Sex Differences in Immune Responses to Infectious Diseases: The Role of Genetics, Hormones, and Aging
Abstract
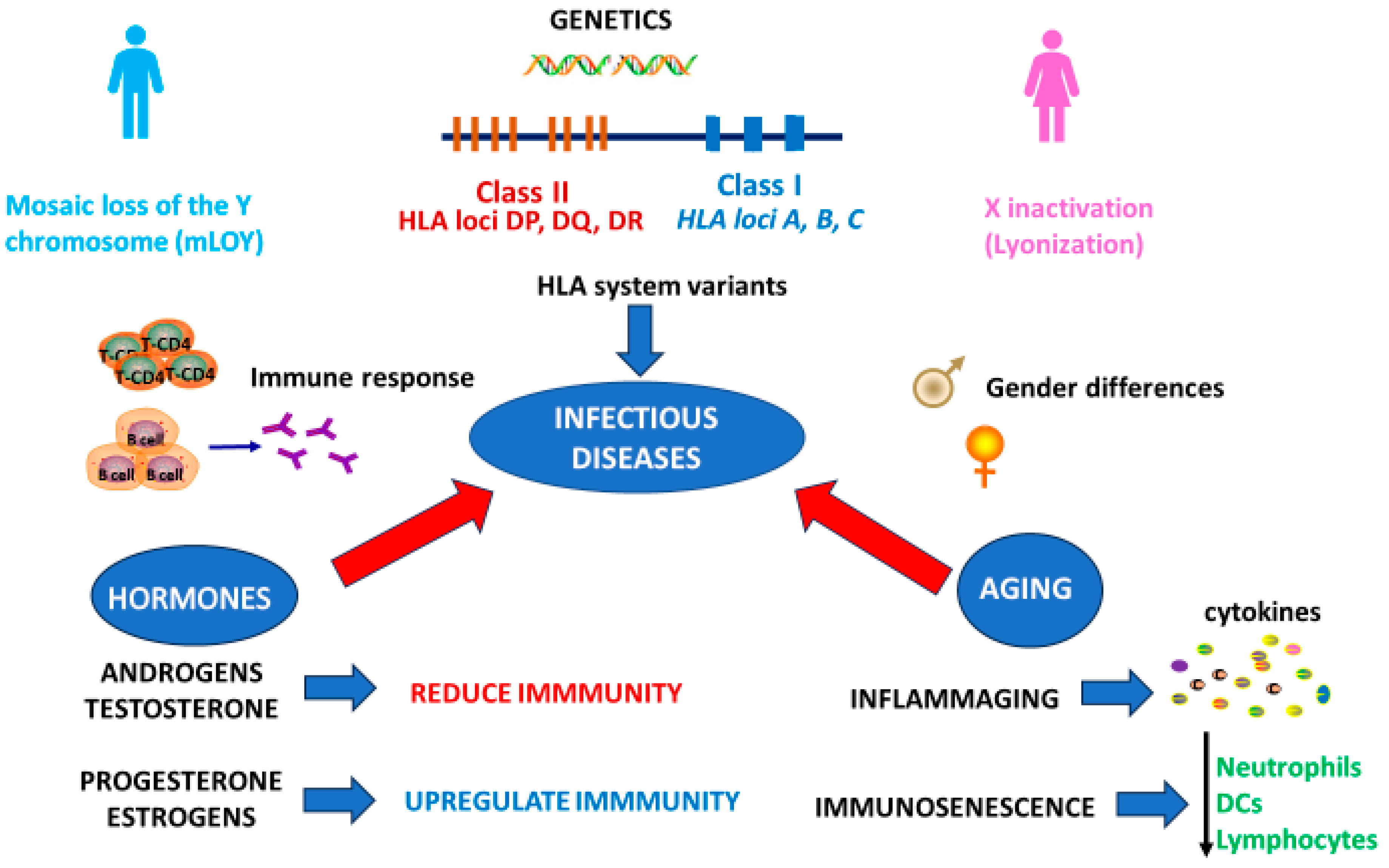
1. Introduction
2. Sex Differences in the Immune Response to Infectious Diseases: The Perspective of Genetics
3. Sex-Related Hormonal Differences in Immune Responses to Infectious Diseases
3.1. Estrogen
3.2. Progesterone
3.3. Androgens
4. Sex-Related Differences in the Immune Response to Infections During Aging
5. Conclusions and Future Prospectives
Author Contributions
Funding
Conflicts of Interest
References
- Jit, M.; Cook, A.R. Informing Public Health Policies with Models for Disease Burden, Impact Evaluation, and Economic Evaluation. Annu. Rev. Public Health 2024, 45, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Prem, K.; Cook, A.R.; Jit, M. Projecting Social Contact Matrices in 152 Countries Using Contact Surveys and Demographic Data. PLoS Comput. Biol. 2017, 13, e1005697. [Google Scholar] [CrossRef] [PubMed]
- Doerre, A.; Doblhammer, G. The Influence of Gender on COVID-19 Infections and Mortality in Germany: Insights from Age- and Gender-Specific Modeling of Contact Rates, Infections, and Deaths in the Early Phase of the Pandemic. PLoS ONE 2022, 17, e0268119. [Google Scholar] [CrossRef]
- Meijs, D.A.M.; van Bussel, B.C.T.; Stessel, B.; Mehagnoul-Schipper, J.; Hana, A.; Scheeren, C.I.E.; Peters, S.A.E.; van Mook, W.N.K.A.; van der Horst, I.C.C.; Marx, G.; et al. Better COVID-19 Intensive Care Unit Survival in Females, Independent of Age, Disease Severity, Comorbidities, and Treatment. Sci. Rep. 2022, 12, 734. [Google Scholar] [CrossRef]
- Risman, B.J. Gender as a Social Structure: Theory Wrestling with Activism. Gend. Soc. 2004, 18, 429–450. [Google Scholar] [CrossRef]
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The Male-Specific Region of the Human Y Chromosome Is a Mosaic of Discrete Sequence Classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sackton, T.B.; Martinsen, L.; Lemos, B.; Eickbush, T.H.; Hartl, D.L. Y Chromosome Mediates Ribosomal DNA Silencing and Modulates the Chromatin State in Drosophila. Proc. Natl. Acad. Sci. USA 2012, 109, 9941–9946. [Google Scholar] [CrossRef]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila Imd Signaling Pathway. J. Immunol. 2014, 192, 3455–3462. [Google Scholar] [CrossRef]
- Case, L.K.; Wall, E.H.; Dragon, J.A.; Saligrama, N.; Krementsov, D.N.; Moussawi, M.; Zachary, J.F.; Huber, S.A.; Blankenhorn, E.P.; Teuscher, C. The Y Chromosome as a Regulatory Element Shaping Immune Cell Transcriptomes and Susceptibility to Autoimmune Disease. Genome Res. 2013, 23, 1474–1485. [Google Scholar] [CrossRef]
- Wang, Y.; Sano, S. Why Y Matters? The Implication of Loss of Y Chromosome in Blood and Cancer. Cancer Sci. 2024, 115, 706–714. [Google Scholar] [CrossRef]
- Danielsson, M.; Halvardson, J.; Davies, H.; Torabi Moghadam, B.; Mattisson, J.; Rychlicka-Buniowska, E.; Jaszczyński, J.; Heintz, J.; Lannfelt, L.; Giedraitis, V.; et al. Longitudinal Changes in the Frequency of Mosaic Chromosome Y Loss in Peripheral Blood Cells of Aging Men Varies Profoundly between Individuals. Eur. J. Hum. Genet. EJHG 2020, 28, 349–357. [Google Scholar] [CrossRef]
- Mas-Peiro, S.; Abplanalp, W.T.; Rasper, T.; Berkowitsch, A.; Leistner, D.M.; Dimmeler, S.; Zeiher, A.M. Mosaic Loss of Y Chromosome in Monocytes Is Associated with Lower Survival after Transcatheter Aortic Valve Replacement. Eur. Heart J. 2023, 44, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Horitani, K.; Ogawa, H.; Halvardson, J.; Chavkin, N.W.; Wang, Y.; Sano, M.; Mattisson, J.; Hata, A.; Danielsson, M.; et al. Hematopoietic Loss of Y Chromosome Leads to Cardiac Fibrosis and Heart Failure Mortality. Science 2022, 377, 292–297. [Google Scholar] [CrossRef]
- Mattisson, J.; Danielsson, M.; Hammond, M.; Davies, H.; Gallant, C.J.; Nordlund, J.; Raine, A.; Edén, M.; Kilander, L.; Ingelsson, M.; et al. Leukocytes with Chromosome Y Loss Have Reduced Abundance of the Cell Surface Immunoprotein CD99. Sci. Rep. 2021, 11, 15160. [Google Scholar] [CrossRef] [PubMed]
- Dumanski, J.P.; Halvardson, J.; Davies, H.; Rychlicka-Buniowska, E.; Mattisson, J.; Moghadam, B.T.; Nagy, N.; Węglarczyk, K.; Bukowska-Strakova, K.; Danielsson, M.; et al. Immune Cells Lacking Y Chromosome Show Dysregulation of Autosomal Gene Expression. Cell. Mol. Life Sci. CMLS 2021, 78, 4019–4033. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, K.S.; Jiwrajka, N.; Lovell, C.D.; Toothacre, N.E.; Anguera, M.C. The X-Quisite X-Ception: Sex Differences with Immune Responses. Nat. Rev. Immunol. 2024, 24, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Flo, T.H.; Aderem, A. Pathogen Recognition by Toll-like Receptors. In NeuroImmune Biology; Bertók, L., Chow, D.A., Eds.; Natural Immunity; Elsevier: Amsterdam, The Netherlands, 2005; Volume 5, pp. 167–182. [Google Scholar]
- Goldstein, J.D.; Burlion, A.; Zaragoza, B.; Sendeyo, K.; Polansky, J.K.; Huehn, J.; Piaggio, E.; Salomon, B.L.; Marodon, G. Inhibition of the JAK/STAT Signaling Pathway in Regulatory T Cells Reveals a Very Dynamic Regulation of Foxp3 Expression. PLoS ONE 2016, 11, e0153682. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sadhukhan, D.; Saraswathy, R. Role of Sex in Immune Response and Epigenetic Mechanisms. Epigenetics Chromatin 2024, 17, 1. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Konno, H.; Yamamoto, T.; Yamazaki, K.; Gohda, J.; Akiyama, T.; Semba, K.; Goto, H.; Kato, A.; Yujiri, T.; Imai, T.; et al. TRAF6 Establishes Innate Immune Responses by Activating NF-kappaB and IRF7 upon Sensing Cytosolic Viral RNA and DNA. PLoS ONE 2009, 4, e5674. [Google Scholar] [CrossRef]
- Pontoriero, M.; Fiume, G.; Vecchio, E.; De Laurentiis, A.; Albano, F.; Iaccino, E.; Mimmi, S.; Pisano, A.; Agosti, V.; Giovannone, E.; et al. Activation of NF-κB in B Cell Receptor Signaling through Bruton’s Tyrosine Kinase-Dependent Phosphorylation of IκB-α. J. Mol. Med. 2019, 97, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, K.; Gardam, S.; Borghi, A.; Kreike, M.; Carpentier, I.; Beyaert, R. XEDAR Activates the Non-Canonical NF-κB Pathway. Biochem. Biophys. Res. Commun. 2015, 465, 275–280. [Google Scholar] [CrossRef]
- Bianchi, I.; Lleo, A.; Gershwin, M.E.; Invernizzi, P. The X Chromosome and Immune Associated Genes. J. Autoimmun. 2012, 38, J187–J192. [Google Scholar] [CrossRef]
- Sierra, I.; Anguera, M.C. Enjoy the Silence: X-Chromosome Inactivation Diversity in Somatic Cells. Curr. Opin. Genet. Dev. 2019, 55, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Disteche, C.M.; Berletch, J.B. X Inactivation and Escape: Epigenetic and Structural Features. Front. Cell Dev. Biol. 2019, 7, 219. [Google Scholar] [CrossRef]
- Meester, I.; Manilla-Muñoz, E.; León-Cachón, R.B.R.; Paniagua-Frausto, G.A.; Carrión-Alvarez, D.; Ruiz-Rodríguez, C.O.; Rodríguez-Rangel, X.; García-Martínez, J.M. SeXY Chromosomes and the Immune System: Reflections after a Comparative Study. Biol. Sex Differ. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Syrett, C.M.; Paneru, B.; Sandoval-Heglund, D.; Wang, J.; Banerjee, S.; Sindhava, V.; Behrens, E.M.; Atchison, M.; Anguera, M.C. Altered X-Chromosome Inactivation in T Cells May Promote Sex-Biased Autoimmune Diseases. JCI Insight 2019, 4, e126751. [Google Scholar] [CrossRef]
- Syrett, C.M.; Sindhava, V.; Sierra, I.; Dubin, A.H.; Atchison, M.; Anguera, M.C. Diversity of Epigenetic Features of the Inactive X-Chromosome in NK Cells, Dendritic Cells, and Macrophages. Front. Immunol. 2018, 9, 3087. [Google Scholar] [CrossRef]
- Schurz, H.; Salie, M.; Tromp, G.; Hoal, E.G.; Kinnear, C.J.; Möller, M. The X Chromosome and Sex-Specific Effects in Infectious Disease Susceptibility. Hum. Genom. 2019, 13, 2. [Google Scholar] [CrossRef]
- Case, L.K.; Teuscher, C. Y Genetic Variation and Phenotypic Diversity in Health and Disease. Biol. Sex Differ. 2015, 6, 6. [Google Scholar] [CrossRef]
- Case, L.K.; Toussaint, L.; Moussawi, M.; Roberts, B.; Saligrama, N.; Brossay, L.; Huber, S.A.; Teuscher, C. Chromosome y Regulates Survival Following Murine Coxsackievirus B3 Infection. G3 (Bethesda) 2012, 2, 115–121. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Butterfield, R.J.; Roper, R.J.; Rhein, D.M.; Melvold, R.W.; Haynes, L.; Ma, R.Z.; Doerge, R.W.; Teuscher, C. Sex-Specific Quantitative Trait Loci Govern Susceptibility to Theiler’s Murine Encephalomyelitis Virus-Induced Demyelination. Genetics 2003, 163, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.F.; Loredo Osti, J.C.; Guillot, L.; Morgan, K.; Qureshi, S.T. Sex Differences in the Genetic Architecture of Susceptibility to Cryptococcus Neoformans Pulmonary Infection. Genes Immun. 2008, 9, 536–545. [Google Scholar] [CrossRef][Green Version]
- Khramtsova, E.A.; Davis, L.K.; Stranger, B.E. The Role of Sex in the Genomics of Human Complex Traits. Nat. Rev. Genet. 2019, 20, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Schuurhof, A.; Bont, L.; Siezen, C.L.E.; Hodemaekers, H.; van Houwelingen, H.C.; Kimman, T.G.; Hoebee, B.; Kimpen, J.L.L.; Janssen, R. Interleukin-9 Polymorphism in Infants with Respiratory Syncytial Virus Infection: An Opposite Effect in Boys and Girls. Pediatr. Pulmonol. 2010, 45, 608–613. [Google Scholar] [CrossRef]
- Moretti, S.; Renga, G.; Oikonomou, V.; Galosi, C.; Pariano, M.; Iannitti, R.G.; Borghi, M.; Puccetti, M.; De Zuani, M.; Pucillo, C.E.; et al. A Mast Cell-ILC2-Th9 Pathway Promotes Lung Inflammation in Cystic Fibrosis. Nat. Commun. 2017, 8, 14017. [Google Scholar] [CrossRef]
- Vermeesch, J.R.; Petit, P.; Kermouni, A.; Renauld, J.C.; Van Den Berghe, H.; Marynen, P. The IL-9 Receptor Gene, Located in the Xq/Yq Pseudoautosomal Region, Has an Autosomal Origin, Escapes X Inactivation and Is Expressed from the Y. Hum. Mol. Genet. 1997, 6, 1–8. [Google Scholar] [CrossRef][Green Version]
- Alwani, M.; Yassin, A.; Al-Zoubi, R.M.; Aboumarzouk, O.M.; Nettleship, J.; Kelly, D.; Al-Qudimat, A.R.; Shabsigh, R. Sex-Based Differences in Severity and Mortality in COVID-19. Rev. Med. Virol. 2021, 31, e2223. [Google Scholar] [CrossRef]
- Gemmati, D.; Bramanti, B.; Serino, M.L.; Secchiero, P.; Zauli, G.; Tisato, V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-Chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int. J. Mol. Sci. 2020, 21, 3474. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.-M.; Raoult, D. ACE2 Receptor Polymorphism: Susceptibility to SARS-CoV-2, Hypertension, Multi-Organ Failure, and COVID-19 Disease Outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Sauermann, U.; Altmüller, J.; Fritzer, E.; Nothnagel, M.; Dalibor, N.; Fellay, J.; Kaup, F.-J.; Stahl-Hennig, C.; Nürnberg, P.; et al. X Chromosomal Variation Is Associated with Slow Progression to AIDS in HIV-1-Infected Women. Am. J. Hum. Genet. 2009, 85, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Fortin, A.; Abel, L.; Casanova, J.L.; Gros, P. Host Genetics of Mycobacterial Diseases in Mice and Men: Forward Genetic Studies of BCG-Osis and Tuberculosis. Annu. Rev. Genom. Hum. Genet. 2007, 8, 163–192. [Google Scholar] [CrossRef]
- Cussigh, A.; Falleti, E.; Fabris, C.; Bitetto, D.; Cmet, S.; Fontanini, E.; Bignulin, S.; Fornasiere, E.; Fumolo, E.; Minisini, R.; et al. Interleukin 6 Promoter Polymorphisms Influence the Outcome of Chronic Hepatitis C. Immunogenetics 2011, 63, 33–41. [Google Scholar] [CrossRef]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and Consequences of Sex Differences in Immune Responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Taneja, V. Sex Hormones Determine Immune Response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Chen, L.; Xu, T.; Lou, J.; Zhang, T.; Wu, S.; Xie, R.; Xu, J. The Beneficial Roles and Mechanisms of Estrogens in Immune Health and Infection Disease. Steroids 2024, 207, 109426. [Google Scholar] [CrossRef]
- Ben-Batalla, I.; Vargas-Delgado, M.E.; Von Amsberg, G.; Janning, M.; Loges, S. Influence of Androgens on Immunity to Self and Foreign: Effects on Immunity and Cancer. Front. Immunol. 2020, 11, 1184. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, A.; Yalcinkaya, R.; Sardh, F.; Landegren, N. Immune Dynamics throughout Life in Relation to Sex Hormones and Perspectives Gained from Gender-Affirming Hormone Therapy. Front. Immunol. 2025, 15, 1501364. [Google Scholar] [CrossRef]
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The Many Faces of Estrogen Signaling. Biochem. Medica 2014, 24, 329–342. [Google Scholar] [CrossRef]
- Hoffmann, J.P.; Liu, J.A.; Seddu, K.; Klein, S.L. Sex Hormone Signaling and Regulation of Immune Function. Immunity 2023, 56, 2472–2491. [Google Scholar] [CrossRef]
- Bartkowiak-Wieczorek, J.; Jaros, A.; Gajdzińska, A.; Wojtyła-Buciora, P.; Szymański, I.; Szymaniak, J.; Janusz, W.; Walczak, I.; Jonaszka, G.; Bienert, A. The Dual Faces of Oestrogen: The Impact of Exogenous Oestrogen on the Physiological and Pathophysiological Functions of Tissues and Organs. Int. J. Mol. Sci. 2024, 25, 8167. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.; Cheung, A.S.; Pang, K.; Saffery, R.; Novakovic, B. Sexual Dimorphism in Innate Immunity: The Role of Sex Hormones and Epigenetics. Front. Immunol. 2020, 11, 604000. [Google Scholar] [CrossRef] [PubMed]
- Pepe, G.; Locati, M.; Della Torre, S.; Mornata, F.; Cignarella, A.; Maggi, A.; Vegeto, E. The Estrogen–Macrophage Interplay in the Homeostasis of the Female Reproductive Tract. Hum. Reprod. Update 2018, 24, 652–672. [Google Scholar] [CrossRef]
- Sciarra, F.; Campolo, F.; Franceschini, E.; Carlomagno, F.; Venneri, M. Gender-Specific Impact of Sex Hormones on the Immune System. Int. J. Mol. Sci. 2023, 24, 6302. [Google Scholar] [CrossRef]
- Campbell, L.; Emmerson, E.; Williams, H.; Saville, C.R.; Krust, A.; Chambon, P.; Mace, K.A.; Hardman, M.J. Estrogen Receptor-Alpha Promotes Alternative Macrophage Activation during Cutaneous Repair. J. Investig. Dermatol. 2014, 134, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Keselman, A.; Fang, X.; White, P.B.; Heller, N.M. Estrogen Signaling Contributes to Sex Differences in Macrophage Polarization during Asthma. J. Immunol. 2017, 199, 1573–1583. [Google Scholar] [CrossRef]
- Batty, M.J.; Chabrier, G.; Sheridan, A.; Gage, M.C. Metabolic Hormones Modulate Macrophage Inflammatory Responses. Cancers 2021, 13, 4661. [Google Scholar] [CrossRef]
- Straub, R.H. The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Enright, S.; Werstuck, G.H. Investigating the Effects of Sex Hormones on Macrophage Polarization. Int. J. Mol. Sci. 2024, 25, 951. [Google Scholar] [CrossRef]
- Adachi, A.; Honda, T.; Egawa, G.; Kanameishi, S.; Takimoto, R.; Miyake, T.; Hossain, M.R.; Komine, M.; Ohtsuki, M.; Gunzer, M.; et al. Estradiol Suppresses Psoriatic Inflammation in Mice by Regulating Neutrophil and Macrophage Functions. J. Allergy Clin. Immunol. 2022, 150, 909–919.e8. [Google Scholar] [CrossRef]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.-Y.; McDonnell, D.P. Estrogen Receptor Signaling in the Immune System. Endocr. Rev. 2023, 44, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.T.; Heaton, N.S. The Impact of Estrogens and Their Receptors on Immunity and Inflammation during Infection. Cancers 2022, 14, 909. [Google Scholar] [CrossRef]
- Vermillion, M.S.; Ursin, R.L.; Attreed, S.E.; Klein, S.L. Estriol Reduces Pulmonary Immune Cell Recruitment and Inflammation to Protect Female Mice From Severe Influenza. Endocrinology 2018, 159, 3306–3320. [Google Scholar] [CrossRef]
- Robinson, D.P.; Lorenzo, M.E.; Jian, W.; Klein, S.L. Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses. PLoS Pathog. 2011, 7, e1002149. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Boon, A.C.M.; Michelson, A.P.; Foraker, R.E.; Zhan, M.; Payne, P.R.O. Estrogen Hormone Is an Essential Sex Factor Inhibiting Inflammation and Immune Response in COVID-19. Sci. Rep. 2022, 12, 9462. [Google Scholar] [CrossRef] [PubMed]
- Khan, M. A Plausible Explanation for Male Dominance in Typhoid Ileal Perforation. Clin. Exp. Gastroenterol. 2012, 5, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.J.; O’Neill, A.J.; Grantham, J.J.; Sheridan-Pereira, M.; Fitzpatrick, J.M.; Webb, D.W.; Watson, R.W.G. Sex-Specific Alterations in Neutrophil Apoptosis: The Role of Estradiol and Progesterone. Blood 2003, 102, 2653–2659. [Google Scholar] [CrossRef]
- Dai, R.; Cowan, C.; Heid, B.; Khan, D.; Liang, Z.; Pham, C.T.N.; Ahmed, S.A. Neutrophils and Neutrophil Serine Proteases Are Increased in the Spleens of Estrogen-Treated C57BL/6 Mice and Several Strains of Spontaneous Lupus-Prone Mice. PLoS ONE 2017, 12, e0172105. [Google Scholar] [CrossRef]
- Molero, L. Expression of Estrogen Receptor Subtypes and Neuronal Nitric Oxide Synthase in Neutrophils from Women and Men Regulation by Estrogen. Cardiovasc. Res. 2002, 56, 43–51. [Google Scholar] [CrossRef]
- Gupta, S.; Nakabo, S.; Blanco, L.P.; O’Neil, L.J.; Wigerblad, G.; Goel, R.R.; Mistry, P.; Jiang, K.; Carmona-Rivera, C.; Chan, D.W.; et al. Sex Differences in Neutrophil Biology Modulate Response to Type I Interferons and Immunometabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 16481–16491. [Google Scholar] [CrossRef]
- Curran, E.M.; Berghaus, L.J.; Vernetti, N.J.; Saporita, A.J.; Lubahn, D.B.; Estes, D.M. Natural Killer Cells Express Estrogen Receptor-α and Estrogen Receptor-β and Can Respond to Estrogen Via a Non-Estrogen Receptor-α-Mediated Pathway. Cell. Immunol. 2001, 214, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Zhao, J.; Zhou, J.; Zhao, S.; Hu, Y.; Hou, Y. Modulation of 17β-Estradiol on the Number and Cytotoxicity of NK Cells in Vivo Related to MCM and Activating Receptors. Int. Immunopharmacol. 2007, 7, 1765–1775. [Google Scholar] [CrossRef]
- Albrecht, A.E.; Hartmann, B.W.; Scholten, C.; Huber, J.C.; Kalinowska, W.; Zielinski, C.C. Effect of Estrogen Replacement Therapy on Natural Killer Cell Activity in Postmenopausal Women. Maturitas 1996, 25, 217–222. [Google Scholar] [CrossRef]
- Sorachi, K.; Kumagai, S.; Sugita, M.; Yodoi, J.; Imura, H. Enhancing Effect of 17β-Estradiol on Human NK Cell Activity. Immunol. Lett. 1993, 36, 31–35. [Google Scholar] [CrossRef]
- Gutiérrez-Brito, J.A.; Lomelí-Nieto, J.Á.; Muñoz-Valle, J.F.; Oregon-Romero, E.; Corona-Angeles, J.A.; Hernández-Bello, J. Sex Hormones and Allergies: Exploring the Gender Differences in Immune Responses. Front. Allergy 2025, 5, 1483919. [Google Scholar] [CrossRef] [PubMed]
- Zoller, A.L.; Kersh, G.J. Estrogen Induces Thymic Atrophy by Eliminating Early Thymic Progenitors and Inhibiting Proliferation of β-Selected Thymocytes. J. Immunol. 2006, 176, 7371–7378. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Maret, A.; Coudert, J.D.; Garidou, L.; Foucras, G.; Gourdy, P.; Krust, A.; Dupont, S.; Chambon, P.; Druet, P.; Bayard, F.; et al. Estradiol Enhances Primary Antigen-specific CD4 T Cell Responses and Th1 Development in Vivo. Essential Role of Estrogen Receptor α Expression in Hematopoietic Cells. Eur. J. Immunol. 2003, 33, 512–521. [Google Scholar] [CrossRef]
- Klein, P.W.; Easterbrook, J.D.; Lalime, E.N.; Klein, S.L. Estrogen and Progesterone Affect Responses to Malaria Infection in Female C57BL/6 Mice. Gend. Med. 2008, 5, 423–433. [Google Scholar] [CrossRef]
- Khan, D.; Dai, R.; Karpuzoglu, E.; Ahmed, S.A. Estrogen Increases, Whereas IL-27 and IFN-γ Decrease, Splenocyte IL-17 Production in WT Mice. Eur. J. Immunol. 2010, 40, 2549–2556. [Google Scholar] [CrossRef]
- Alanazi, H.; Zhang, Y.; Fatunbi, J.; Luu, T.; Kwak-Kim, J. The Impact of Reproductive Hormones on T Cell Immunity; Normal and Assisted Reproductive Cycles. J. Reprod. Immunol. 2024, 165, 104295. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.; Wang, J.; Jin, H.; Song, X.; Yan, J.; Kang, Y.; Zhao, L.; An, X.; Du, X.; Chen, X.; et al. Induction of Regulatory T Cells by Physiological Level Estrogen. J. Cell. Physiol. 2008, 214, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhong, Q.; Palmer, T.; Benner, A.; Wang, L.; Suresh, K.; Damico, R.; D’Alessio, F.R. Estradiol Resolves Pneumonia via ERβ in Regulatory T Cells. JCI Insight 2021, 6, e133251. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Diebel, L.N.; Liberati, D.M. Estrogen Modulation of Pneumonia? An Immunoglobulin A Effect. J. Trauma Acute Care Surg. 2012, 72, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Rio, P.; Caldarelli, M.; Chiantore, M.; Ocarino, F.; Candelli, M.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Immune Cells, Gut Microbiota, and Vaccines: A Gender Perspective. Cells 2024, 13, 526. [Google Scholar] [CrossRef]
- Mai, T.; Zan, H.; Zhang, J.; Hawkins, J.S.; Xu, Z.; Casali, P. Estrogen Receptors Bind to and Activate the HOXC4/HoxC4 Promoter to Potentiate HoxC4-Mediated Activation-Induced Cytosine Deaminase Induction, Immunoglobulin Class Switch DNA Recombination, and Somatic Hypermutation. J. Biol. Chem. 2010, 285, 37797–37810. [Google Scholar] [CrossRef]
- Park, S.-R.; Kim, P.-H.; Lee, K.-S.; Lee, S.-H.; Seo, G.-Y.; Yoo, Y.-C.; Lee, J.; Casali, P. APRIL Stimulates NF-κB-Mediated HoxC4 Induction for AID Expression in Mouse B Cells. Cytokine 2013, 61, 608–613. [Google Scholar] [CrossRef]
- Motomura, K.; Miller, D.; Galaz, J.; Liu, T.N.; Romero, R.; Gomez-Lopez, N. The Effects of Progesterone on Immune Cellular Function at the Maternal-Fetal Interface and in Maternal Circulation. J. Steroid Biochem. Mol. Biol. 2023, 229, 106254. [Google Scholar] [CrossRef]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone Receptor Signaling Mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef]
- Azeez, J.M.; Susmi, T.R.; Remadevi, V.; Ravindran, V.; Sasikumar Sujatha, A.; Ayswarya, R.N.S.; Sreeja, S. New Insights into the Functions of Progesterone Receptor (PR) Isoforms and Progesterone Signaling. Am. J. Cancer Res. 2021, 11, 5214–5232. [Google Scholar]
- Mauvais-Jarvis, F.; Klein, S.L.; Levin, E.R. Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology 2020, 161, bqaa127. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.K.; Patel, M.V.; Fahey, J.V.; Wira, C.R. Innate and Adaptive Immunity at Mucosal Surfaces of the Female Reproductive Tract: Stratification and Integration of Immune Protection against the Transmission of Sexually Transmitted Infections. J. Reprod. Immunol. 2011, 88, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.D.; Kelly, R.W.; Brenner, R.M.; Baird, D.T. Antiprogestins as a Model for Progesterone Withdrawal. Steroids 2003, 68, 1061–1068. [Google Scholar] [CrossRef]
- Bazer, F.; Spencer, T.; Johnson, G. Interferons and Uterine Receptivity. Semin. Reprod. Med. 2009, 27, 090–102. [Google Scholar] [CrossRef]
- Gómez-Oro, C.; Latorre, M.C.; Arribas-Poza, P.; Ibáñez-Escribano, A.; Baca-Cornejo, K.R.; Gallego-Valle, J.; López-Escobar, N.; Mondéjar-Palencia, M.; Pion, M.; López-Fernández, L.A.; et al. Progesterone Promotes CXCl2-Dependent Vaginal Neutrophil Killing by Activating Cervical Resident Macrophage–Neutrophil Crosstalk. JCI Insight 2024, 9, e177899. [Google Scholar] [CrossRef]
- Wan, C.; Latter, J.L.; Amirshahi, A.; Symonds, I.; Finnie, J.; Bowden, N.; Scott, R.J.; Cunningham, K.A.; Timms, P.; Beagley, K.W. Progesterone Activates Multiple Innate Immune Pathways in C Hlamydia Trachomatis-Infected Endocervical Cells. Am. J. Reprod. Immunol. 2014, 71, 165–177. [Google Scholar] [CrossRef]
- Li, M.; Li, A.; Huang, H.; Munson, J.; Obadan, A.; Fuller, D.H.; Adams Waldorf, K.M. Impact of Progesterone on Innate Immunity and Cell Death after Influenza A Virus H1N1 2009 Infection of Lung and Placental Cells in Vitro. Front. Virol. 2022, 2, 953208. [Google Scholar] [CrossRef]
- Groh, L.A.; Verel, D.E.; Van Der Heijden, C.D.C.C.; Matzaraki, V.; Moorlag, S.J.C.F.M.; De Bree, L.C.; Koeken, V.A.C.M.; Mourits, V.P.; Keating, S.T.; Van Puffelen, J.H.; et al. Immune Modulatory Effects of Progesterone on oxLDL-Induced Trained Immunity in Monocytes. J. Leukoc. Biol. 2022, 112, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Earhart, A.P.; Karasseva, N.G.; Storey, K.M.; Olthoff, B.; Sarker, M.B.; Laffey, K.G.; Lange, M.J.; Rector, R.S.; Schulz, L.C.; Gil, D.; et al. Lower Female Survival from an Opportunistic Infection Reveals Progesterone-Driven Sex Bias in Trained Immunity. Cell Rep. 2023, 42, 113007. [Google Scholar] [CrossRef]
- Su, S.; Hua, D.; Li, J.-P.; Zhang, X.-N.; Bai, L.; Cao, L.-B.; Guo, Y.; Zhang, M.; Dong, J.-Z.; Liang, X.-W.; et al. Modulation of Innate Immune Response to Viruses Including SARS-CoV-2 by Progesterone. Signal Transduct. Target. Ther. 2022, 7, 137. [Google Scholar] [CrossRef]
- Hall, O.J.; Limjunyawong, N.; Vermillion, M.S.; Robinson, D.P.; Wohlgemuth, N.; Pekosz, A.; Mitzner, W.; Klein, S.L. Progesterone-Based Therapy Protects Against Influenza by Promoting Lung Repair and Recovery in Females. PLOS Pathog. 2016, 12, e1005840. [Google Scholar] [CrossRef]
- Littauer, E.Q.; Esser, E.S.; Antao, O.Q.; Vassilieva, E.V.; Compans, R.W.; Skountzou, I. H1N1 Influenza Virus Infection Results in Adverse Pregnancy Outcomes by Disrupting Tissue-Specific Hormonal Regulation. PLOS Pathog. 2017, 13, e1006757. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, H.; Lin, J.; Wang, Y.; Dong, J.; Li, J.; Li, J. Progesterone Inhibits Inflammatory Response in E.Coli- or LPS-Stimulated Bovine Endometrial Epithelial Cells by NF-κB and MAPK Pathways. Dev. Comp. Immunol. 2020, 105, 103568. [Google Scholar] [CrossRef]
- Lee, J.H.; Ulrich, B.; Cho, J.; Park, J.; Kim, C.H. Progesterone Promotes Differentiation of Human Cord Blood Fetal T Cells into T Regulatory Cells but Suppresses Their Differentiation into Th17 Cells. J. Immunol. 2011, 187, 1778–1787. [Google Scholar] [CrossRef]
- Laškarin, G.; Faust, Z.; ŠTrbo, N.; Sotošek, V.; Szekeres-Bartho, J.; Podack, E.R.; Rukavina, D. Progesterone Directly and Indirectly Affects Perforin Expression in Cytolytic Cells. Am. J. Reprod. Immunol. 1999, 42, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.X.; Ma, Z.; Rourke, T.; Srinivasan, S.; McChesney, M.; Miller, C.J. Immunoglobulin Concentrations and Antigen-Specific Antibody Levels in Cervicovaginal Lavages of Rhesus Macaques Are Influenced by the Stage of the Menstrual Cycle. Infect. Immun. 1999, 67, 6321–6328. [Google Scholar] [CrossRef]
- Lü, F.X.; Abel, K.; Ma, Z.; Rourke, T.; Lu, D.; Torten, J.; Mcchesney, M.; Miller, C.J. The Strength of B Cell Immunity in Female Rhesus Macaques Is Controlled by CD8 + T Cells under the Influence of Ovarian Steroid Hormones. Clin. Exp. Immunol. 2002, 128, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.-J.; Lai, K.-P.; Zeng, W.; Chuang, K.-H.; Altuwaijri, S.; Chang, C. Androgen Receptor Influences on Body Defense System via Modulation of Innate and Adaptive Immune Systems. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef]
- Vancolen, S.; Sébire, G.; Robaire, B. Influence of Androgens on the Innate Immune System. Andrology 2023, 11, 1237–1244. [Google Scholar] [CrossRef]
- D’Agostino, P.; Milano, S.; Barbera, C.; Di Bella, G.; La Rosa, M.; Ferlazzo, V.; Farruggio, R.; Miceli, D.M.; Miele, M.; Castagnetta, L.; et al. Sex Hormones Modulate Inflammatory Mediators Produced by Macrophagesa. Ann. N. Y. Acad. Sci. 1999, 876, 426–429. [Google Scholar] [CrossRef]
- Rettew, J.A.; Huet-Hudson, Y.M.; Marriott, I. Testosterone Reduces Macrophage Expression in the Mouse of Toll-Like Receptor 4, a Trigger for Inflammation and Innate Immunity. Biol. Reprod. 2008, 78, 432–437. [Google Scholar] [CrossRef]
- Thompson, M.G.; Peiffer, D.S.; Larson, M.; Navarro, F.; Watkins, S.K. FOXO3, Estrogen Receptor Alpha, and Androgen Receptor Impact Tumor Growth Rate and Infiltration of Dendritic Cell Subsets Differentially between Male and Female Mice. Cancer Immunol. Immunother. 2017, 66, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.; Chang, J.J.; Chan, E.S.; Pollard, R.B.; Sidhu, H.K.; Kulkarni, S.; Wen, T.F.; Lindsay, R.J.; Orellana, L.; Mildvan, D.; et al. Sex Differences in the Toll-like Receptor–Mediated Response of Plasmacytoid Dendritic Cells to HIV-1. Nat. Med. 2009, 15, 955–959. [Google Scholar] [CrossRef]
- Lin, A.A.; Wojciechowski, S.E.; Hildeman, D.A. Androgens Suppress Antigen-Specific T Cell Responses and IFN-γ Production during Intracranial LCMV Infection. J. Neuroimmunol. 2010, 226, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Chuang, K.-H.; Altuwaijri, S.; Li, G.; Lai, J.-J.; Chu, C.-Y.; Lai, K.-P.; Lin, H.-Y.; Hsu, J.-W.; Keng, P.; Wu, M.-C.; et al. Neutropenia with Impaired Host Defense against Microbial Infection in Mice Lacking Androgen Receptor. J. Exp. Med. 2009, 206, 1181–1199. [Google Scholar] [CrossRef] [PubMed]
- Scalerandi, M.V.; Peinetti, N.; Leimgruber, C.; Cuello Rubio, M.M.; Nicola, J.P.; Menezes, G.B.; Maldonado, C.A.; Quintar, A.A. Inefficient N2-Like Neutrophils Are Promoted by Androgens During Infection. Front. Immunol. 2018, 9, 1980. [Google Scholar] [CrossRef]
- Hreha, T.N.; Collins, C.A.; Cole, E.B.; Jin, R.J.; Hunstad, D.A. Androgen Exposure Impairs Neutrophil Maturation and Function within the Infected Kidney. mBio 2024, 15, e03170-23. [Google Scholar] [CrossRef]
- Olson, P.D.; Hruska, K.A.; Hunstad, D.A. Androgens Enhance Male Urinary Tract Infection Severity in a New Model. J. Am. Soc. Nephrol. 2016, 27, 1625–1634. [Google Scholar] [CrossRef]
- Blanquart, E.; Laffont, S.; Guéry, J.-C. Sex Hormone Regulation of Innate Lymphoid Cells. Biomed. J. 2021, 44, 144–156. [Google Scholar] [CrossRef]
- Michla, M.; Wilhelm, C. Food for Thought—ILC Metabolism in the Context of Helminth Infections. Mucosal Immunol. 2022, 15, 1234–1242. [Google Scholar] [CrossRef]
- Olsen, N.J.; Olson, G.; Viselli, S.M.; Gu, X.; Kovacs, W.J. Androgen Receptors in Thymic Epithelium Modulate Thymus Size and Thymocyte Development*. Endocrinology 2001, 142, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Heng, T.S.P.; Goldberg, G.L.; Gray, D.H.D.; Sutherland, J.S.; Chidgey, A.P.; Boyd, R.L. Effects of Castration on Thymocyte Development in Two Different Models of Thymic Involution. J. Immunol. 2005, 175, 2982–2993. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.C.; Moser, M.T.; Tri, S.D.; Mercader, M.; Kuntz, S.M.; Dong, H.; Hurwitz, A.A.; McKean, D.J.; Celis, E.; Leibovich, B.C.; et al. Augmentation of T Cell Levels and Responses Induced by Androgen Deprivation. J. Immunol. 2004, 173, 6098–6108. [Google Scholar] [CrossRef]
- Kissick, H.T.; Sanda, M.G.; Dunn, L.K.; Pellegrini, K.L.; On, S.T.; Noel, J.K.; Arredouani, M.S. Androgens Alter T-Cell Immunity by Inhibiting T-Helper 1 Differentiation. Proc. Natl. Acad. Sci. USA 2014, 111, 9887–9892. [Google Scholar] [CrossRef]
- Fijak, M.; Damm, L.; Wenzel, J.; Aslani, F.; Walecki, M.; Wahle, E.; Eisel, F.; Bhushan, S.; Hackstein, H.; Baal, N.; et al. Influence of Testosterone on Inflammatory Response in Testicular Cells and Expression of Transcription Factor Foxp3 in T Cells. Am. J. Reprod. Immunol. 2015, 74, 12–25. [Google Scholar] [CrossRef]
- Benten, W.P.M.; Stephan, C.; Wunderlich, F. B Cells Express Intracellular but Not Surface Receptors for Testosterone and Estradiol. Steroids 2002, 67, 647–654. [Google Scholar] [CrossRef]
- Altuwaijri, S.; Chuang, K.-H.; Lai, K.-P.; Lai, J.-J.; Lin, H.-Y.; Young, F.M.; Bottaro, A.; Tsai, M.-Y.; Zeng, W.-P.; Chang, H.-C.; et al. Susceptibility to Autoimmunity and B Cell Resistance to Apoptosis in Mice Lacking Androgen Receptor in B Cells. Mol. Endocrinol. 2009, 23, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmson, A.S.; Lantero Rodriguez, M.; Stubelius, A.; Fogelstrand, P.; Johansson, I.; Buechler, M.B.; Lianoglou, S.; Kapoor, V.N.; Johansson, M.E.; Fagman, J.B.; et al. Testosterone Is an Endogenous Regulator of BAFF and Splenic B Cell Number. Nat. Commun. 2018, 9, 2067. [Google Scholar] [CrossRef]
- Stárka, L.; Dušková, M. Androgens in SARS-CoV-2 Coronavirus Infections. Physiol. Res. 2021, 70, S145–S151. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Guastamacchia, E.; Magrone, T.; Jirillo, E.; Lisco, G.; De Pergola, G.; Triggiani, V. Worse Progression of COVID-19 in Men: Is Testosterone a Key Factor? Andrology 2021, 9, 53–64. [Google Scholar] [CrossRef]
- Moskalev, A.; Stambler, I.; Caruso, C. Innate and Adaptive Immunity in Aging and Longevity: The Foundation of Resilience. Aging Dis. 2020, 11, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Ligotti, M.E.; Accardi, G.; Aiello, A.; Candore, G. An Immunologist’s Guide to Immunosenescence and Its Treatment. Expert Rev. Clin. Immunol. 2022, 18, 961–981. [Google Scholar] [CrossRef]
- Ligotti, M.E.; Accardi, G.; Aiello, A.; Aprile, S.; Calabrò, A.; Caldarella, R.; Caruso, C.; Ciaccio, M.; Corsale, A.M.; Dieli, F.; et al. Sicilian Semi- and Supercentenarians: Identification of Age-Related T-Cell Immunophenotype to Define Longevity Trait. Clin. Exp. Immunol. 2023, 214, 61–78. [Google Scholar] [CrossRef]
- Franceschi, C.; Salvioli, S.; Garagnani, P.; de Eguileor, M.; Monti, D.; Capri, M. Immunobiography and the Heterogeneity of Immune Responses in the Elderly: A Focus on Inflammaging and Trained Immunity. Front. Immunol. 2017, 8, 982. [Google Scholar] [CrossRef] [PubMed]
- Yanicke, S.; Ucar, D. Sex Differences in Immune System Aging and Responsiveness to Vaccination. Public Policy Aging Rep. 2023, 33, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Ramirez, A.; Rathinam, V.; Fitzgerald, K.A.; Golenbock, D.T.; Mathew, A. Defective Pro-IL-1β Responses in Macrophages from Aged Mice. Immun. Ageing A 2012, 9, 27. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, N.; Qiao, B.; Zhang, F.; Wu, J.; Liu, C.; Li, Y.; Du, J. Age-Related Decline of Interferon-Gamma Responses in Macrophage Impairs Satellite Cell Proliferation and Regeneration. J. Cachexia Sarcopenia Muscle 2020, 11, 1291–1305. [Google Scholar] [CrossRef]
- Perskin, M.H.; Cronstein, B.N. Age-Related Changes in Neutrophil Structure and Function. Mech. Ageing Dev. 1992, 64, 303–313. [Google Scholar] [CrossRef]
- Gullotta, G.S.; De Feo, D.; Friebel, E.; Semerano, A.; Scotti, G.M.; Bergamaschi, A.; Butti, E.; Brambilla, E.; Genchi, A.; Capotondo, A.; et al. Age-Induced Alterations of Granulopoiesis Generate Atypical Neutrophils That Aggravate Stroke Pathology. Nat. Immunol. 2023, 24, 925–940. [Google Scholar] [CrossRef]
- Weisel, K.C.; Bautz, F.; Seitz, G.; Yildirim, S.; Kanz, L.; Möhle, R. Modulation of CXC Chemokine Receptor Expression and Function in Human Neutrophils during Aging in Vitro Suggests a Role in Their Clearance from Circulation. Mediat. Inflamm. 2009, 2009, 790174. [Google Scholar] [CrossRef] [PubMed]
- Barkaway, A.; Rolas, L.; Joulia, R.; Bodkin, J.; Lenn, T.; Owen-Woods, C.; Reglero-Real, N.; Stein, M.; Vázquez-Martínez, L.; Girbl, T.; et al. Age-Related Changes in the Local Milieu of Inflamed Tissues Cause Aberrant Neutrophil Trafficking and Subsequent Remote Organ Damage. Immunity 2021, 54, 1494–1510.e7. [Google Scholar] [CrossRef]
- Hornigold, K.; Chu, J.Y.; Chetwynd, S.A.; Machin, P.A.; Crossland, L.; Pantarelli, C.; Anderson, K.E.; Hawkins, P.T.; Segonds-Pichon, A.; Oxley, D.; et al. Age-Related Decline in the Resistance of Mice to Bacterial Infection and in LPS/TLR4 Pathway-Dependent Neutrophil Responses. Front. Immunol. 2022, 13, 888415. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, C.; Zou, Z.; Fan, E.K.Y.; Chen, L.; Li, Y.; Billiar, T.R.; Wilson, M.A.; Shi, X.; Fan, J. Aging-Related Atg5 Defect Impairs Neutrophil Extracellular Traps Formation. Immunology 2017, 151, 417–432. [Google Scholar] [CrossRef]
- ElGindi, M.; Sapudom, J.; Garcia Sabate, A.; Chesney Quartey, B.; Alatoom, A.; Al-Sayegh, M.; Li, R.; Chen, W.; Teo, J. Effects of an Aged Tissue Niche on the Immune Potency of Dendritic Cells Using Simulated Microgravity. Npj Aging 2023, 9, 14. [Google Scholar] [CrossRef]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging Is Associated with a Numerical and Functional Decline in Plasmacytoid Dendritic Cells, Whereas Myeloid Dendritic Cells Are Relatively Unaltered in Human Peripheral Blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef]
- Márquez, E.J.; Chung, C.-H.; Marches, R.; Rossi, R.J.; Nehar-Belaid, D.; Eroglu, A.; Mellert, D.J.; Kuchel, G.A.; Banchereau, J.; Ucar, D. Sexual-Dimorphism in Human Immune System Aging. Nat. Commun. 2020, 11, 751. [Google Scholar] [CrossRef]
- Mkhikian, H.; Hayama, K.L.; Khachikyan, K.; Li, C.; Zhou, R.W.; Pawling, J.; Klaus, S.; Tran, P.Q.N.; Ly, K.M.; Gong, A.D.; et al. Age-Associated Impairment of T Cell Immunity Is Linked to Sex-Dimorphic Elevation of N-Glycan Branching. Nat. Aging 2022, 2, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.V.; Ghosh, R.; Coll, K.; Chun, C.; Yousefzadeh, M.J. The 3 I’s of Immunity and Aging: Immunosenescence, Inflammaging, and Immune Resilience. Front. Aging 2024, 5, 1490302. [Google Scholar] [CrossRef]
- Candore, G.; Balistreri, C.R.; Colonna-Romano, G.; Lio, D.; Listì, F.; Vasto, S.; Caruso, C. Gender-Related Immune-Inflammatory Factors, Age-Related Diseases, and Longevity. Rejuvenation Res. 2010, 13, 292–297. [Google Scholar] [CrossRef]
- Calippe, B.; Douin-Echinard, V.; Delpy, L.; Laffargue, M.; Lélu, K.; Krust, A.; Pipy, B.; Bayard, F.; Arnal, J.-F.; Guéry, J.-C.; et al. 17Beta-Estradiol Promotes TLR4-Triggered Proinflammatory Mediator Production through Direct Estrogen Receptor Alpha Signaling in Macrophages in Vivo. J. Immunol. 2010, 185, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Laffont, S.; Seillet, C.; Guéry, J.-C. Estrogen Receptor-Dependent Regulation of Dendritic Cell Development and Function. Front. Immunol. 2017, 8, 108. [Google Scholar] [CrossRef]
- Marttila, S.; Jylhävä, J.; Nevalainen, T.; Nykter, M.; Jylhä, M.; Hervonen, A.; Tserel, L.; Peterson, P.; Hurme, M. Transcriptional Analysis Reveals Gender-Specific Changes in the Aging of the Human Immune System. PLoS ONE 2013, 8, e66229. [Google Scholar] [CrossRef] [PubMed]
- Jentsch-Ullrich, K.; Koenigsmann, M.; Mohren, M.; Franke, A. Lymphocyte Subsets’ Reference Ranges in an Age- and Gender-Balanced Population of 100 Healthy Adults--a Monocentric German Study. Clin. Immunol. Orlando Fla 2005, 116, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, K.; Utsuyama, M.; Hayashi, Y.; Kitagawa, M.; Makinodan, T.; Fulop, T. Slower Immune System Aging in Women versus Men in the Japanese Population. Immun. Ageing A 2013, 10, 19. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Huang, M.-C.; Kon, J.; Patel, K.; Schwartz, J.B.; Fast, K.; Ferrucci, L.; Madara, K.; Taub, D.D.; Longo, D.L. Gender Specificity of Altered Human Immune Cytokine Profiles in Aging. FASEB J. 2010, 24, 3580–3589. [Google Scholar] [CrossRef]
- Pietschmann, P.; Gollob, E.; Brosch, S.; Hahn, P.; Kudlacek, S.; Willheim, M.; Woloszczuk, W.; Peterlik, M.; Tragl, K.H. The Effect of Age and Gender on Cytokine Production by Human Peripheral Blood Mononuclear Cells and Markers of Bone Metabolism. Exp. Gerontol. 2003, 38, 1119–1127. [Google Scholar] [CrossRef]
- Feng, Z.; Liao, M.; Zhang, L. Sex Differences in Disease: Sex Chromosome and Immunity. J. Transl. Med. 2024, 22, 1150. [Google Scholar] [CrossRef]
- Obergassel, J.; O’Reilly, M.; Sommerfeld, L.C.; Kabir, S.N.; O’Shea, C.; Syeda, F.; Eckardt, L.; Kirchhof, P.; Fabritz, L. Effects of Genetic Background, Sex, and Age on Murine Atrial Electrophysiology. Europace 2021, 23, 958–969. [Google Scholar] [CrossRef]
- Brown, E.J.; Nguyen, A.H.; Bachtrog, D. The Drosophila Y Chromosome Affects Heterochromatin Integrity Genome-Wide. Mol. Biol. Evol. 2020, 37, 2808–2824. [Google Scholar] [CrossRef]
- Gutiérrez-Hurtado, I.A.; Sánchez-Méndez, A.D.; Becerra-Loaiza, D.S.; Rangel-Villalobos, H.; Torres-Carrillo, N.; Gallegos-Arreola, M.P.; Aguilar-Velázquez, J.A. Loss of the Y Chromosome: A Review of Molecular Mechanisms, Age Inference, and Implications for Men’s Health. Int. J. Mol. Sci. 2024, 25, 4230. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.M. Sex/Gender Differences in Infectious Diseases. In Sex/Gender-Specific Medicine in Clinical Areas; Kim, N., Ed.; Springer Nature: Singapore, 2024; pp. 311–324. ISBN 978-981-97-0130-8. [Google Scholar]
- Lakshmikanth, T.; Consiglio, C.; Sardh, F.; Forlin, R.; Wang, J.; Tan, Z.; Barcenilla, H.; Rodriguez, L.; Sugrue, J.; Noori, P.; et al. Immune System Adaptation during Gender-Affirming Testosterone Treatment. Nature 2024, 633, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Marcon, G.; Tettamanti, M.; Capacci, G.; Fontanel, G.; Spanò, M.; Nobili, A.; Forloni, G.; Franceschi, C. COVID-19 Mortality in Lombardy: The Vulnerability of the Oldest Old and the Resilience of Male Centenarians. Aging 2020, 12, 15186–15195. [Google Scholar] [CrossRef]
- Cardelli, M.; Pierpaoli, E.; Marchegiani, F.; Marcheselli, F.; Piacenza, F.; Giacconi, R.; Recchioni, R.; Casoli, T.; Stripoli, P.; Provinciali, M.; et al. Biomarkers of Cell Damage, Neutrophil and Macrophage Activation Associated with in-Hospital Mortality in Geriatric COVID-19 Patients. Immun. Ageing A 2022, 19, 65. [Google Scholar] [CrossRef]
- Olivieri, F.; Marchegiani, F.; Matacchione, G.; Giuliani, A.; Ramini, D.; Fazioli, F.; Sabbatinelli, J.; Bonafè, M. Sex/Gender-Related Differences in Inflammaging. Mech. Ageing Dev. 2023, 211, 111792. [Google Scholar] [CrossRef] [PubMed]
| Chromosome | Regulated Genes and Pathway | Function | Reference |
|---|---|---|---|
| Y | IMD | Regulates innate immune response via NF-κB signaling (in Drosophila model) | [8] |
| Y | Expression of CD99 | Facilitates immune cell transendothelial migration and adhesion | [14] |
| X | FOXP3 | Master regulator of T regulatory (Treg) cell development and function | [16] |
| X | CD99, CXCR3 | CD99: immune cell migration; CXCR3: chemokine receptor involved in leukocyte trafficking | [16] |
| X | IL-2RG | Common gamma chain in several interleukin receptors, essential for T and NK cell development | [16] |
| X | OGT, CYBB | OGT: protein glycosylation in signaling; CYBB: component of NADPH oxidase complex for pathogen killing | [16] |
| X | TLR7 | Recognizes single-stranded RNA viruses, activates innate immune signaling | [17] |
| X | IRAK1 | Mediates downstream TLR/IL-1 receptor signaling, promotes inflammatory cytokine production | [20] |
| X | BTK | Regulates B cell development, activation, and NF-κB signaling | [22] |
| X | XEDAR | TNF receptor family member, activates both canonical and non-canonical NF-κB pathways | [23] |
| Sex Hormones | Effects on Immune Cells and Pathways | References |
|---|---|---|
| Estrogen | Macrophage M2 polarization | [55] |
| Modulation of cytokine production and limiting “cytokine storm” | [58,64,65] | |
| Promotion of type I IFNs and TNFα through TLR signaling | [62] | |
| Neutrophil apoptosis, chemotaxis, and release of NETs | [53] | |
| Increasing nNOS | [70] | |
| Increasing the number of NK cells and suppressing their cytotoxicity | [73,74] | |
| Survival and proliferation of B lymphocytes | [76] | |
| Antibody and autoantibody production | [76] | |
| Th1 differentiation | [78] | |
| IFN-γ-dependent inflammation | [78] | |
| Differentiation and expansion of Tregs | [82] | |
| Progesterone | Promotion of macrophage–neutrophil crosstalk | [96] |
| Inhibition of TNFα | [99] | |
| Expression of antiviral genes (e.g., IFNB1) | [101] | |
| T cell differentiation into Tregs | [105] | |
| Th2-type responses | [92] | |
| Release of anti-inflammatory cytokines (e.g., IL-4 and IL-10) | [92] | |
| Inhibition of ISCs frequency | [108] | |
| Androgens | Induction of IL-10 and reduction in NO and TNFα | [111] |
| Induction of anti-inflammatory macrophages and dendritic cells | [111,113] | |
| Reduction in TLR4 expression | [112] | |
| Neutrophil recruitment with weakened bactericidal ability | [116,117] | |
| Inhibition of ILCs | [120] | |
| Suppression of thymic development | [122,123] | |
| Inhibition of T cell proliferation | [124] | |
| Inhibition of IL-12-induced STAT4 phosphorylation | [125] | |
| Negative control on BAFF | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rio, P.; Caldarelli, M.; Miccoli, E.; Guazzarotti, G.; Gasbarrini, A.; Gambassi, G.; Cianci, R. Sex Differences in Immune Responses to Infectious Diseases: The Role of Genetics, Hormones, and Aging. Diseases 2025, 13, 179. https://doi.org/10.3390/diseases13060179
Rio P, Caldarelli M, Miccoli E, Guazzarotti G, Gasbarrini A, Gambassi G, Cianci R. Sex Differences in Immune Responses to Infectious Diseases: The Role of Genetics, Hormones, and Aging. Diseases. 2025; 13(6):179. https://doi.org/10.3390/diseases13060179
Chicago/Turabian StyleRio, Pierluigi, Mario Caldarelli, Edoardo Miccoli, Giulia Guazzarotti, Antonio Gasbarrini, Giovanni Gambassi, and Rossella Cianci. 2025. "Sex Differences in Immune Responses to Infectious Diseases: The Role of Genetics, Hormones, and Aging" Diseases 13, no. 6: 179. https://doi.org/10.3390/diseases13060179
APA StyleRio, P., Caldarelli, M., Miccoli, E., Guazzarotti, G., Gasbarrini, A., Gambassi, G., & Cianci, R. (2025). Sex Differences in Immune Responses to Infectious Diseases: The Role of Genetics, Hormones, and Aging. Diseases, 13(6), 179. https://doi.org/10.3390/diseases13060179









