Abstract
Diagnosing tuberculosis (TB) in children presents significant challenges, necessitating the identification of reliable biomarkers for accurate diagnosis. In this study, we investigated plasma matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) as potential diagnostic markers. A prospective case–control study involved 167 children classified into confirmed TB, unconfirmed TB, and unlikely TB control groups. Plasma levels of MMPs (MMP 1, 2, 3, 7, 8, 9, 12, and 13) and TIMPs (TIMP 1, 2, 3, and 4) were measured using multiplex assays. Elevated baseline levels of MMP-1, MMP-2, MMP-7, MMP-9, TIMP-1, TIMP-2, TIMP-3, and TIMP-4 were observed in active TB cases compared to unlikely TB controls. Receiver operating characteristics (ROC) analysis identified MMP-1, MMP-2, MMP-9, and TIMP-1 as potential biomarkers with over 80% sensitivity and specificity. A three-MMP signature (MMP-1, MMP-2, and MMP-9) demonstrated 100% sensitivity and specificity. The findings suggest that a baseline MMP signature could serve as an accurate biomarker for diagnosing pediatric TB, enabling early intervention and effective management.
1. Introduction
Tuberculosis (TB) stands as a leading cause of global mortality, with pediatric TB significantly contributing to the overall burden, particularly in high-burden countries [1]. Pediatric TB poses a diagnostic conundrum owing to its paucibacillary nature, which renders current clinical and laboratory diagnostic tools less effective [2]. Compounding the issue is the challenge of obtaining sputum samples from young children, further complicating bacteriological confirmation. Hence, the demand for reliable, non-sputum-based point-of-care (POC) tests for pediatric TB diagnosis is urgent [3]. Host immune biomarkers in blood have emerged as an alternative for early TB detection and are prioritized in the WHO’s End TB strategy. Nevertheless, developing an effective POC diagnostic tool for pediatric TB remains daunting, given the unique characteristics of this population.
Matrix metalloproteinases (MMPs), pivotal in tissue oxidation and immunological defense, have surfaced as potential biomarkers. These proteolytic enzymes are essential in various physiological processes, including immune responses, inflammation, tissue repair, and matrix protein breakdown [4,5]. Concurrently, tissue inhibitors of metalloproteinases (TIMPs), integral to tissue remodeling and healing, counterbalance MMP-induced tissue damage [6]. Previous research in the pediatric population has indicated elevated levels of MMP1, 7, 8, and TIMP 1, 3 in children with pulmonary TB compared to those with extrapulmonary TB and healthy controls [7,8]. Similarly, studies in adults with pulmonary TB have shown increased circulating levels of MMP1, 2, 3, 7, 10, 12, and 13 compared to healthy controls [9]. Recent findings have also suggested that MMP and TIMP levels may serve as correlates of risk and prognostic biomarkers for treatment failure, relapse, and death in individuals with pulmonary TB, with MMPs being key mediators of TB pathology [10]. This study aims to assess the plasma concentrations of MMPs and TIMPs to distinguish between microbiologically confirmed, unconfirmed, and unlikely TB cases among prospectively recruited pediatric participants.
2. Methods
2.1. Study Population and Procedures
We conducted a prospective case–control study spanning the pediatric wards of the Institute of Child Health and Hospital for Children, Chennai, and Government Stanley Medical College and Hospital, Chennai, between February 2016 and March 2018. This study aimed to assess plasma matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) as potential diagnostic markers for pediatric tuberculosis (TB).
A total of 195 children were screened during the recruitment phase, of which 167 were enrolled in the study (Table 1). This cohort comprised 44 children with microbiologically confirmed TB, 47 with unconfirmed TB, and 76 unlikely TB controls who presented with other respiratory ailments. To ascertain TB status, children underwent various diagnostic tests, including sputum samples or gastric aspirates for microbiological testing and Tuberculin Skin Test (TST) for immune response assessment.

Table 1.
Study demographics.
For confirmed TB cases, microbiological positivity for tuberculosis was required, as evidenced by Xpert MTB/RIF, smear, or culture testing. Unconfirmed TB cases exhibited clinical features suggestive of TB, abnormal chest X-rays, or a history of household TB contact or positive response to anti-tuberculosis treatment (ATT), meeting at least two of the criteria. Unlikely TB controls were children with alternative diagnoses, such as COPD, viral pneumonia, bacterial pneumonia, or asthma/wheeze, who were either TST negative or TST positive with non-TB diagnoses [11].
At enrollment, all active TB cases (confirmed and unconfirmed) had no prior TB or ATT history, and positive TST results were defined as an induration of at least 10 mm in diameter at the site of tuberculin inoculation. Blood samples were collected from all participants using sodium heparin tubes and transported within 2 h to the Immunology lab for processing. Plasma samples were subsequently stored in a −80 °C freezer for future analysis. Notably, all confirmed and unconfirmed TB cases received ATT for 6 months.
The study also classified disease severity into two categories: severe TB, characterized by abnormal chest x-rays with AFB smear positivity alongside positive Xpert/MGIT/LJ results, and minimal TB, which included abnormal chest x-rays with negative AFB smear or normal chest x-rays with positive Xpert/MGIT/LJ results.
The detailed demographic and epidemiological data for the participants have been previously reported [12,13].
2.2. Metalloproteinase Assays
Plasma levels of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) were quantified using commercially available kits. The assay’s lowest detection limits ranged from 7.2 to 211.3 pg/mL for different MMPs and TIMPs. Circulating plasma levels were assessed using a commercially available Luminex Magpix Multiplex Assay system (Bio-Rad, Hercules, CA, USA). Additionally, MMP and TIMP levels were measured with commercially available kits: the Luminex Human Magnetic Assay 8-Plex and 4-Plex kits, both from R&D Systems.
2.3. Statistical Analysis
Geometric means were used for central tendency measurements. The Kruskal–Wallis test with Dunn’s multiple comparisons assessed significant differences between confirmed TB, unconfirmed TB, and unlikely TB. Receiver operator characteristics (ROC) curves evaluated the discriminatory power of each immune biomarker, with optimal biomarker combinations selected using CombiROC v.1.2. GraphPad PRISM Version 8.0 facilitated data analysis.
3. Results
3.1. Elevated Plasma Levels of MMPs and TIMPs in Pediatric TB
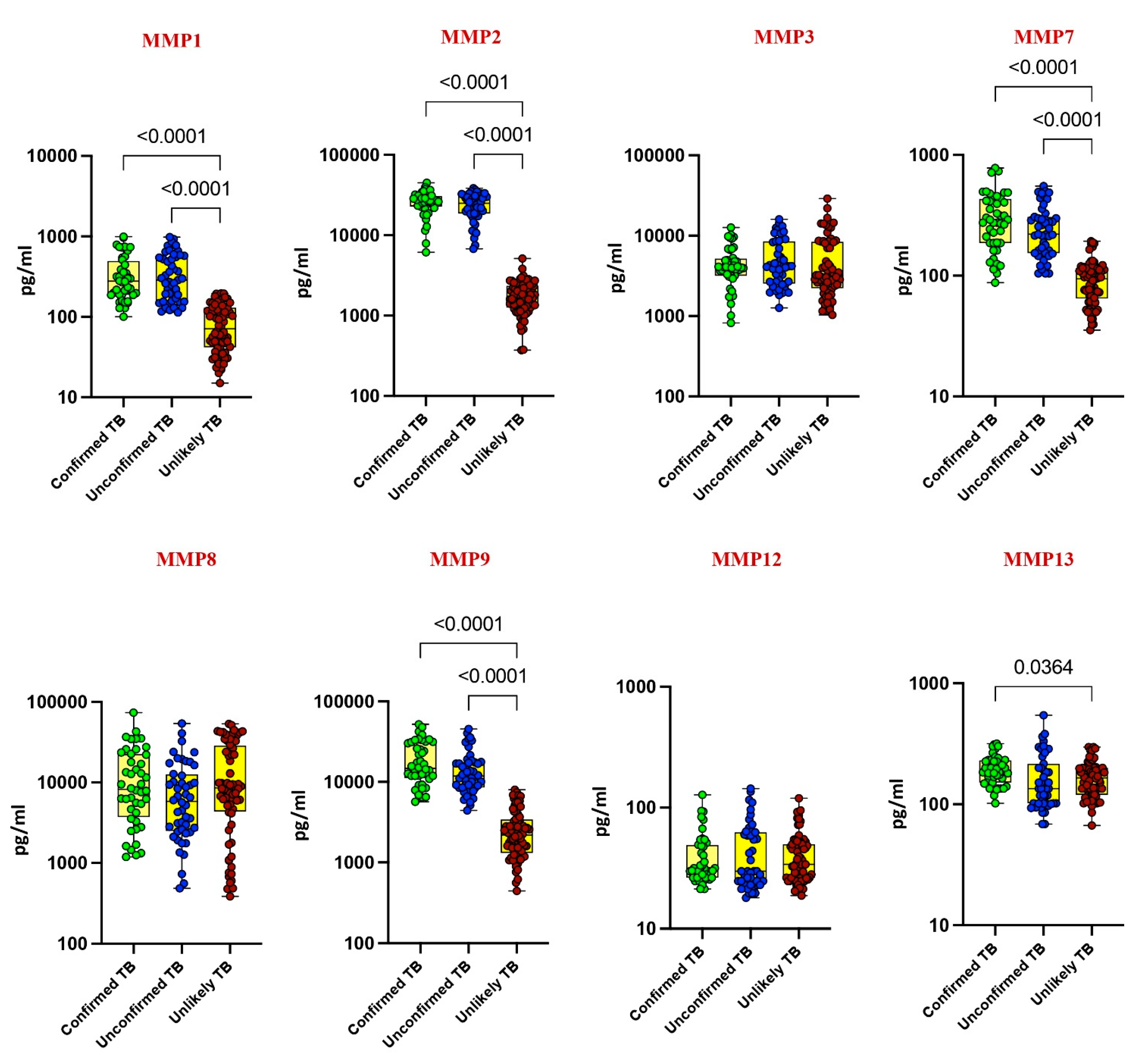
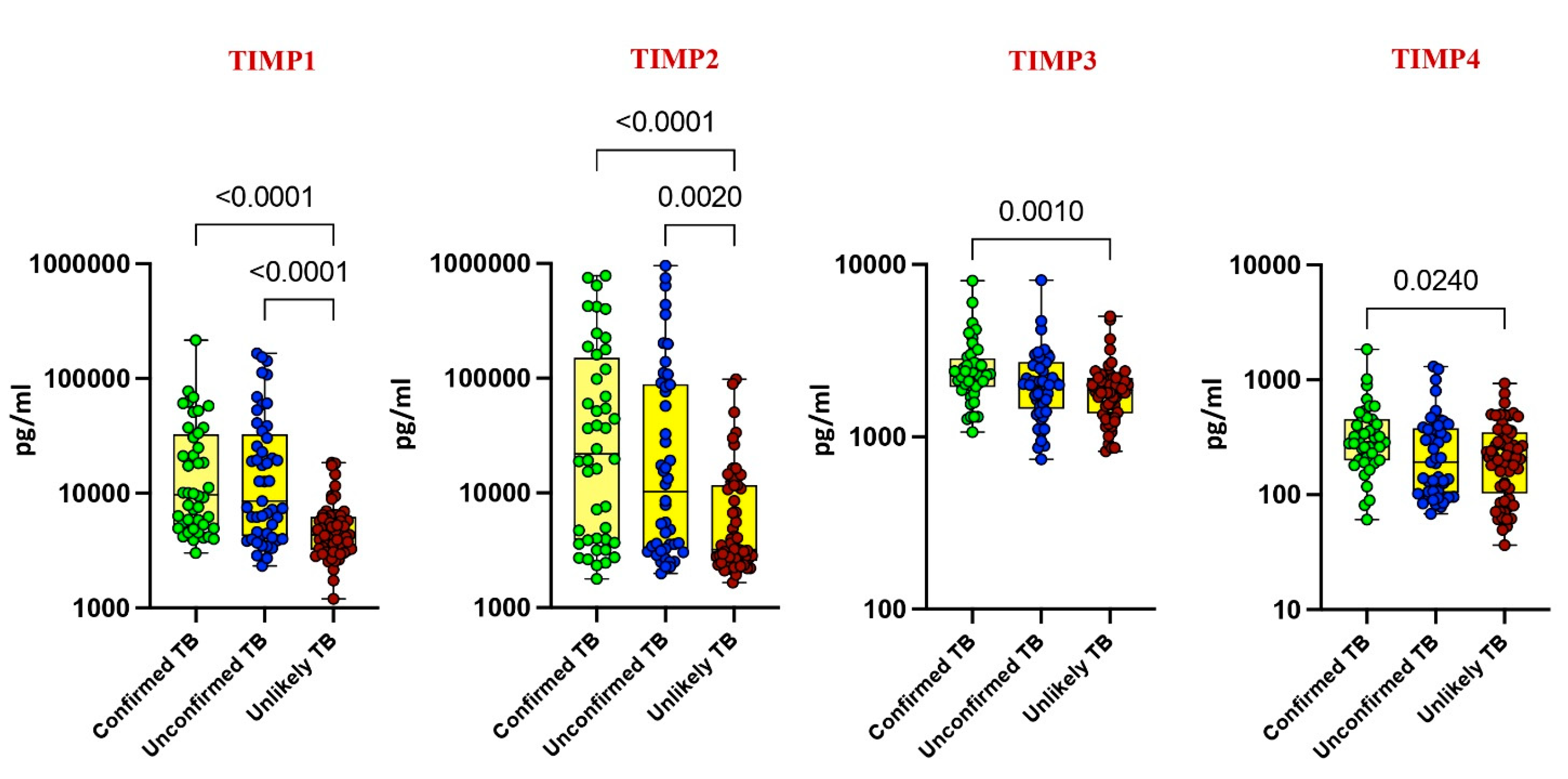
Our investigation delved into the plasma levels of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in pediatric tuberculosis (TB), comparing confirmed TB, unconfirmed TB, unlikely TB, and healthy controls. Significantly elevated levels of MMP-1, MMP-2, MMP-7, MMP-9, TIMP-1, and TIMP-2 were noted in confirmed and unconfirmed TB cases compared to unlikely TB and healthy controls (p < 0.001). For instance, MMP-1 exhibited a geometric mean (GM) of 295.8 pg/mL in confirmed TB, while it was 303 pg/mL in unconfirmed TB and 70 pg/mL in unlikely TB. Similarly, MMP-2 displayed a GM of 24,719 pg/mL in confirmed TB, 22,163 pg/mL in unconfirmed TB, and 1646 pg/mL in unlikely TB (Figure 1 and Figure 2).
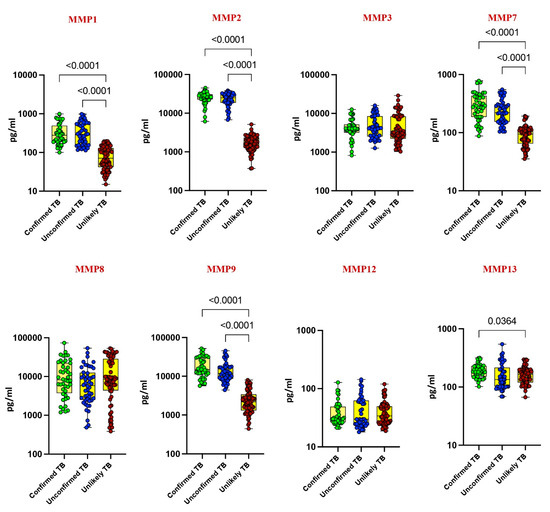
Figure 1.
Elevated circulating levels of MMPs in children with active TB disease. The plasma levels of MMP 1, 2, 3, 7, 8, 9, 12, and 13 were measured in confirmed TB (n = 44), unconfirmed TB (n = 47), and unlikely TB (n = 76) individuals at baseline. The data are represented as scatter plots with each circle representing a single individual. p values were calculated using the Kruskal–Wallis test with Dunn’s post hoc for multiple comparisons.
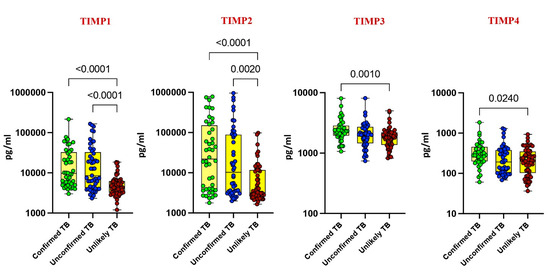
Figure 2.
Elevated circulating levels of TIMPs in children with active TB disease. The plasma levels of TIMP 1, 2, 3, and 4 were measured in confirmed TB (n = 44), unconfirmed TB (n = 47), and unlikely TB (n = 76) individuals at baseline. The data are represented as scatter plots with each circle representing a single individual. p values were calculated using the Kruskal–Wallis test with Dunn’s post hoc for multiple comparisons.
Conversely, as shown in Figure 1 and Figure 2, MMP-13, TIMP-3, and TIMP-4 showed elevated levels exclusively in confirmed TB cases (p < 0.001). For instance, MMP-13 had a GM of 188 pg/mL in confirmed TB, while it was 150 pg/mL in unlikely TB. Likewise, TIMP-3 exhibited a GM of 2373 pg/mL in confirmed TB, contrasting with 1791 pg/mL in unlikely TB. No significant differences were observed for MMP-3, MMP-8, and MMP-12 among the study groups. These findings underscore the potential of MMPs and TIMPs as diagnostic markers for pediatric TB.
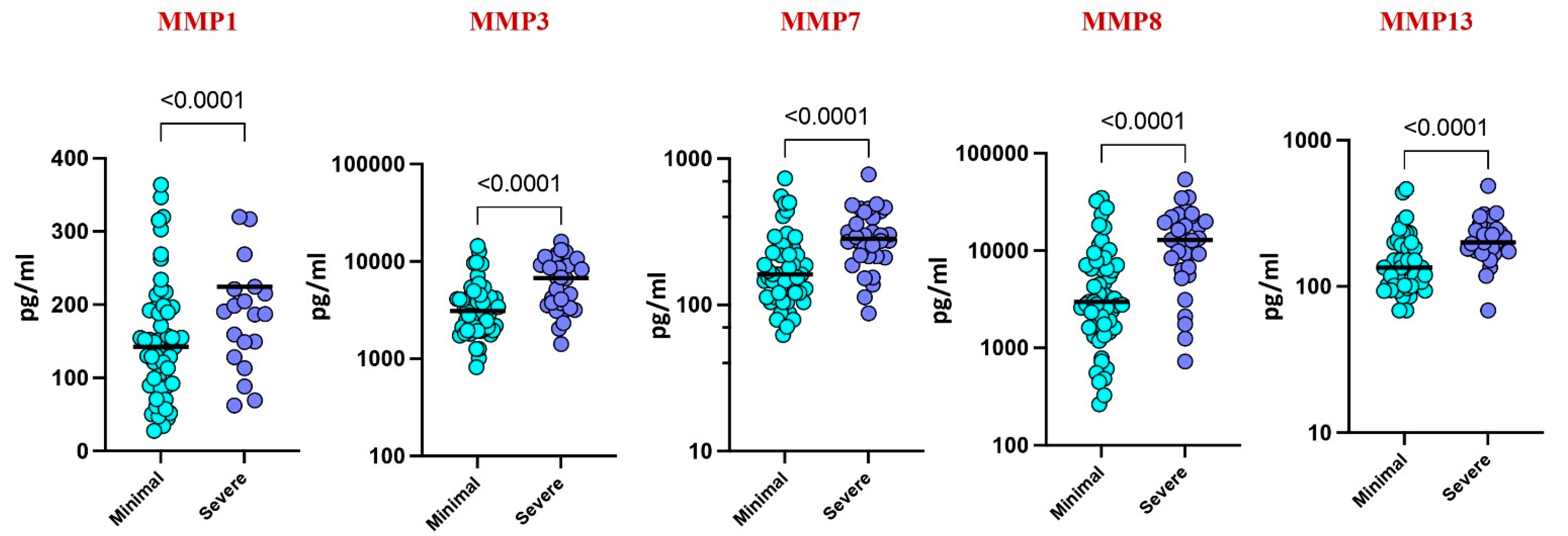
3.2. Plasma MMPs Associated with Disease Severity
Upon stratifying by disease severity, based on chest X-ray and smear reports, higher levels of MMP-1 (p = 0.0155), MMP-3 (p = 0.419), MMP-7 (p = 0.0038), MMP-8 (p = 0.0018), and MMP-13 (p = 0.0281) were observed in severe cases compared to those with minimal illness. These findings suggest a correlation between elevated MMP levels and disease severity in pediatric TB (Figure 3).
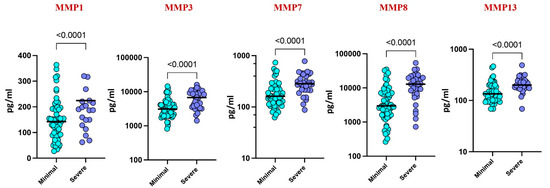
Figure 3.
Plasma MMPs are associated with disease severity. The plasma levels of MMP 1, 3, 7, 8, and 13 were measured among the active TB children with minimal TB (n = 59) and severe TB (n = 32) individuals at baseline. The data are represented by scatter plots with each circle representing a single individual. p values were calculated using the Mann–Whitney U test.
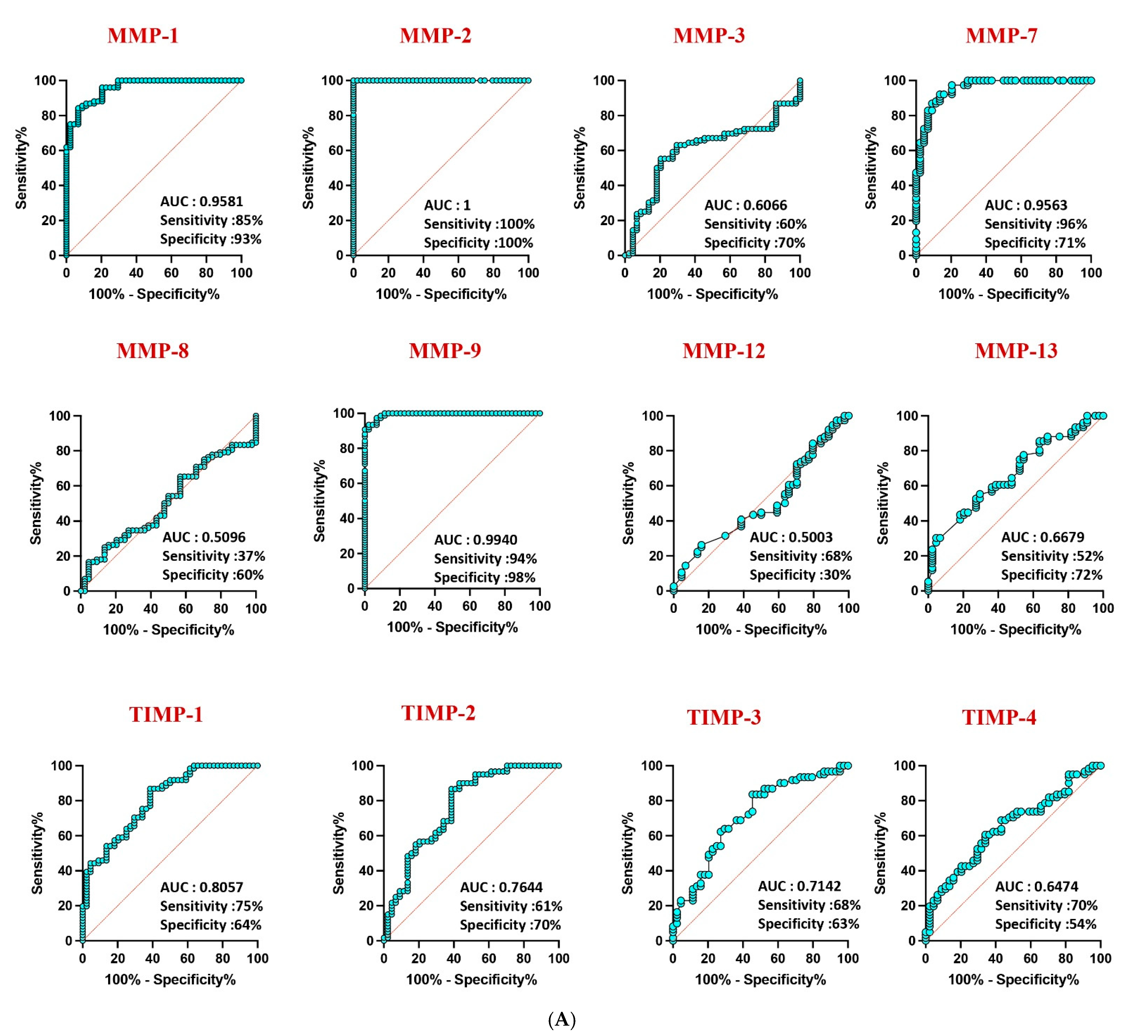
3.3. MMPs as Discriminatory Markers
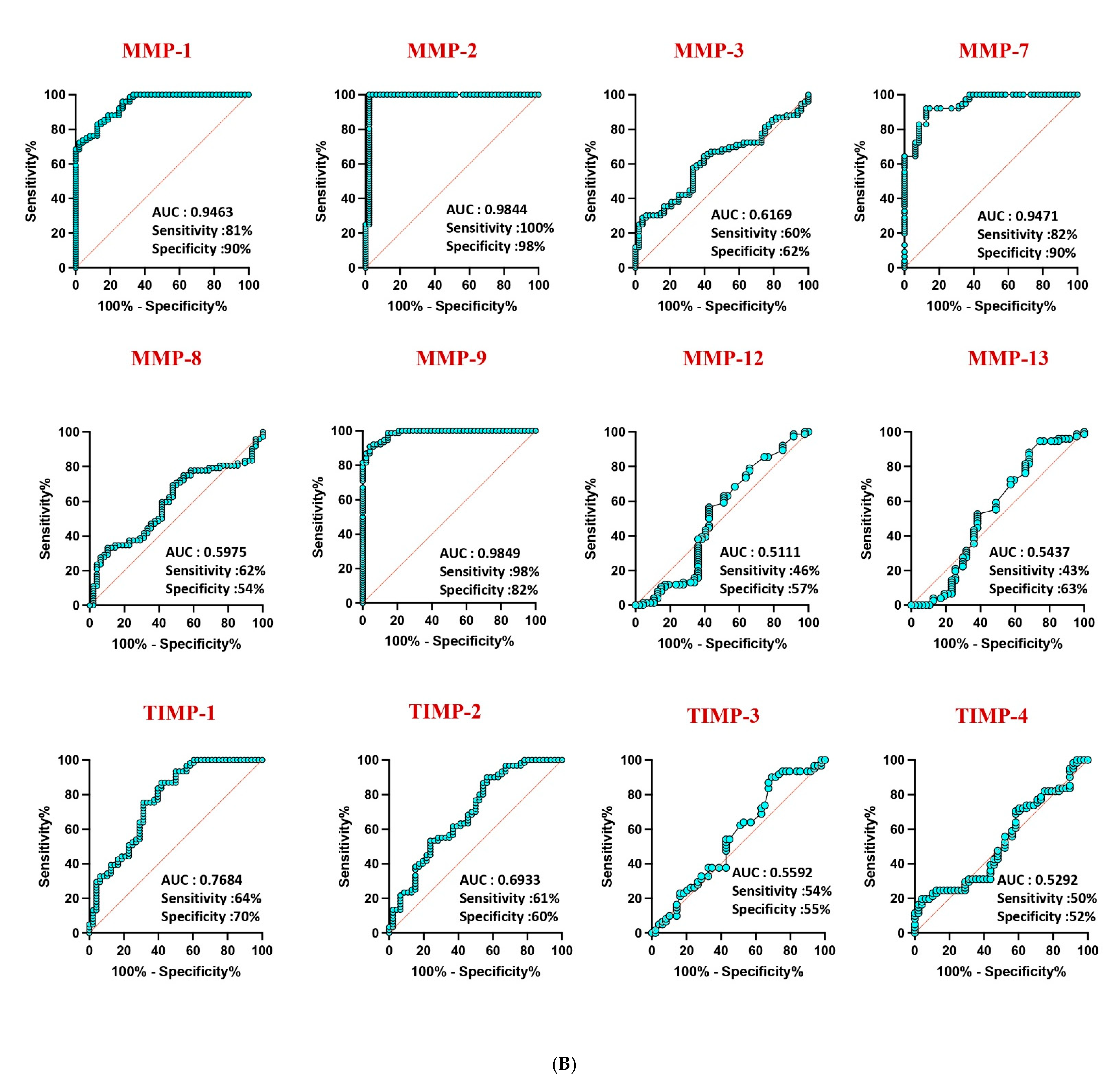
Receiver operating characteristic (ROC) analysis highlighted the robust discriminatory power of MMP-1 (AUC = 0.9581), MMP-2 (AUC = 1), and MMP-9 (AUC = 0.9940) in distinguishing confirmed TB from unlikely TB, with high area under the curve (AUC) values (Figure 4A). Similar discriminatory potential was observed for unconfirmed TB vs. unlikely TB. However, other MMPs and TIMPs exhibited weaker sensitivity and specificity, underscoring the differential diagnostic capacity of specific MMPs in pediatric TB (Figure 4B).
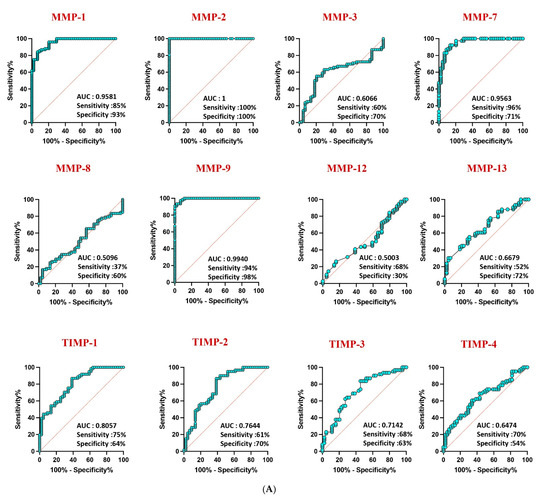
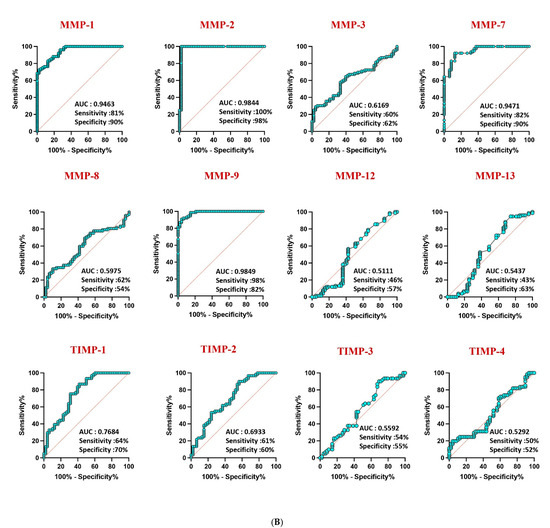
Figure 4.
ROC analysis to estimate the discriminatory power of MMP in children with active TB disease and unlikely TB. ROC analysis to estimate the sensitivity, specificity and AUC was performed using MMP 1, 2, 3, 7, 8, 9, 12, and 13 and TIMP 1, 2, 3, and 4 to estimate the capacity of these factors to distinguish individuals with (A) confirmed TB vs. unlikely TB and (B) unconfirmed TB vs. unlikely TB. ROC = receiver operator characteristics.
3.4. Enhanced Discrimination with Biomarker Combinations
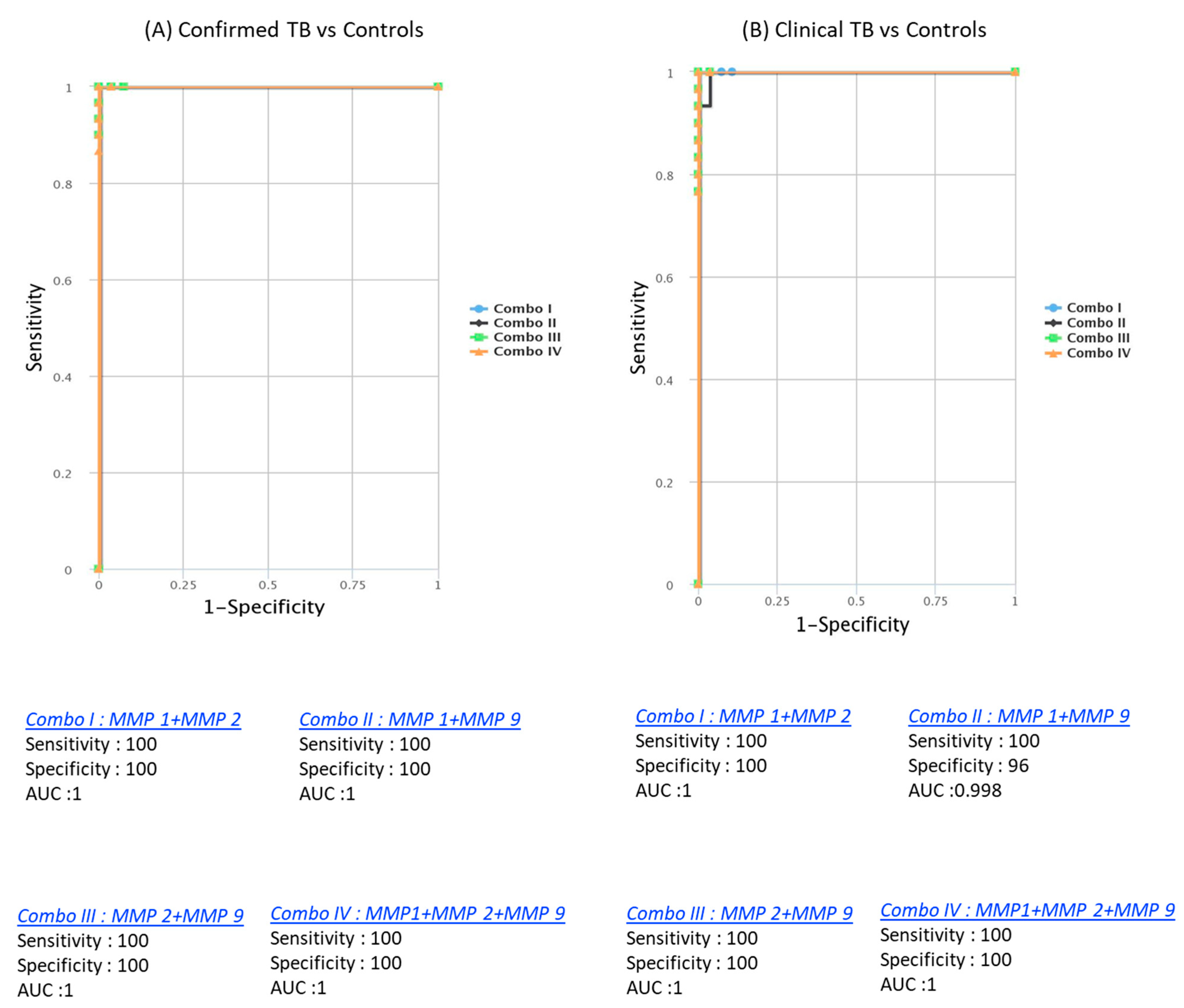
CombiROC analysis unveiled the significant improvement in discriminatory power with combinations of MMPs. Dual combinations, such as MMP1/MMP2 (AUC = 1, sensitivity = 100%, specificity = 100%), MMP1/MMP9 (AUC = 0.986, sensitivity = 97%, specificity = 90%), MMP2/MMP9 (AUC = 1, sensitivity = 100%, specificity = 100%), and triple combinations like MMP1/MMP2/MMP9 (AUC = 1, sensitivity = 100%, specificity = 100%), demonstrated outstanding predictive performance. These combinations achieved high AUC, sensitivity, and specificity in discriminating both confirmed from unlikely TB cases. Similarly, for discriminating both unconfirmed from unlikely TB cases, dual combinations like MMP1/MMP2 (AUC = 1, sensitivity = 100%, specificity = 100%), MMP1/MMP9 (AUC = 0.973, sensitivity = 94%, specificity = 90%), MMP2/MMP9 (AUC = 0.992, sensitivity = 97%, specificity = 100%), and triple combinations such as MMP1/MMP2/MMP9 (AUC = 1, sensitivity = 100%, specificity = 100%) were highly effective (Figure 5). These findings underscore the utility of a signature comprising two or three MMPs as accurate biomarkers for discriminating active TB disease in pediatric populations from controls, offering enhanced sensitivity and specificity.
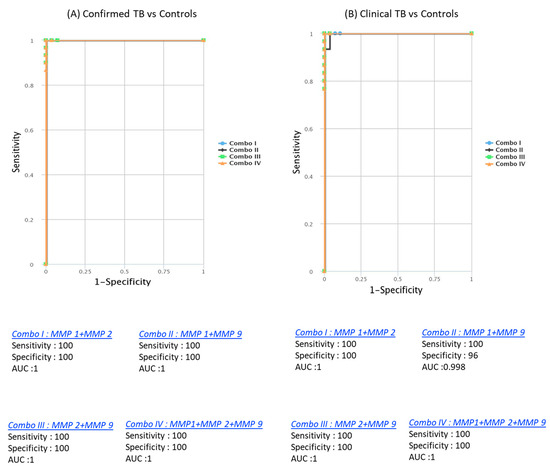
Figure 5.
Identification of biomarkers showing the strongest association using a combination of MMP biomarkers in active TB disease. CombiROC model analysis shows the chemokines with the highest accuracy in discriminating confirmed TB and unconfirmed TB disease from unlikely TB. ROC curves for comparing multiple markers and their combinations between confirmed TB and unconfirmed TB versus unlikely TB. (A) Confirmed TB vs. unlikely TB; (B) unconfirmed TB vs. unlikely TB are shown.
4. Discussion
Children and adolescents constitute a clinically significant demographic with heightened susceptibility to tuberculosis (TB) [14]. Pediatric TB manifests with a rapid progression from Mycobacterium tuberculosis infection to active disease, influenced by various factors including age, nutritional status, immune competence, genetic predisposition, and the severity of the initial infection. Despite contributing significantly to the overall TB burden, diagnosing TB in children poses considerable challenges due to low positivity rates in conventional diagnostic methods [15,16].
Current diagnostic approaches for pediatric TB rely on clinical symptoms, radiological findings, and the detection of M. tuberculosis in respiratory samples, gastric aspirates, or sputum [17]. However, these methods often fall short, particularly in resource-limited settings, highlighting the critical need for effective non-sputum-based point-of-care (POC) tests. Moreover, the swift progression from infection to disease in children underscores the urgency for early and accurate diagnostic modalities.
Previously, in 2013, our research identified elevated levels of MMP-1, MMP-7, MMP-8, TIMP-1, and TIMP-3 in pulmonary or extrapulmonary TB compared to controls [8]. This suggests that higher MMP levels in TB-infected children could potentially serve as biomarkers to distinguish individuals with TB disease from healthy controls. However, our earlier study had limitations, including a small sample size and the absence of children with latent TB or other pulmonary infections. In the current study, well-defined groups within the study cohort address these limitations.
Additionally, our research has explored combinations of MMP-7, C-reactive protein (CRP), and lipopolysaccharide-binding protein (LBP) as markers with high accuracy in discriminating between children with active TB and healthy controls [7]. Furthermore, recent findings from our group have highlighted baseline cytokine signatures (TNFα, IL-2, IL-17A) and chemokine signatures (CCL1/CXCL1/CXCL10) as potential accurate biomarkers for pediatric TB diagnosis [12,13]. These studies collectively underscore the ongoing efforts to identify reliable biomarkers and diagnostic tools to improve TB detection and management in pediatric populations.
The primary objective of this study was to assess the diagnostic utility of emerging immune biomarkers, specifically matrix metalloproteinases (MMPs), in pediatric tuberculosis (TB). Focusing on children with microbiologically confirmed TB, unconfirmed TB, and those unlikely to have TB, the study unveiled significantly elevated levels of MMPs (1, 2, 7, 9) and tissue inhibitors of metalloproteinases (TIMPs) in both confirmed and unconfirmed TB cases compared to controls. These findings suggest the promising potential of MMPs as blood-based biomarkers for distinguishing TB cases from those without the disease. Furthermore, the study demonstrated that combining different MMPs enhanced the discriminatory power, offering high sensitivity and specificity in distinguishing TB cases from controls. Particularly, the multi-biomarker panel comprising MMP-1/MMP-2, MMP-1/MMP-9, MMP-2/MMP-9, and MMP-1/MMP-2/MMP-9 showed robust results, exhibiting the highest values for sensitivity and specificity.
While some MMPs and TIMPs have been previously investigated in the immunopathology of pediatric TB, a comprehensive analysis of a wide panel of MMPs and TIMPs in confirmed TB, unconfirmed TB, and unlikely TB cases has not been undertaken until now. This study introduces a novel three-immune biosignature of MMPs capable of efficiently discriminating confirmed and unconfirmed TB from unlikely TB with good accuracy. Additionally, our findings suggest that regardless of diagnosis, host immune responses remain unaltered, indicating a consistent pattern across TB cases.
Expanding on our earlier report in 2013, which highlighted the elevation of MMP-1, MMP-7, MMP-8, TIMP-1, and TIMP-3 in pulmonary or extrapulmonary TB, this current study addresses previous limitations by incorporating a well-defined study cohort, including children with latent TB and other pulmonary infections [8]. Notably, combinations of MMP-7, C-reactive protein (CRP), and lipopolysaccharide-binding protein (LBP) emerged as markers with high accuracy in differentiating between children with active TB and healthy controls [7]. This underscores the pivotal role of MMPs as potent immune biomarkers for diagnosing pediatric TB.
In studies involving adult pulmonary tuberculosis (TB) patients, increased levels of matrix metalloproteinases (MMPs), including MMP-1, 2, 3, 7, 8, and 9, have been observed in various biological fluids such as sputum, pleural fluid, and bronchoalveolar lavage (BAL) fluids [18,19]. Additional investigations have reported elevated systemic levels of MMP-1 and MMP-19 in TB patients compared to controls [20,21]. Additionally, several published studies in adult populations have reported elevated MMP-9 levels in various respiratory infections, such as community-acquired pneumonia. In our cohort, to ensure that the responses are specific to TB disease, we used a comparative group of children with unlikely TB. These children visited the respiratory clinic for seasonal respiratory illnesses, helping to distinguish TB-specific responses from those associated with other respiratory conditions [22] (Yang et al., 2005). Furthermore, the severity of TB disease has been correlated with MMP levels, with comorbid conditions such as diabetes mellitus exacerbating clinical severity [9]. Recent findings from our group have further solidified the role of MMPs and tissue inhibitors of metalloproteinases (TIMPs) as correlates of risk and prognostic biomarkers for treatment failure, relapse, and death in individuals with pulmonary TB [10]. These data align with existing evidence, underscoring the association of elevated MMPs with disease severity and bacterial burden in TB. Collectively, MMPs emerge as pivotal contributors linked to the pathology of TB, making them promising candidates for biomarkers in pediatric TB diagnosis. Limitations of our study include a limited sample size, the absence of a validation cohort in a different geographical location, and the lack of inclusion of healthy control children. Additionally, the cost-effectiveness of this assay was also not addressed. Addressing these limitations in future research will be important for strengthening the validity and applicability of our conclusions.
The strengths of this study lie in the well-characterized participant groups and the inclusion of individuals deemed unlikely to have TB, enabling a robust comparison of MMP and TIMP responses among confirmed, unconfirmed, and improbable TB cases. While the findings are encouraging, further research in diverse endemic communities is warranted to validate MMPs as potential diagnostic tools for pediatric TB.
5. Conclusions
This study offers valuable insights into the potential of MMPs as immune biomarkers for pediatric TB diagnosis. The identification of reliable biomarkers holds promise for the development of effective, non-invasive diagnostic tools, addressing the unique challenges posed by TB in children.
Author Contributions
Designed the study (S.B. (Subash Babu), N.P.K., and S.H.); conducted experiments (N.P.K., A.N., and N.S.G.); acquired data (N.P.K. and K.T.); analyzed data (N.P.K. and K.T.); contributed reagents and also revised subsequent drafts of the manuscript (S.H., S.S., and S.B. (Subash Babu)); responsible for the enrolment of participants and also contributed to acquisition and interpretation of clinical data (V.V.B., S.B. (Sarath Balaji), S.E., J.G., M.A.A., and D.B.); wrote the manuscript (S.B. (Subash Babu) and N.P.K.). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID). This study was also partially supported by USAID, WHO, and ICMR under the Model DOTS project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
All individuals were examined as part of a clinical research protocol (NIRTIEC2012004) approved by the Institutional Ethics committees of the ICMR-National Institute for Research in Tuberculosis, Chennai, and all methods were performed in accordance with the relevant guidelines and regulations (approved on 7 May 2013). All legal guardians of the children included provided written informed consent.
Informed Consent Statement
All legal guardians of the included children provided written informed consent.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We thank the staff of the Department of Clinical Research, NIRT–A Gunasundari, Valarmathi Nagarajan, G Mangalambal, Shakila Shanker, A Stella Mary, R Selvi, J Jemima, Krishna Yadav, S Vaishnavi, R Supriya, S Bharathi, C Hema Giranab, S Gopika, A Seetha, Sravankumar Adavath, Mumthas Banu Kottayil, M Shanthi, M Vedavalli, Hema Latha, Dino Xavier, Guru K, Sasi Kumar, and A Kesavan for their valuable assistance in recruitment and follow-up of participants.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dye, C.; Scheele, S.; Dolin, P.; Pathania, V.; Raviglione, M.C. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999, 282, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Newton, S.M.; Brent, A.J.; Anderson, S.; Whittaker, E.; Kampmann, B. Paediatric tuberculosis. Lancet Infect. Dis. 2008, 8, 498–510. [Google Scholar] [CrossRef]
- Boehme, C.C.; Nicol, M.P.; Nabeta, P.; Michael, J.S.; Gotuzzo, E.; Tahirli, R.; Gler, M.T.; Blakemore, R.; Worodria, W.; Gray, C.; et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: A multicentre implementation study. Lancet 2011, 377, 1495–1505. [Google Scholar] [CrossRef]
- Craig, V.J.; Zhang, L.; Hagood, J.S.; Owen, C.A. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2015, 53, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Hattle, J.M.; Dreitz, S.A.; Troudt, J.M.; Izzo, L.S.; Basaraba, R.J.; Orme, I.M.; Matrisian, L.M.; Izzo, A.A. Role for matrix metalloproteinase 9 in granuloma formation during pulmonary Mycobacterium tuberculosis infection. Infect. Immun. 2006, 74, 6135–6144. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta BBA Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Albuquerque, V.V.; Kumar, N.P.; Fukutani, K.F.; Vasconcelos, B.; Arriaga, M.B.; Silveira-Mattos, P.S.; Babu, S.; Andrade, B.B. Plasma levels of C-reactive protein, matrix metalloproteinase-7 and lipo-polysaccharide-binding protein distinguish active pulmonary or extrapulmonary tuberculosis from uninfected controls in children. Cytokine 2019, 123, 154773. [Google Scholar] [CrossRef]
- Kumar, N.P.; Anuradha, R.; Andrade, B.B.; Suresh, N.; Ganesh, R.; Shankar, J.; Kumaraswami, V.; Nutman, T.B.; Babu, S. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in Children. Clin. Vaccine Immunol. 2013, 20, 704–711. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Viswanathan, V.; Shruthi, B.S.; Sivakumar, S.; Menon, P.A.; Kornfeld, H.; Babu, S. Elevated levels of matrix metalloproteinases reflect severity and extent of disease in tuberculosis-diabetes co-morbidity and are predominantly reversed following standard anti-tuberculosis or metformin treatment. BMC Infect. Dis. 2018, 18, 345. [Google Scholar] [CrossRef]
- Kumar, N.P.; Moideen, K.; Nancy, A.; Viswanathan, V.; Thiruvengadam, K.; Sivakumar, S.; Hissar, S.; Nair, D.; Banurekha, V.V.; Kornfeld, H.; et al. Association of Plasma Matrix Metalloproteinase and Tissue Inhibitors of Matrix Metalloproteinase Levels With Adverse Treatment Outcomes Among Patients With Pulmonary Tuberculosis. JAMA Netw. Open 2020, 3, e2027754. [Google Scholar] [CrossRef]
- Graham, S.M.; Cuevas, L.E.; Jean-Philippe, P.; Browning, R.; Casenghi, M.; Detjen, A.K.; Gnanashanmugam, D.; Hesseling, A.C.; Kampmann, B.; Mandalakas, A.; et al. Clinical Case Definitions for Classification of Intrathoracic Tuberculosis in Chil-dren: An Update. Clin. Infect. Dis. 2015, 61 (Suppl. S3), S179–S187. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.P.; Hissar, S.; Thiruvengadam, K.; Banurekha, V.V.; Balaji, S.; Elilarasi, S.; Gomathi, N.S.; Ganesh, J.; Aravind, M.A.; Baskaran, D.; et al. Plasma chemokines as immune biomarkers for diagnosis of pediatric tuberculosis. BMC Infect. Dis. 2021, 21, 1055. [Google Scholar] [CrossRef]
- Kumar, N.P.; Hissar, S.; Thiruvengadam, K.; Banurekha, V.V.; Suresh, N.; Shankar, J.; Elilarasi, S.; Gomathi, N.S.; Kalpana, S.; Ganesh, J.; et al. Discovery and Validation of a Three-Cytokine Plasma Signature as a Biomarker for Diagnosis of Pediatric Tuberculosis. Front. Immunol. 2021, 12, 653898. [Google Scholar] [CrossRef] [PubMed]
- Dodd, P.J.; Sismanidis, C.; A Seddon, J. Global burden of drug-resistant tuberculosis in children: A mathematical modelling study. Lancet Infect. Dis. 2016, 16, 1193–1201. [Google Scholar] [CrossRef]
- Chiang, S.S.; Khan, F.A.; Milstein, M.B.; Tolman, A.W.; Benedetti, A.; Starke, J.R.; Becerra, M.C. Treatment outcomes of childhood tuberculous meningitis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 947–957. [Google Scholar] [CrossRef]
- Marais, B.J.; Gie, R.P.; Schaaf, H.S.; Hesseling, A.C.; Obihara, C.C.; Starke, J.J.; A Enarson, D.; Donald, P.R.; Beyers, N. The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era. Int. J. Tuberc. Lung Dis. 2004, 8, 392–402. [Google Scholar] [PubMed]
- Parrish, N.M.; Carroll, K.C. Role of the clinical mycobacteriology laboratory in diagnosis and management of tuberculosis in low-prevalence settings. J. Clin. Microbiol. 2011, 49, 772–776. [Google Scholar] [CrossRef]
- Ugarte-Gil, C.A.; Elkington, P.; Gilman, R.H.; Coronel, J.; Tezera, L.B.; Bernabe-Ortiz, A.; Gotuzzo, E.; Friedland, J.S.; Moore, D.A.J. Induced Sputum MMP-1, -3 & -8 Concentrations during Treatment of Tuberculosis. PLoS ONE 2013, 8, e61333. [Google Scholar] [CrossRef]
- Hoheisel, G.; Sack, U.; Hui, D.S.; Huse, K.; Chan, K.S.; Chan, K.K.; Hartwig, K.; Schuster, E.; Scholz, G.H.; Schauer, J. Occurrence of matrix metalloproteinases and tissue inhibitors of metalloproteinases in tu-berculous pleuritis. Tuberculosis 2001, 81, 203–209. [Google Scholar] [CrossRef]
- Hrabec, E.; Strek, M.; Zieba, M.; Kwiatkowska, S.; Hrabec, Z. Circulation level of matrix metalloproteinase-9 is correlated with disease severity in tuberculosis patients. Int. J. Tuberc. Lung Dis. 2002, 6, 713–719. [Google Scholar]
- Thuong, N.T.; Dunstan, S.J.; Chau, T.T.; Thorsson, V.; Simmons, C.P.; Quyen, N.T.; Thwaites, G.E.; Thi Ngoc Lan, N.; Hibberd, M.; Teo, Y.Y.; et al. Identification of tuberculosis susceptibility genes with human macrophage gene ex-pression profiles. PLoS Pathog. 2008, 4, e1000229. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Chu, S.-C.; Chiang, I.-C.; Kuo, W.-F.; Chiou, H.-L.; Chou, F.-P.; Kuo, W.-H.; Hsieh, Y.-S. Excessive matrix metalloproteinase-9 in the plasma of community-acquired pneumonia. Clin. Chim. Acta 2005, 352, 209–215. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).