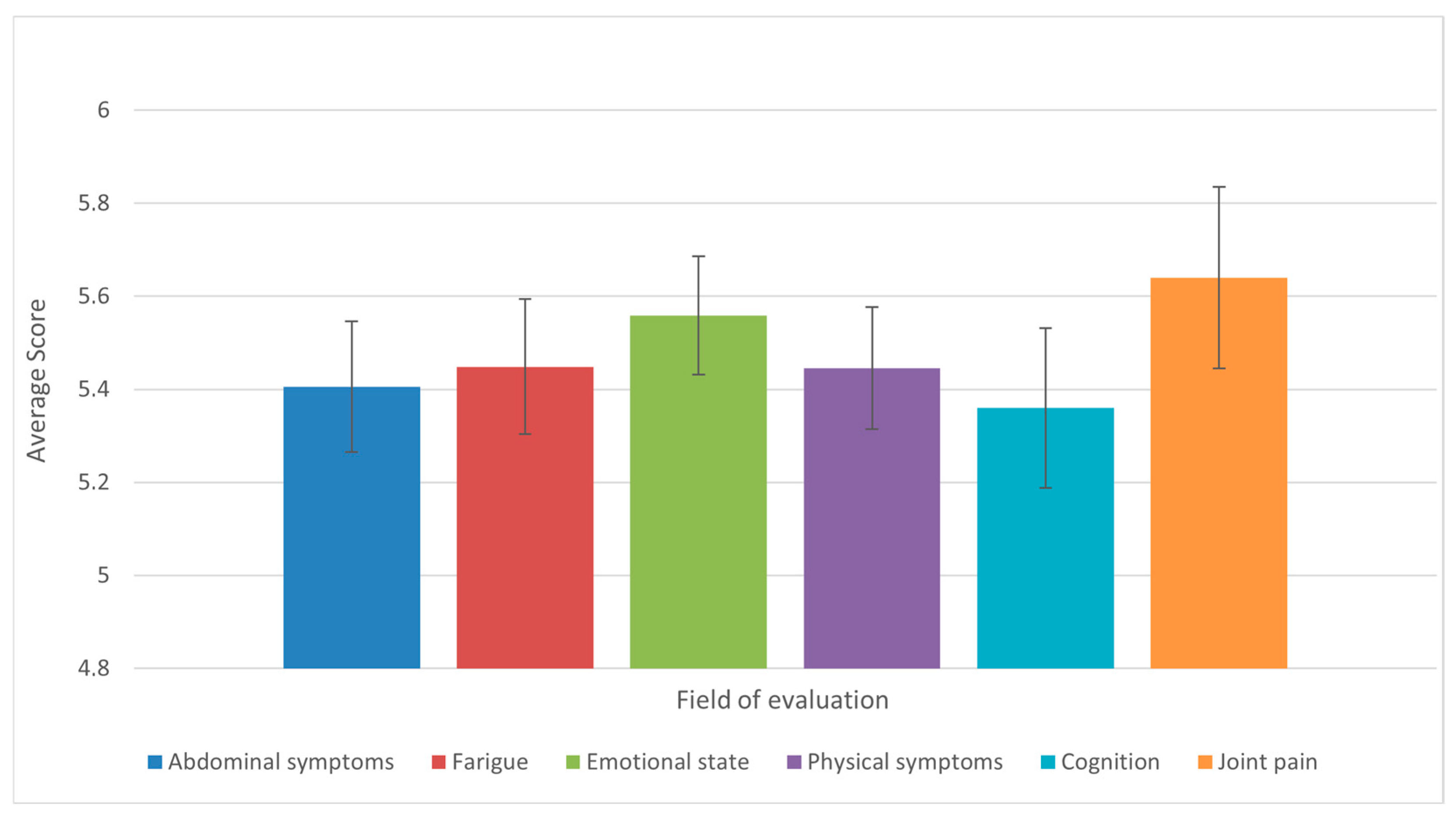
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Diseases |
| ALT | Alanine Aminotransferase |
| AST | Aspartate Aminotransferase |
| CLDQ-NASH | The Chronic Liver Disease Questionnaire for Nonalcoholic Steatohepatitis |
| CNAQ | Council of Nutrition Appetite Questionnaire |
| CNS | Central nervous system |
| EALS | European Association for the Study of the Liver Guidelines |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| GBD | Global Burden of Diseases, Injuries, and Risk Factors Study |
| GPP | Good practice point |
| MELD | The Model for End-Stage Liver Disease |
| NAFLD | Non-alcoholic fatty liver disease |
References
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; The Members of the EASL HEPAHEALTH Steering Committee. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: Observational study. BMJ 2018, 362, k2817. [Google Scholar] [CrossRef]
- Safiri, S.; Sepanlou, S.G.; Ikuta, K.S.; Bisignano, C.; Salimzadeh, H.; Delavari, A.; Ansari, R.; Roshandel, G.; Merat, S.; Fitzmaurice, C.; et al. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 913–933. [Google Scholar] [CrossRef] [PubMed]
- Cirrhosis: Diagnosis and Management—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31845776/ (accessed on 5 August 2025).
- Tapper, E.B.; Parikh, N.D. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA 2023, 329, 1589–1602. [Google Scholar] [CrossRef]
- Muir, A.J. Understanding the Complexities of Cirrhosis. Clin. Ther. 2015, 37, 1822–1836. [Google Scholar] [CrossRef]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Santangeli, E.; Abbati, C.; Chen, R.; Di Carlo, A.; Leoni, S.; Piscaglia, F.; Ferri, S. Pathophysiological-Based Nutritional Interventions in Cirrhotic Patients with Sarcopenic Obesity: A State-of-the-Art Narrative Review. Nutrients 2024, 16, 427. [Google Scholar] [CrossRef]
- Traub, J.; Reiss, L.; Aliwa, B.; Stadlbauer, V. Malnutrition in patients with liver cirrhosis. Nutrients 2021, 13, 540. [Google Scholar] [CrossRef]
- Naqvi, I.H.; Mahmood, K.; Salekeen, S.; Akhter, S.T. Determining the frequency and severity of malnutrition and correlating it with the severity of liver cirrhosis. Turk. J. Gastroenterol. 2013, 24, 415–422. [Google Scholar] [CrossRef]
- Nutritional Status and Its Impact on Clinical Outcomes for Patients Admitted to Hospital with Cirrhosis—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27266216/ (accessed on 5 August 2025).
- Maharshi, S.; Sharma, B.C.; Srivastava, S. Malnutrition in cirrhosis increases morbidity and mortality. J. Gastroenterol. Hepatol. 2015, 30, 1507–1513. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Maurício, S.F.; da Silva, T.A.; de Vasconcelos Generoso, S.; Lima, A.S.; Correia, M.I.T.D. Combined nutritional assessment methods to predict clinical outcomes in patients on the waiting list for liver transplantation. Nutrition 2018, 47, 21–26. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Martins, A.I.F.; Cunha, C.E.; Anastácio, L.R.; Lima, A.S.; Correia, M.I.T.D. Negative energy balance secondary to inadequate dietary intake of patients on the waiting list for liver transplantation. Nutrition 2013, 29, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Izbéki, F.; Kiss, I.; Wittmann, T.; Várkonyi, T.T.; Légrády, P.; Lonovics, J. Impaired accommodation of proximal stomach in patients with alcoholic liver cirrhosis. Scand. J. Gastroenterol. 2002, 37, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Aprile, L.R.O.; Meneghelli, U.G.; Martinelli, A.L.C.; Monteiro, C.R. Gastric motility in patients with presinusoidal portal hypertension. Am. J. Gastroenterol. 2002, 97, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Aqel, B.A.; Scolapio, J.S.; Dickson, R.C.; Burton, D.D.; Bouras, E.P. Contribution of ascites to impaired gastric function and nutritional intake in patients with cirrhosis and ascites. Clin. Gastroenterol. Hepatol. 2005, 3, 1095–1100. [Google Scholar] [CrossRef]
- Grüngreiff, K.; Reinhold, D.; Wedemeyer, H. The role of zinc in liver cirrhosis. Ann. Hepatol. 2016, 15, 7–16. [Google Scholar] [CrossRef]
- Lanspa, S.J.; Chan, A.T.H.; Bell, J.S.; Go, V.L.W.; Dickson, E.R.; Dimagno, E.P. Pathogenesis of steatorrhea in primary biliary cirrhosis. Hepatology 1985, 5, 837–842. [Google Scholar] [CrossRef]
- Hofmann, A.F. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef]
- Glucose Intolerance Insulin Resistance in Patients with Liver Disease. II. A Study of Etiologic Factors and Evaluation of Insulin Actions—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/5506964/ (accessed on 5 August 2025).
- Müller, M.J.; Böttcher, J.; Selberg, O.; Weselmann, S.; Böker, K.H.; Schwarze, M.; Mühlen, A.v.Z.; Manns, M.P. Hypermetabolism in clinically stable patients with liver cirrhosis. Am. J. Clin. Nutr. 1999, 69, 1194–1201. [Google Scholar] [CrossRef]
- Nakaya, Y.; Harada, N.; Kakui, S.; Okada, K.; Takahashi, A.; Inoi, J.; Ito, S. Severe catabolic state after prolonged fasting in cirrhotic patients: Effect of oral branched-chain amino-acid-enriched nutrient mixture. J. Gastroenterol. 2002, 37, 531–536. [Google Scholar] [CrossRef]
- E Owen, O.; A Reichle, F.; Mozzoli, M.A.; Kreulen, T.; Patel, M.S.; Elfenbein, I.B.; Golsorkhi, M.; Chang, K.H.; Rao, N.S.; Sue, H.S.; et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J. Clin. Investig. 1981, 68, 240–252. [Google Scholar] [CrossRef]
- Petersen, K.F.; Krssak, M.; Navarro, V.; Chandramouli, V.; Hundal, R.; Schumann, W.C.; Landau, B.R.; Shulman, G.I. Contributions of net hepatic glycogenolysis and gluconeogenesis to glucose production in cirrhosis. Am. J. Physiol. Metab. 1999, 276, E529–E535. [Google Scholar] [CrossRef] [PubMed]
- Yeung, R.T.T.; Wang, C.C.L. A study of carbohydrate metabolism in postnecrotic cirrhosis of liver. Gut 1974, 15, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Bianchi, G.; Lucidi, P.; Villanova, N.; Zoli, M.; De Feo, P. Plasma Ghrelin Concentrations, Food Intake, and Anorexia in Liver Failure. J. Clin. Endocrinol. Metab. 2004, 89, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Iwata, K.; Hara, N.; Hattori, A.; Ishidome, M.; Sekoguchi-Fujikawa, N.; Mifuji-Moroka, R.; Sugimoto, R.; Fujita, N.; Kobayashi, Y.; et al. Nutrition therapy using a multidisciplinary team improves survival rates in patients with liver cirrhosis. Nutrition 2013, 29, 1418–1421. [Google Scholar] [CrossRef]
- Reuter, B.; Shaw, J.; Hanson, J.; Tate, V.; Acharya, C.; Bajaj, J.S. Nutritional Assessment in Inpatients with Cirrhosis Can Be Improved After Training and Is Associated with Lower Readmissions. Liver Transplant. 2019, 25, 1790–1799. [Google Scholar] [CrossRef]
- Sam, J.; Nguyen, G.C. Protein-calorie malnutrition as a prognostic indicator of mortality among patients hospitalized with cirrhosis and portal hypertension. Liver Int. 2009, 29, 1396–1402. [Google Scholar] [CrossRef]
- Nielsen, K.; Kondrup, J.; Martinsen, L.; Stilling, B.; Wikman, B. Nutritional assessment and adequacy of dietary intake in hospitalized patients with alcoholic liver cirrhosis. Br. J. Nutr. 1993, 69, 665–679. [Google Scholar] [CrossRef]
- Ney, M.; Abraldes, J.G.; Ma, M.; Belland, D.; Harvey, A.; Robbins, S.; Heyer, V.D.; Tandon, P. Insufficient Protein Intake Is Associated with Increased Mortality in 630 Patients with Cirrhosis Awaiting Liver Transplantation. Nutr. Clin. Pract. 2015, 30, 530–536. [Google Scholar] [CrossRef]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef]
- Swart, G.R.; Zillikens, M.C.; Van Vuure, J.K.; Van den Berg, J.W.O. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. Br. Med. J. 1989, 299, 1202–1203. [Google Scholar] [CrossRef]
- Tsien, C.D.; Mccullough, A.J.; Dasarathy, S. Late evening snack: Exploiting a period of anabolic opportunity in cirrhosis. J. Gastroenterol. Hepatol. 2012, 27, 430–441. [Google Scholar] [CrossRef]
- Guo, Y.J.; Tian, Z.B.; Jiang, N.; Ding, X.L.; Mao, T.; Jing, X. Effects of late evening snack on cirrhotic patients: A systematic review and meta-analysis. Gastroenterol. Res. Pract. 2018, 2018, 9189062. [Google Scholar] [CrossRef] [PubMed]
- Food Groups Intake of Cirrhotic Patients, Comparison with the Nutritional Status and Disease Stage—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/31528306/ (accessed on 5 August 2025).
- Haberl, J.; Zollner, G.; Fickert, P.; Stadlbauer, V. To salt or not to salt?—That is the question in cirrhosis. Liver Int. 2018, 38, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.B.; Yang, X.J.; Zhu, H.Y.; Xu, B.Y. Effect of a diet with unrestricted sodium on ascites in patients with hepatic cirrhosis. Gut Liver 2012, 6, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Putadechakum, S.; Klangjareonchai, T.; Soponsaritsuk, A.; Roongpisuthipong, C. Nutritional status assessment in cirrhotic patients after protein supplementation. ISRN Gastroenterol. 2012, 2012, 690402. [Google Scholar] [CrossRef]
- Cunha, L.; Nono, M.H.; Guibert, A.L.; Nidegger, D.; Beau, P.; Beauchant, M. Effects of prolonged oral nutritional support in malnourished cirrhotic patients: Results of a pilot study. Gastroenterol. Clin. Biol. 2004, 28, 36–39. [Google Scholar] [CrossRef]
- Ney, M.; Vandermeer, B.; Van Zanten, S.J.V.; Ma, M.M.; Gramlich, L.; Tandon, P. Meta-Analysis: Oral or enteral nutritional supplementation in cirrhosis. Aliment. Pharmacol. Ther. 2013, 37, 672–679. [Google Scholar] [CrossRef]
- Maharshi, S.; Sharma, B.C.; Sachdeva, S.; Srivastava, S.; Sharma, P. Efficacy of Nutritional Therapy for Patients with Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin. Gastroenterol. Hepatol. 2016, 14, 454–460.e3. [Google Scholar] [CrossRef]
- Norman, K.; Kirchner, H.; Freudenreich, M.; Ockenga, J.; Lochs, H.; Pirlich, M. Three month intervention with protein and energy rich supplements improve muscle function and quality of life in malnourished patients with non-neoplastic gastrointestinal disease-A randomized controlled trial. Clin. Nutr. 2008, 27, 48–56. [Google Scholar] [CrossRef]
- Bering, T.; Diniz, K.G.; Coelho, M.P.P.; Vieira, D.A.; Soares, M.M.S.; Kakehasi, A.M.; Correia, M.I.T.; Teixeira, R.; Queiroz, D.M.; Rocha, G.A.; et al. Association between pre-sarcopenia, sarcopenia, and bone mineral density in patients with chronic hepatitis C. J. Cachexia Sarcopenia Muscle 2018, 9, 255–268. [Google Scholar] [CrossRef]
- Carey, E.J.; Lai, J.C.; Wang, C.W.; Dasarathy, S.; Lobach, I.; Montano-Loza, A.J.; Dunn, M.A. The Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017, 23, 625–633. [Google Scholar] [CrossRef]
- Chang, K.V.; De Chen, J.; Wu, W.T.; Huang, K.C.; Lin, H.Y.; Han, D.S. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? A systematic review and meta-analysis. J. Formos. Med. Assoc. 2019, 118, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Chinnaratha, M.A.; Chaudhary, S.; Doogue, M.; Mccormick, R.J.; Woodman, R.J.; Wigg, A.J. Prevalence of hepatic osteodystrophy and vitamin D deficiency in cirrhosis. Intern. Med. J. 2015, 45, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Review article: Malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment. Pharmacol. Ther. 2019, 51, 64–77. [Google Scholar] [CrossRef]
- Kato, M.; Miwa, Y.; Tajika, M.; Hiraoka, T.; Muto, Y.; Moriwaki, H. Preferential Use of Branched-Chain Amino Acids as an Energy Substrate in Patients with Liver Cirrhosis. Intern. Med. 1998, 37, 429–434. [Google Scholar] [CrossRef]
- Abuelazm, M.; Fares, A.; Elhady, M.M.; Amin, A.M.; Khan, U.; Gowaily, I.; Jaber, F. Branched-Chain Amino Acid Supplements for Sarcopenia in Liver Cirrhosis: A Systematic Review and Meta-analysis. J. Clin. Exp. Hepatol. 2025, 15, 102417. [Google Scholar] [CrossRef]
- Yamauchi, M.; Takeda, K.; Sakamoto, K.; Ohata, M.; Toda, G. Effect of oral branched chain amino acid supplementation in the late evening on the nutritional state of patients with liver cirrhosis. Hepatol. Res. 2001, 21, 199–204. [Google Scholar] [CrossRef]
- Nakaya, Y.; Okita, K.; Suzuki, K.; Moriwaki, H.; Kato, A.; Miwa, Y.; Shiraishi, K.; Okuda, H.; Onji, M.; Kanazawa, H.; et al. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition 2007, 23, 113–120. [Google Scholar] [CrossRef]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 2006, 35, 204–214. [Google Scholar] [CrossRef]
- Improvement of Hepatic Encephalopathy Using a Modified High-Calorie High-Protein Diet—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16200232/ (accessed on 5 August 2025).
- Amodio, P.; Bemeur, C.; Butterworth, R.; Cordoba, J.; Kato, A.; Montagnese, S.; Uribe, M.; Vilstrup, H.; Morgan, M.Y. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology 2013, 58, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Iebba, V.; Giusto, M. What is new about diet in hepatic encephalopathy. Metab. Brain Dis. 2015, 31, 1289–1294. [Google Scholar] [CrossRef]
- Plauth, M.; Cabré, E.; Riggio, O.; Assis-Camilo, M.; Pirlich, M.; Kondrup, J.; Ferenci, P.; Holm, E.; Dahl, S.V.; Müller, M.; et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin. Nutr. 2006, 25, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Kearns, P.J.; Young, H.; Garcia, G.; Blaschke, T.; O’HAnlon, G.; Rinki, M.; Sucher, K.; Gregory, P. Accelerated improvement of alcoholic liver disease with enteral nutrition. Gastroenterology 1992, 102, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Baltz, J.G.; Argo, C.K.; Al-Osaimi, A.M.S.; Northup, P.G. Mortality after percutaneous endoscopic gastrostomy in patients with cirrhosis: A case series. Gastrointest. Endosc. 2010, 72, 1072–1075. [Google Scholar] [CrossRef]
- Cabré, E.; Rodríguez-Iglesias, P.; Caballería, J.; Quer, J.C.; Sánchez-Lombraña, J.L.; Parés, A.; Papo, M.; Planas, R.; Gassull, M.A. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: A multicenter randomized trial. Hepatology 2000, 32, 36–42. [Google Scholar] [CrossRef]
- Wilson, M.M.; Thomas, D.R.; Rubenstein, L.Z.; Chibnall, J.T.; Anderson, S.; Baxi, A.; Diebold, M.R.; Morley, J.E. Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am. J. Clin. Nutr. 2005, 82, 1074–1081. [Google Scholar] [CrossRef]
- Graur, M. Ghid de Alimentație Sănătoasă; Performantica Publisher: Iasi, Romania, 2006; pp. 1–173. ISBN 978-973-730-204-5. [Google Scholar]
- Younossi, Z.M.; Stepanova, M.; Younossi, I.; Racila, A. Validation of Chronic Liver Disease Questionnair for Nonalcoholic Steatohepatitis in Patients with Biopsy-Proven Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2019, 17, 2093–2100.e3. [Google Scholar] [CrossRef]
- Vasilachi, G.; Vasilachi, A. Alimentația Omului Sănătos și a Omului Bolnav (Recomandări Pentru Cele Mai Diverse Cazuri de Boală); Editura ARC: Chișinău, Moldova, 2016. [Google Scholar]
- Available online: https://www.nutritionvalue.org/ (accessed on 6 August 2025).
- Carbs, C.C.; Carb, C. Calorie Counter, 6th ed.; Chello Publishing: London, UK, 1882; ISBN 978-1908261151. [Google Scholar]
- Wang, T.; Shen, J. Usefulness of simplified nutritional appetite questionnaire (SNAQ) in appetite assesment in elder patients with liver cirrhosis. J. Nutr. Health Aging 2018, 22, 911–915. [Google Scholar] [CrossRef]
- Rudzińska, A.; Czesak, J.; Wieczorek-Stawińska, W.; Gąsowski, J.; Piotrowicz, K. Taste assessment as a part of geriatric nutritional care: Potential implications for clinical practice. Clin. Nutr. Open Sci. 2024, 55, 274–283. [Google Scholar] [CrossRef]
- Towey, J.; Ngonadi, C.; Greig, C.; Armstrong, M. O4 Impact of hepatic encephalopathy in advanced chronic liver disease on appetite and nutritional intake: An exploratory prospective observational UK study. Gut 2024, 73, A1–A2. [Google Scholar]
- Georgiou, A.; Yannakoulia, M.; Papatheodoridis, G.V.; Deutsch, M.; Alexopoulou, A.; Vlachogiannakos, J.; Ioannidou, P.; Papageorgiou, M.V.; Voulgaris, T.; Papadopoulos, N.; et al. Assessment of dietary habits and the adequacy of dietary intake of patients with cirrhosis-the KIRRHOS study. Clin. Nutr. 2021, 40, 3992–3998. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, C.; Kumar, A.; Arora, A.; Anikhindi, S.A.; Singla, V.; Bansal, N.; Jasrotia, S. Nutritional assessment and factors affecting dietary intake in patients with cirrhosis: A single-center observational study. Nutrition 2021, 84, 111099. [Google Scholar] [CrossRef]
- Pham, D.M.; Nguyen, T.D. Nutritional Status, Dietary and Nutrition Impact Symptoms of Cirrhotic Patients. Tạp Chí Dinh Dưỡng và Thực Phẩm 2023, 19, 23–32. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Cardoso Ade, S.R.; Machado Ade, A.S.; Anastácio, L.R. Effect of a 12-week nutritional intervention in the food intake of patients on the waiting list for liver transplantation: A secondary analysis of a randomized controlled trial. Clin. Nutr. 2024, 43, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.N.; Bùi, A.; Nguyen, L.; Chinh, T.; Thi, P.; Oanh, V.; Shigure, Y. Nutritional Status and Nutritional Practice of Cirrhotic Patients at Hanoi Medical University Hospital, 2020. Asian J. Diet. 2021, 3, 7–12. [Google Scholar]
- Mayank Jain, J.K.; Vargese, J.; Srinivasan, V.; Harika, K.; Michael, T.; Venkataraman, J. Health-related quality of life in liver cirrhosis patients using SF-36 and CLDQ questionnaires. Clin. Exp. Hepatol. 2018, 4, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Alavinejad, P.; Hajiani, E.; Danyaee, B.; Morvaridi, M. The effect of nutritional education and continuous monitoring on clinical symptoms, knowledge, and quality of life in patients with cirrhosis. Gastroenterol. Hepatol. Bed Bench 2019, 12, 17–24. [Google Scholar]
- Yamamura, S.; Nakano, D.; Hashida, R.; Tsutsumi, T.; Kawaguchi, T.; Okada, M.; Isoda, H.; Takahashi, H.; Matsuse, H.; Eguchi, Y.; et al. Patient-reported outcomes in patients with non-alcoholic fatty liver disease: A narrative review of Chronic Liver Disease Questionnaire-non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 2021, 36, 629–636. [Google Scholar] [CrossRef]
- Taru, V.; Indre, M.G.; Ignat, M.D.; Forgione, A.; Racz, T.; Olar, B.A.; Farcau, O.; Chereches, R.; Stefanescu, H.; Procopet, B. Validation and performance of chronic liver disease questionnaire (Cldq-ro) in the romanian population. J. Gastrointest. Liver Dis. 2021, 30, 240–246. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Stepanova, M.; Younossi, I.; Racila, A. Validation of the Chronic Liver Disease Questionnaire for MASH (CLDQ-MASH). JHEP Rep. 2025, 7, 101276. [Google Scholar] [CrossRef]
- Scarlata, G.G.M.; Ismaiel, A.; Gambardella, M.L.; Leucuta, D.C.; Luzza, F.; Dumitrascu, D.L.; Abenavoli, L. Use of Non-Invasive Biomarkers and Clinical Scores to Predict the Complications of Liver Cirrhosis: A Bicentric Experience. Medicina 2024, 60, 1854. [Google Scholar] [CrossRef]

 —statistical outliner for female group,
—statistical outliner for female group,  —statistical outliner for male group.
—statistical outliner for male group.
 —statistical outliner for female group,
—statistical outliner for female group,  —statistical outliner for male group.
—statistical outliner for male group.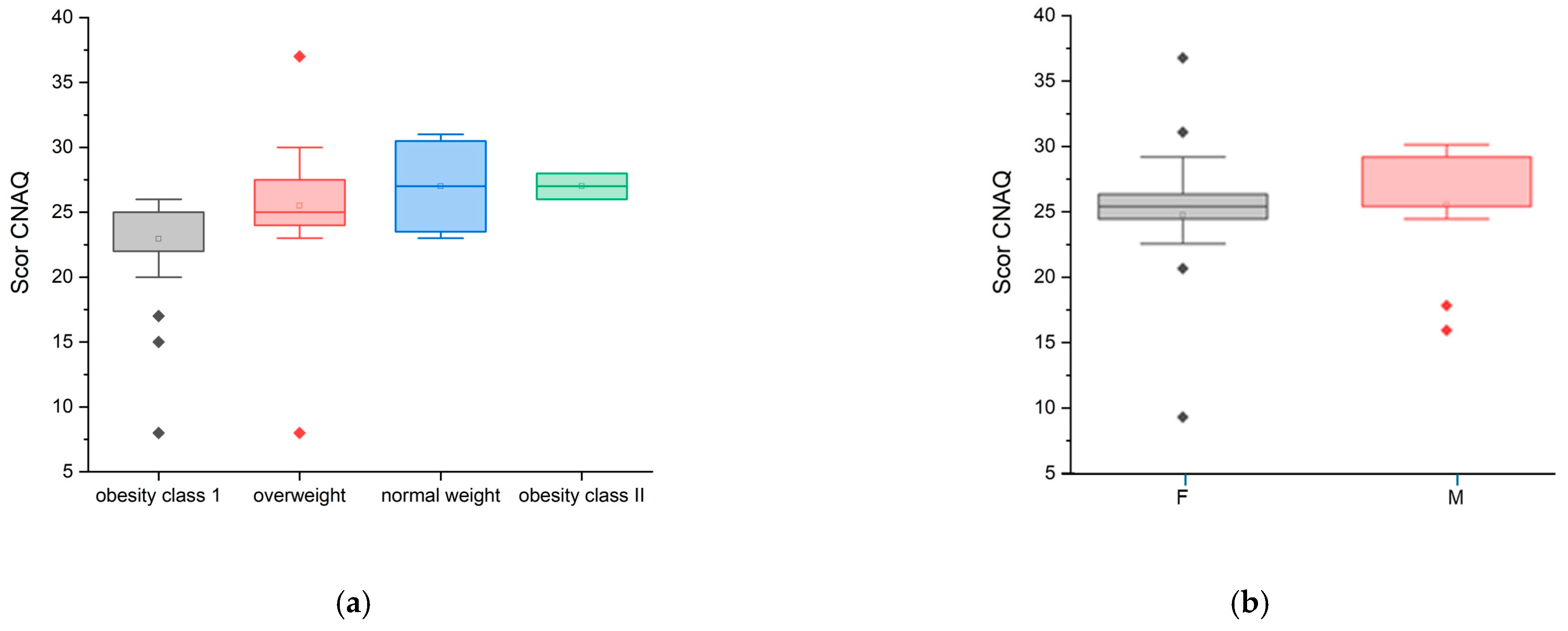


| Age | No. Participants | Percent | Age | No. Participants | Percent |
|---|---|---|---|---|---|
| 29 | 1 | 2% | 59 | 4 | 8% |
| 42 | 1 | 2% | 60 | 6 | 12% |
| 43 | 1 | 2% | 61 | 3 | 6% |
| 44 | 1 | 2% | 62 | 1 | 2% |
| 48 | 2 | 4% | 63 | 3 | 6% |
| 49 | 3 | 6% | 64 | 1 | 2% |
| 50 | 4 | 8% | 65 | 2 | 4% |
| 54 | 1 | 2% | 68 | 1 | 2% |
| 55 | 2 | 4% | 69 | 2 | 4% |
| 56 | 1 | 2% | 70 | 7 | 14% |
| 57 | 1 | 2% | 71 | 2 | 4% |
| No | BMI (kg/m2) | Body Mass Classification | No | BMI (kg/m2) | Body Mass Classification |
|---|---|---|---|---|---|
| 1 | 33.46 | O1 | 26 | 35.64 | O2 |
| 2 | 25.63 | overweight | 27 | 32.44 | O1 |
| 3 | 19.37 | normal range | 28 | 34.37 | O2 |
| 4 | 25.2 | overweight | 29 | 30.37 | O1 |
| 5 | 23.05 | normal range | 30 | 32.81 | O1 |
| 6 | 25.9 | overweight | 31 | 28.71 | overweight |
| 7 | 29.93 | overweight | 32 | 30.45 | O1 |
| 8 | 33.87 | O1 | 33 | 29.90 | overweight |
| 9 | 32.59 | O1 | 34 | 31.25 | O1 |
| 10 | 28.18 | overweight | 35 | 26.94 | overweight |
| 11 | 26.02 | overweight | 36 | 34.58 | O1 |
| 12 | 31.79 | O1 | 37 | 27.65 | overweight |
| 13 | 31.4 | O1 | 38 | 23.87 | normal range |
| 14 | 32.52 | O1 | 39 | 30.50 | O1 |
| 15 | 35.91 | O2 | 40 | 31.48 | O1 |
| 16 | 31.79 | O1 | 41 | 26.41 | overweight |
| 17 | 29.94 | overweight | 42 | 31.48 | O1 |
| 18 | 25 | overweight | 43 | 29.90 | overweight |
| 19 | 26.98 | overweight | 44 | 32.63 | O1 |
| 20 | 27.68 | overweight | 45 | 29.11 | overweight |
| 21 | 30.86 | O1 | 46 | 25.60 | overweight |
| 22 | 18.75 | normal range | 47 | 26.33 | overweight |
| 23 | 31.49 | O1 | 48 | 27.33 | overweight |
| 24 | 32.92 | O1 | 49 | 28.42 | overweight |
| 25 | 27.34 | overweight | 50 | 31.43 | O1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amariței, V.; Gheorghita, R.-E.; Caliman Sturdza, O.A. Nutritional Management in Liver Cirrhosis: A Combined Systematic Review and Observational Study. Diseases 2025, 13, 278. https://doi.org/10.3390/diseases13090278
Amariței V, Gheorghita R-E, Caliman Sturdza OA. Nutritional Management in Liver Cirrhosis: A Combined Systematic Review and Observational Study. Diseases. 2025; 13(9):278. https://doi.org/10.3390/diseases13090278
Chicago/Turabian StyleAmariței, Valentina, Roxana-Elena Gheorghita, and Olga Adriana Caliman Sturdza. 2025. "Nutritional Management in Liver Cirrhosis: A Combined Systematic Review and Observational Study" Diseases 13, no. 9: 278. https://doi.org/10.3390/diseases13090278
APA StyleAmariței, V., Gheorghita, R.-E., & Caliman Sturdza, O. A. (2025). Nutritional Management in Liver Cirrhosis: A Combined Systematic Review and Observational Study. Diseases, 13(9), 278. https://doi.org/10.3390/diseases13090278








