PTEN and ERG Biomarkers as Predictors of Biochemical Recurrence Risk in Patients Undergoing Radical Prostatectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Population
2.2. Ethics Committee Approval and Data Collection
2.3. Statistical Analysis
2.4. Tissue Microarray Construction
2.5. Preparation of Recipient TMA Paraffin Block
2.6. Obtaining Tissue Cores and Constructing the TMA Block
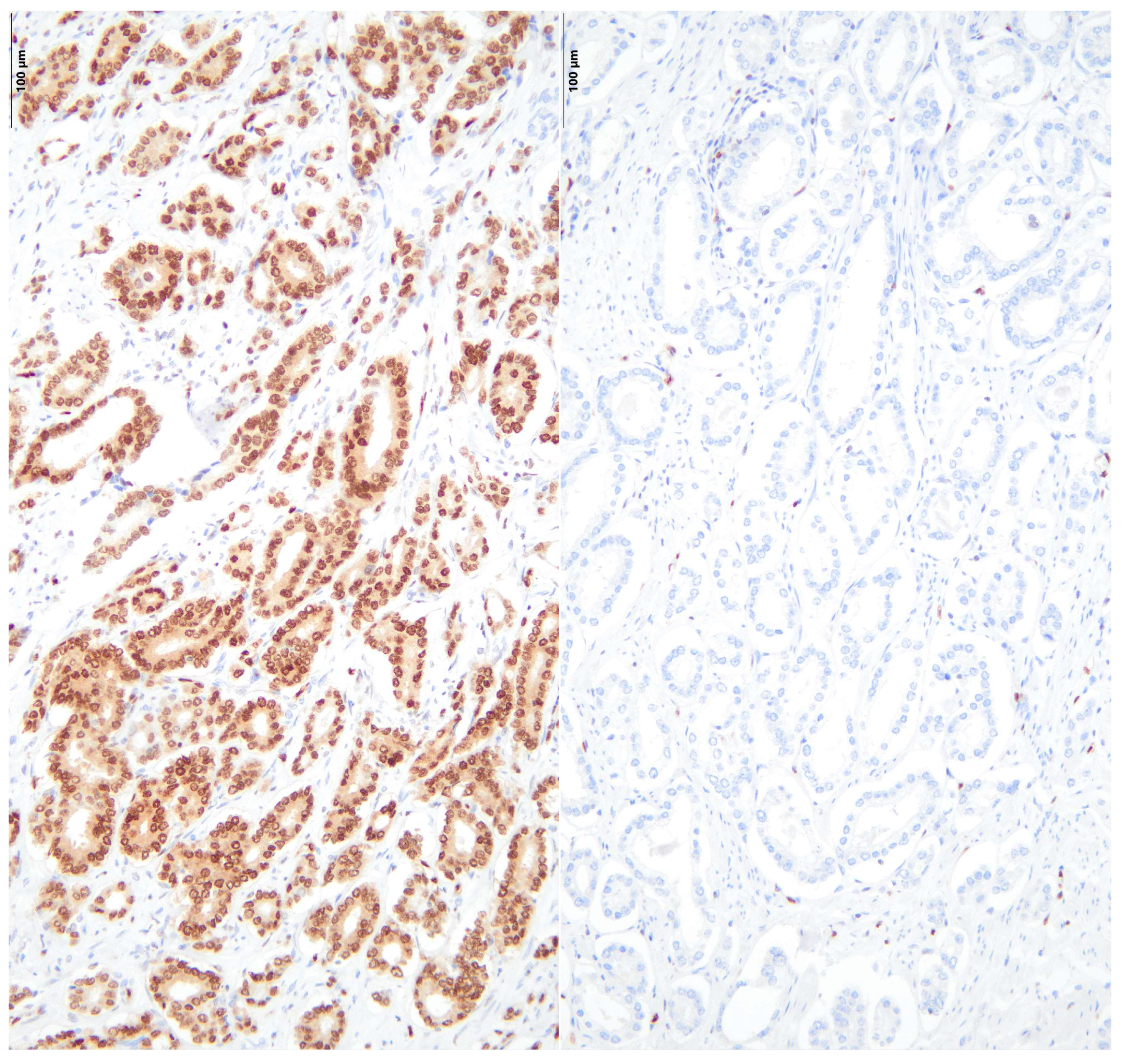
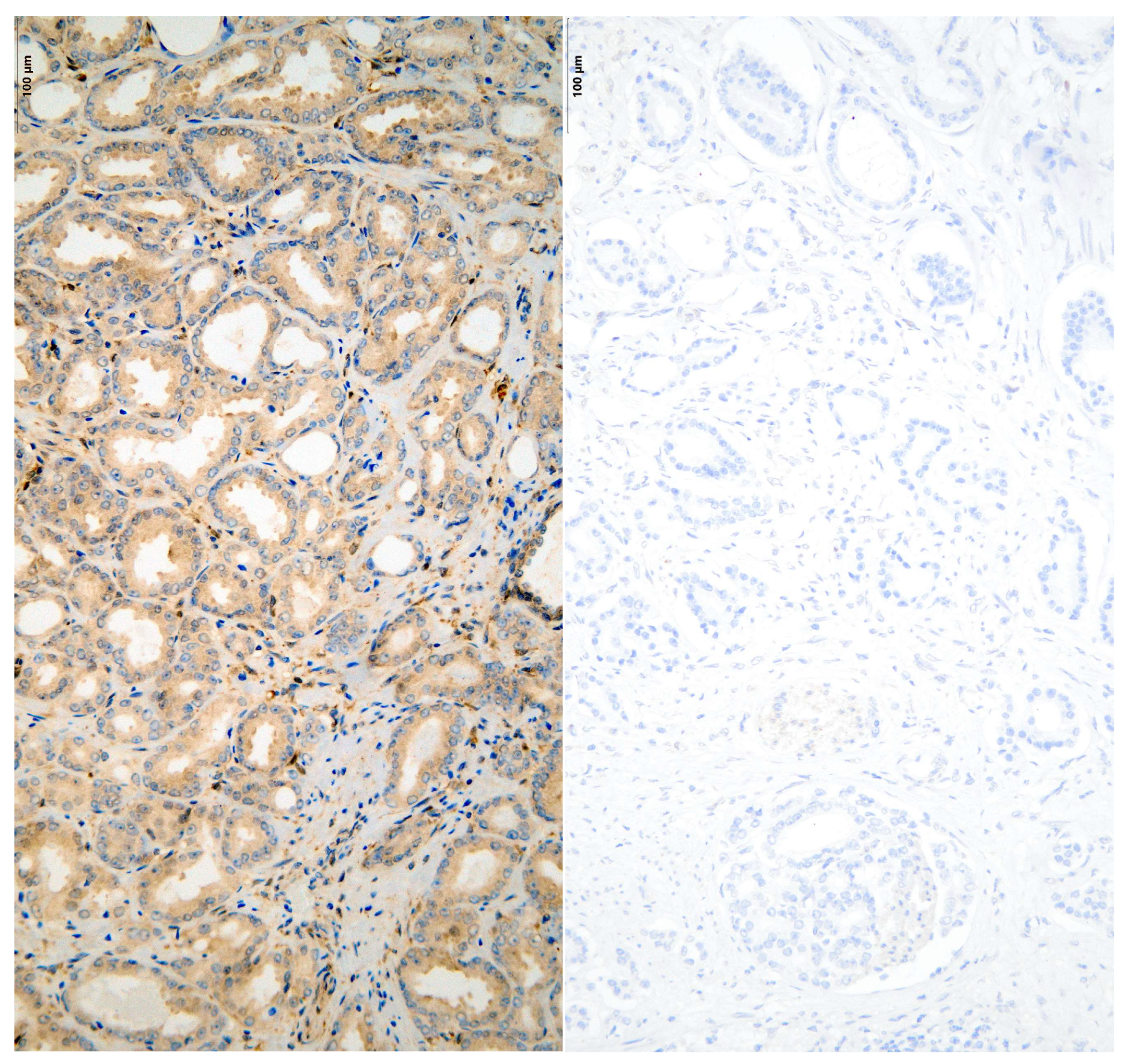
2.7. Immunohistochemical Stainings
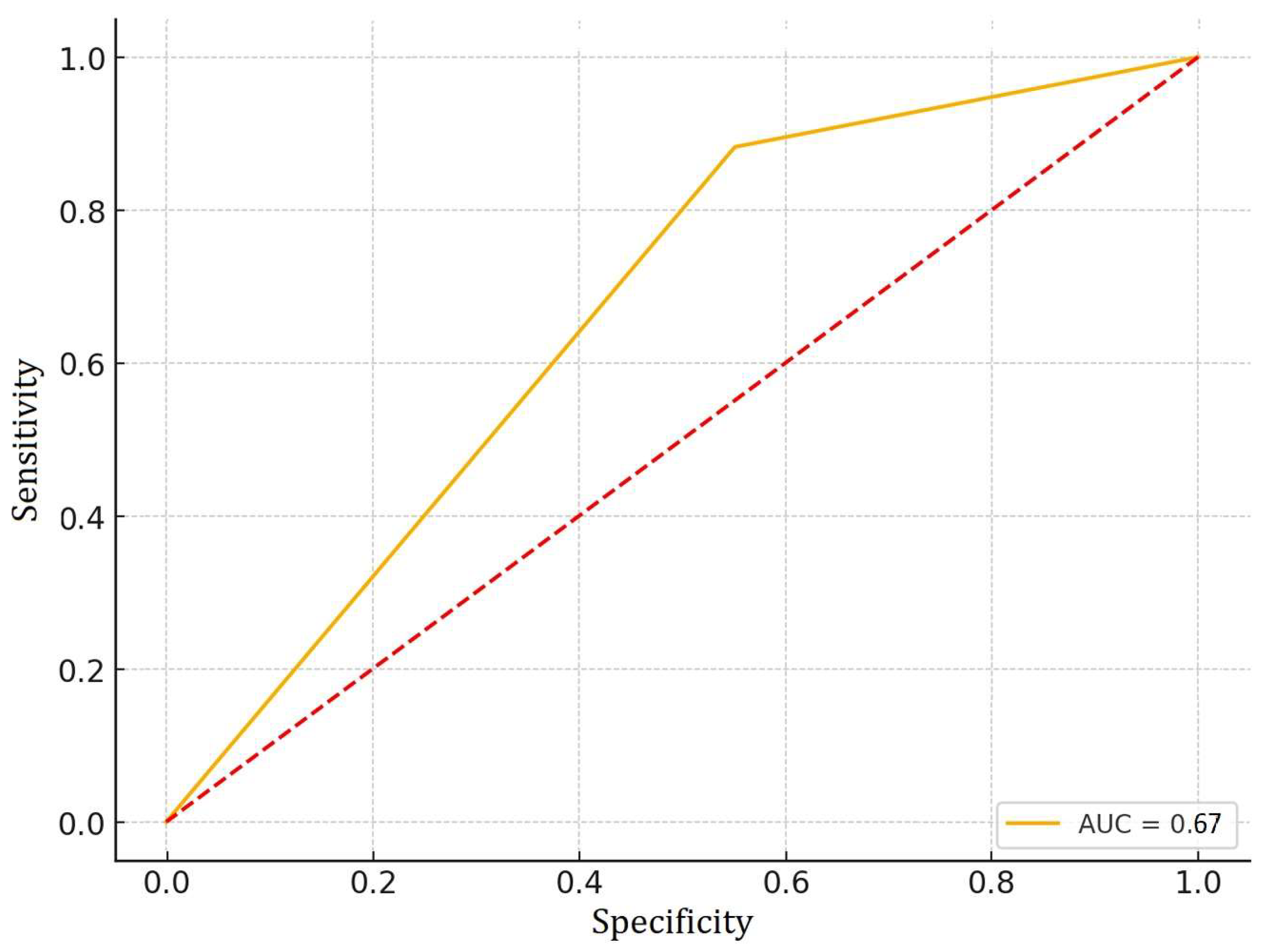
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMACR | alpha-methylacyl-CoA racemase |
| AUC | Area Under the Curve |
| BCR | Biochemical Recurrence |
| CI | Confidence Interval |
| DAB | 3,3′-Diaminobenzidine |
| EPCAM | Epithelial Cell Adhesion Molecule |
| ERG | ETS-related gene |
| GalNac-T3 | N-acetylgalactosaminyltransferase 3 |
| IHC | Immunohistochemistry |
| ISUP grade | International Society of Urological Pathology grade |
| iPSA | Initial Prostate-Specific Antigen |
| OR | Odds Ratio |
| PCa | Prostate Cancer |
| PCA3 | Prostate Cancer Antigen 3 |
| PSMA | Prostate-Specific Membrane Antigen |
| PTEN | Phosphatase and Tensin Homolog |
| ROC | Receiver Operating Characteristic |
| SPSS | Statistical Package for the Social Sciences |
| TGF-beta | Transforming Growth Factor-beta |
| TMA | Tissue Microarray |
References
- Leslie, S.W.; Soon-Sutton, T.L.; RI, A.; Sajjad, H.; Skelton, W.P. Prostate Cancer; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Adamo, P.; Ladomery, M.R. The Oncogene ERG: A Key Factor in Prostate Cancer. Oncogene 2016, 35, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Porzycki, P.; Ciszkowicz, E. Modern Biomarkers in Prostate Cancer Diagnosis. Cent. European J. Urol. 2020, 73, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Brady, L.; Carlsson, J.; Baird, A.M.; Casey, O.; Vlajnic, T.; Murchan, P.; Cormican, D.; Costigan, D.; Gray, S.; Sheils, O.; et al. Correlation of Integrated ERG/PTEN Assessment with Biochemical Recurrence in Prostate Cancer. Cancer Treat. Res. Commun. 2021, 29, 100451. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Salami, S.S.; Lonigro, R.; Bhalla, R.; Siddiqui, J.; Cao, X.; Spratt, D.E.; Palapattu, G.S.; Palanisamy, N.; Wei, J.T.; et al. Association of ERG/PTEN Status with Biochemical Recurrence after Radical Prostatectomy for Clinically Localized Prostate Cancer. Med. Oncol. 2018, 35, 152. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.B.; Bentley, J.; Jeffery, Z.; DeMarzo, A.M. Heterogeneity of PTEN and ERG Expression in Prostate Cancer on Core Needle Biopsies: Implications for Cancer Risk Stratification and Biomarker Sampling. Hum. Pathol. 2015, 46, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Bismar, T.A.; Hegazy, S.; Feng, Z.; Yu, D.; Donnelly, B.; Palanisamy, N.; Trock, B.J. Clinical Utility of Assessing PTEN and ERG Protein Expression in Prostate Cancer Patients: A Proposed Method for Risk Stratification. J. Cancer Res. Clin. Oncol. 2018, 144, 2117–2125. [Google Scholar] [CrossRef] [PubMed]
- Magi-Galluzzi, C. Prostate Cancer: Diagnostic Criteria and Role of Immunohistochemistry. Mod. Pathol. 2018, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Mandel, P.; Wenzel, M.; Hoeh, B.; Welte, M.N.; Preisser, F.; Inam, T.; Wittler, C.; Humke, C.; Köllermann, J.; Wild, P.; et al. Immunohistochemistry for Prostate Biopsy—Impact on Histological Prostate Cancer Diagnoses and Clinical Decision Making. Curr. Oncol. 2021, 28, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Segalés, L.; Juanpere, N.; Gallarín, N.; Lorenzo, M.; López, D.; Perera-Bel, J.; Rodriguez-Vida, A.; Fumadó, L.; Cecchini, L.; Bellmunt, J.; et al. Immunohistochemical Markers as Predictors of Prognosis in Multifocal Prostate Cancer. Virchows Archiv. 2023, 485, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Pentyala, S.; Whyard, T.; Pentyala, S.; Muller, J.; Pfail, J.; Parmar, S.; Helguero, C.G.; Khan, S. Prostate Cancer Markers: An Update. Biomed. Rep. 2016, 4, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, M.; Skotheim, R.I.; Maltau, A.V.; Kidd, S.G.; Lothe, R.A.; Axcrona, K.; Axcrona, U. ‘High Proliferative Cribriform Prostate Cancer’ Defines a Patient Subgroup with an Inferior Prognosis. Histopathology 2023, 83, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Barbosa, Á.R.G.; Takemura, L.S.; Kayano, P.P.; Moran, N.K.S.; Chen, C.K.; Wroclawski, M.L.; Lemos, G.C.; da Cunha, I.W.; Obara, M.T.; et al. The Role of Immunohistochemical Analysis as a Tool for the Diagnosis, Prognostic Evaluation and Treatment of Prostate Cancer: A Systematic Review of the Literature. Front. Oncol. 2018, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.E.; Raspin, K.; Melton, P.E.; Burdon, K.P.; Dickinson, J.L.; FitzGerald, L.M. Acquired Copy Number Variation in Prostate Tumours: A Review of Common Somatic Copy Number Alterations, How They Are Formed and Their Clinical Utility. Br. J. Cancer 2024, 130, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Kimura, T.; Onuma, H.; Egawa, S.; Takahashi, H. Transition Zone Prostate Cancer Is Associated with Better Clinical Outcomes than Peripheral Zone Cancer. BJUI Compass 2021, 2, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.-B.; Lei, H.-Q.; Xiong, H.-Y.; Fu, X.; Shi, F.; Yang, X.-W.; Yang, Y.-F.; Liao, G.-L.; Feng, Y.-P.; Jiang, D.-G.; et al. MicroRNA-Regulated Transcriptome Analysis Identifies Four Major Subtypes with Prognostic and Therapeutic Implications in Prostate Cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4941–4953. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Lee, H.; Byeon, S.J.; Kwon, G.Y.; Jeon, S.S. Genomic Features and Clinical Implications of Intraductal Carcinoma of the Prostate. Int. J. Mol. Sci. 2021, 22, 13125. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, R.A.; Khalifeh, I.; Box, A.; Kalantarian, M.; Ghosh, S.; Abou-Ouf, H.; Lotfi, T.; Shahait, M.; Palanisamy, N.; Bismar, T.A. Molecular Characterization of Prostate Cancer in Middle Eastern Population Highlights Differences with Western Populations with Prognostic Implication. J. Cancer Res. Clin. Oncol. 2020, 146, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Morais, C.E.; Gurgel, D.C.; Teixeira, A.C.; Mattos, T.V.A.; da Silva, A.V.A.; Tavora, F. Prevalence of ERG Expression and PTEN Loss in a Brazilian Prostate Cancer Cohort. Braz. J. Med. Biol. Res. 2019, 52, e8483. [Google Scholar] [CrossRef] [PubMed]
- Salles, D.C.; Mendes, A.A.; Han, M.; Partin, A.W.; Trock, B.J.; Jing, Y.; Lotan, T.L. ERG Status at the Margin Is Associated With Biochemical Recurrence After Radical Prostatectomy With Positive Surgical Margins. Mod. Pathol. 2023, 36, 100147. [Google Scholar] [CrossRef] [PubMed]
- Fragkoulis, C.; Glykas, I.; Tzelves, L.; Stamatakos, P.V.; Papadopoulos, G.; Stathouros, G.; Dellis, A.; Ntoumas, K.; Kostopoulou, A.; Deliveliotis, C.; et al. Clinical Impact of ERG and PTEN Status in Prostate Cancer Patients Underwent Radical Prostatectomy. Arch. Ital. Urol. Androl. 2022, 94, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Voulgari, O.; Goutas, D.; Pergaris, A.; Belogiannis, K.; Thymara, E.; Kavantzas, N.; Lazaris, A.C. Correlations of PTEN and ERG Immunoexpression in Prostate Carcinoma and Lesions Related to Its Natural History: Clinical Perspectives. Curr. Issues Mol. Biol. 2023, 45, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Tolkach, Y.; Zarbl, R.; Bauer, S.; Ritter, M.; Ellinger, J.; Hauser, S.; Hüser, L.; Klauck, S.M.; Altevogt, P.; Sültmann, H.; et al. DNA Promoter Methylation and ERG Regulate the Expression of CD24 in Prostate Cancer. Am. J. Pathol. 2021, 191, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhou, J.; Xia, S.; Li, T. The Impact of PTEN Deletion and ERG Rearrangement on Recurrence after Treatment for Prostate Cancer: A Systematic Review and Meta-Analysis. Clin. Transl. Oncol. 2020, 22, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Eineluoto, J.T.; Sandeman, K.; Pohjonen, J.; Sopyllo, K.; Nordling, S.; Stürenberg, C.; Malén, A.; Kilpeläinen, T.P.; Santti, H.; Petas, A.; et al. Associations of PTEN and ERG with Magnetic Resonance Imaging Visibility and Assessment of Non–Organ-Confined Pathology and Biochemical Recurrence After Radical Prostatectomy. Eur. Urol. Focus. 2021, 7, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Llodrà, S.; Segalés, L.; Juanpere, N.; Marta Lorenzo, T.; Salido, M.; Nonell, L.; David López, T.; Rodríguez-Vida, A.; Bellmunt, J.; Fumadó, L.; et al. SPOP and CHD1 Alterations in Prostate Cancer: Relationship with PTEN Loss, Tumor Grade, Perineural Infiltration, and PSA Recurrence. Prostate 2021, 81, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Büscheck, F.; Sulimankhil, M.; Melling, N.; Höflmayer, D.; Hube-Magg, C.; Simon, R.; Göbel, C.; Hinsch, A.; Weidemann, S.; Izbicki, J.R.; et al. Loss of Cytoplasmic Survivin Expression Is an Independent Predictor of Poor Prognosis in Radically Operated Prostate Cancer Patients. Cancer Med. 2020, 9, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, K.A.; Saramäki, O.R.; Furusato, B.; Kimura, T.; Takahashi, H.; Egawa, S.; Suzuki, H.; Keiger, K.; Ho Hahm, S.; Isaacs, W.B.; et al. Loss of PTEN Is Associated with Aggressive Behavior in ERG-Positive Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Spethmann, T.; Böckelmann, L.C.; Labitzky, V.; Ahlers, A.K.; Schröder-Schwarz, J.; Bonk, S.; Simon, R.; Sauter, G.; Huland, H.; Kypta, R.; et al. Opposing Prognostic Relevance of Junction Plakoglobin in Distinct Prostate Cancer Patient Subsets. Mol. Oncol. 2021, 15, 1956–1969. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, G.; Mosquera, J.-M.; Ramoner, R.; Park, K.; Romanel, A.; Steiner, E.; Horninger, W.; Bektic, J.; Ladurner-Rennau, M.; Rubin, M.A.; et al. Distinct ERG Rearrangement Prevalence in Prostate Cancer: Higher Frequency in Young Age and in Low PSA Prostate Cancer. Prostate Cancer Prostatic Dis. 2013, 16, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Alshalalfa, M.; Seldon, C.; Franco, I.; Vince, R.; Carmona, R.; Punnen, S.; Kaochar, S.; Dess, R.; Kishan, A.; Spratt, D.E.; et al. Clinicogenomic Characterization of Prostate Cancer Liver Metastases. Prostate Cancer Prostatic Dis. 2022, 25, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Moul, J.W.; Pienta, K.J.; Czernin, J.; King, M.T.; Freedland, S.J. Biochemical Recurrence in Patients with Prostate Cancer after Primary Definitive Therapy: Treatment Based on Risk Stratification. Prostate Cancer Prostatic Dis. 2024, 27, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, R.; Rovesti, L.; Cicione, A.; Gravina, C.; Franco, A.; Stira, J.; Simone, G.; D’Annunzio, S.; Nacchia, A.; Papalia, R.; et al. Serum Levels of Chromogranin Are Not Predictive of Poorly Differentiated Prostate Cancer: Results from a Multicenter Radical Prostatectomy Cohort. Prostate 2022, 82, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.J.; Taylor, A.; Haffner, M.C.; Abida, W.; Bryce, A.H.; Karsh, L.I.; Tagawa, S.T.; Twardowski, P.; Serritella, A.V.; Lang, J.M. Germline and Somatic Testing for Homologous Repair Deficiency in Patients with Prostate Cancer (Part 1 of 2). Prostate Cancer Prostatic Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- McKay, R.R.; Xie, W.; Ye, H.; Fennessy, F.M.; Zhang, Z.; Lis, R.; Calagua, C.; Rathkopf, D.; Laudone, V.P.; Bubley, G.J.; et al. Results of a Randomized Phase II Trial of Intense Androgen Deprivation Therapy Prior to Radical Prostatectomy in Men with High-Risk Localized Prostate Cancer. J. Urol. 2021, 206, 80–87. [Google Scholar] [CrossRef] [PubMed]

| Variables | ||||
|---|---|---|---|---|
| 95% C.I. for OR | ||||
| p | Odds Ratio | Inferior | Superior | |
| iPSA | 0.55 | 1.03 | 0.87 | 1.22 |
| pT | 0.61 | 1.04 | 0.27 | 3.99 |
| pN | 0.43 | 1.51 | 0.10 | 2.99 |
| ISUP grade | 0.053 | 0.63 | 0.32 | 1.24 |
| BCR | 0.01 | 0.28 | 0.07 | 1.10 |
| Variables | ||||
|---|---|---|---|---|
| 95% C.I. for OR | ||||
| p | Odds Ratio | Inferior | Superior | |
| iPSA | 0.97 | 0.88 | 0.74 | 1.05 |
| pT | 0.03 | 5.17 | 1.11 | 23.94 |
| pN | 0.33 | 1.27 | 0.09 | 17.13 |
| ISUP grade | 0.29 | 1.24 | 0.68 | 2.26 |
| BCR | 0.27 | 2.57 | 0.60 | 10.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borz, M.B.; Fetica, B.; Gliga, M.C.; Sipos, T.-C.; Buhas, B.A.; Schitcu, V.H. PTEN and ERG Biomarkers as Predictors of Biochemical Recurrence Risk in Patients Undergoing Radical Prostatectomy. Diseases 2025, 13, 235. https://doi.org/10.3390/diseases13080235
Borz MB, Fetica B, Gliga MC, Sipos T-C, Buhas BA, Schitcu VH. PTEN and ERG Biomarkers as Predictors of Biochemical Recurrence Risk in Patients Undergoing Radical Prostatectomy. Diseases. 2025; 13(8):235. https://doi.org/10.3390/diseases13080235
Chicago/Turabian StyleBorz, Mihnea Bogdan, Bogdan Fetica, Maximilian Cosma Gliga, Tamas-Csaba Sipos, Bogdan Adrian Buhas, and Vlad Horia Schitcu. 2025. "PTEN and ERG Biomarkers as Predictors of Biochemical Recurrence Risk in Patients Undergoing Radical Prostatectomy" Diseases 13, no. 8: 235. https://doi.org/10.3390/diseases13080235
APA StyleBorz, M. B., Fetica, B., Gliga, M. C., Sipos, T.-C., Buhas, B. A., & Schitcu, V. H. (2025). PTEN and ERG Biomarkers as Predictors of Biochemical Recurrence Risk in Patients Undergoing Radical Prostatectomy. Diseases, 13(8), 235. https://doi.org/10.3390/diseases13080235






