Trends and Outcomes of TAVR: An Analysis Using the National Inpatient Sample and Readmissions Database
Abstract
1. Introduction
2. Methods
3. Results
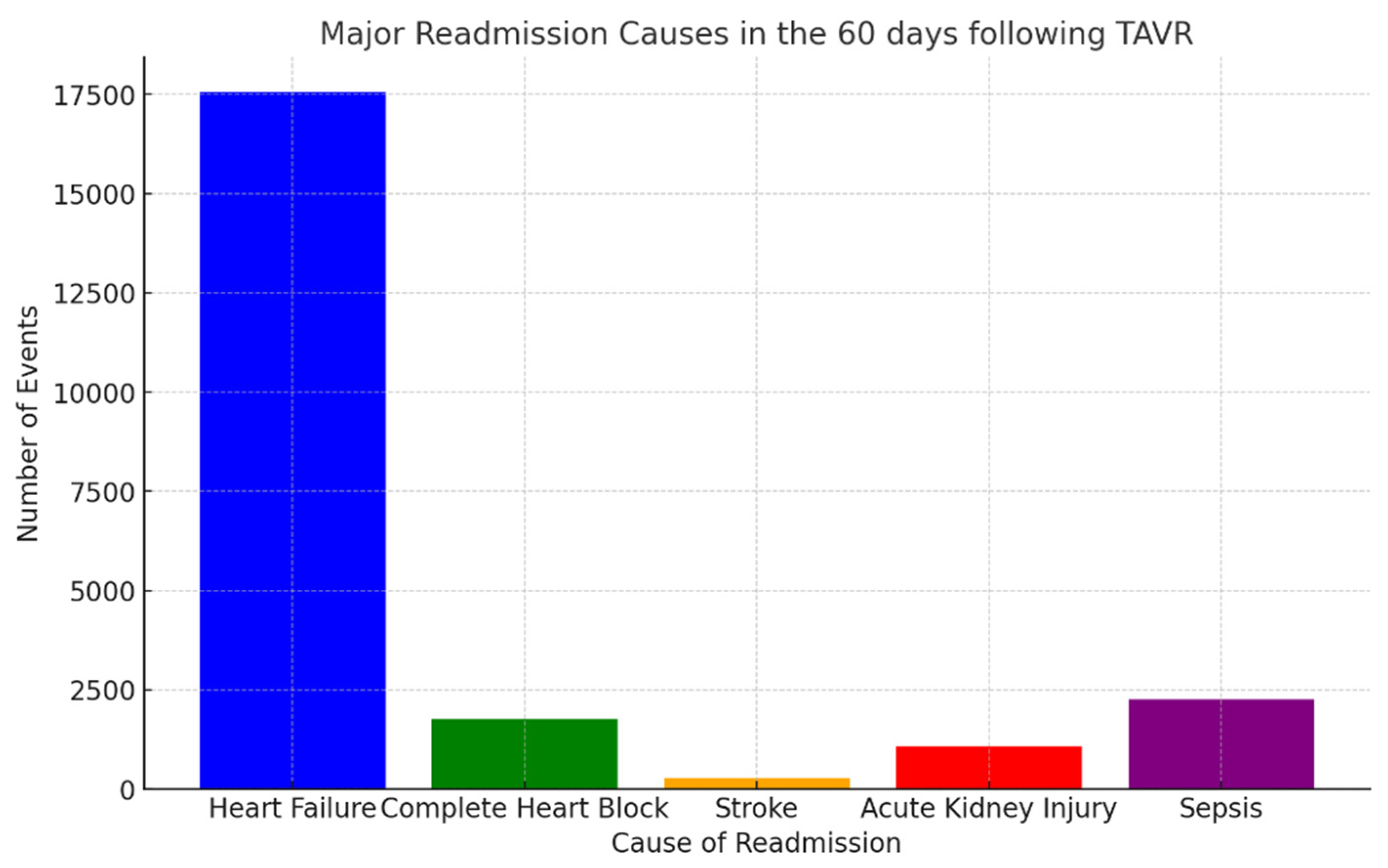
4. Day Readmission Analysis After TAVR Admissions
- Heart failure: 17,566 events (26.57%);
- Complete heart block: 1760 events (2.66%);
- Stroke: 284 events (0.42%);
- Acute kidney injury: 1079 events (1.63%);
- Sepsis: 2256 events (3.41%).
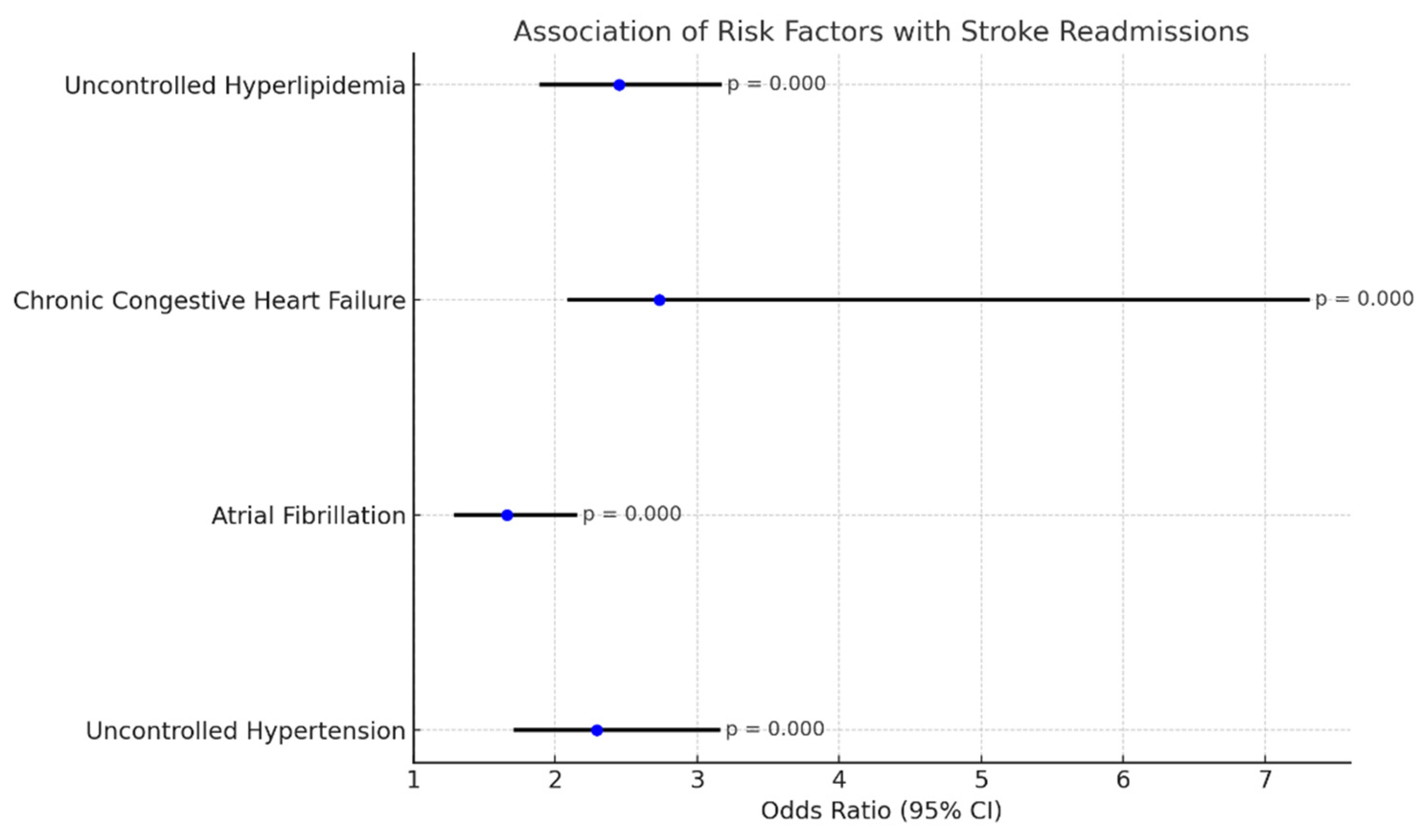
4.1. Stroke Following TAVR
- Uncontrolled hypertension: OR 2.29 (1.72–3.15), p-value: 0.000;
- Atrial fibrillation: OR 1.66 (1.30–2.14), p-value 0.000;
- Chronic congestive heart failure: OR 2.73 (2.1–7.3), p: 0.000;
- Uncontrolled hyperlipidemia: OR 2.45 (1.90–3.16), p-value: 0.000.
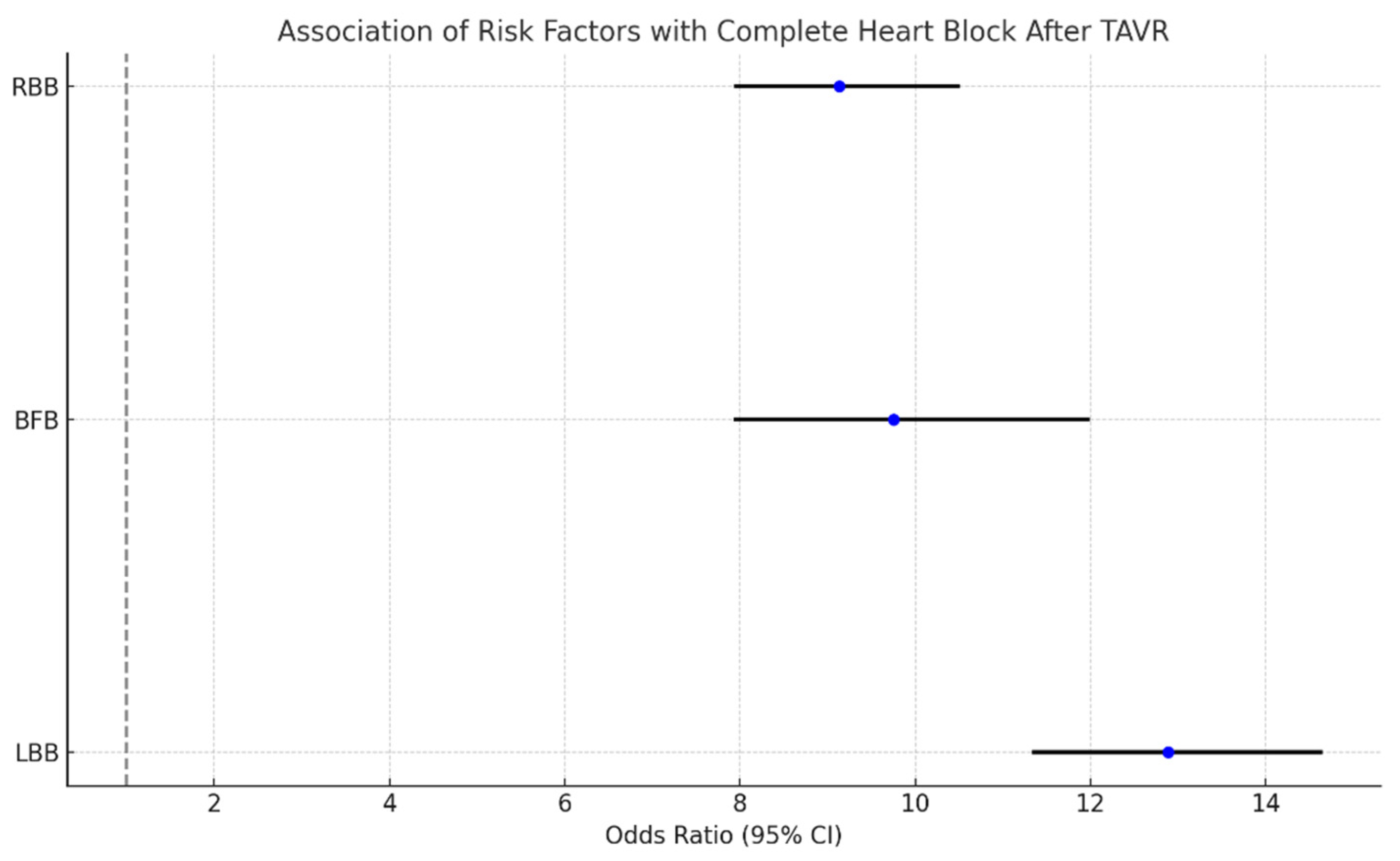
4.2. Complete Heart Block Following TAVR
- LBB: 21.83% (18.06–21.14);
- RBB: 17.36% (13.96–21.40);
- Fascicular block: 6.69% (4.62–9.60).
- LBB: OR 12.89 (11.36–14.63), p-value: 0.008;
- BFB: OR: 9.75 (7.95–11.97), p-value: 0.019;
- RBB: OR: 9.13 (7.96–10.49), p-value: 0.006.
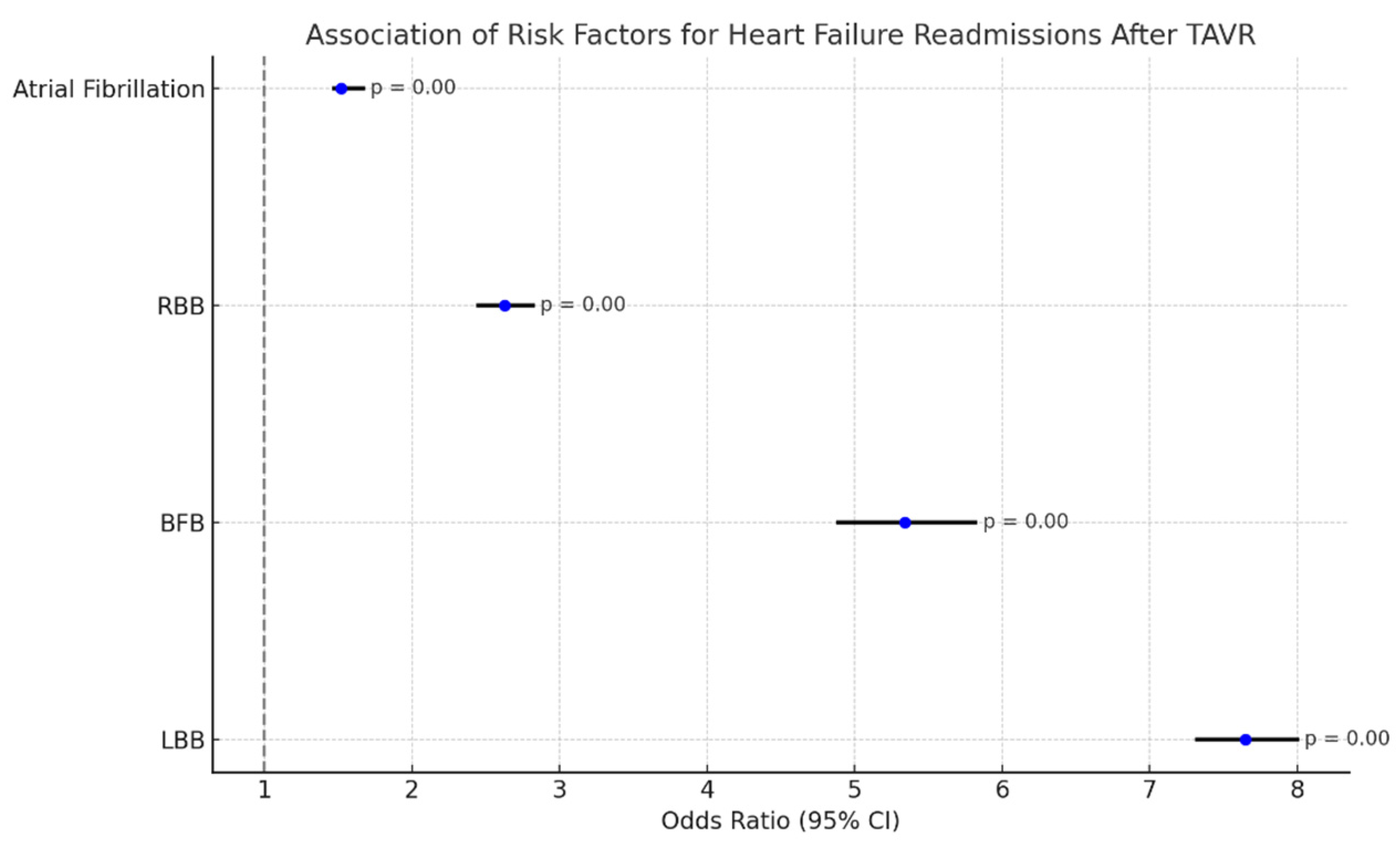
4.3. Heart Failure Readmissions Following TAVR
- LBB: OR: 7.65 (7.32–8.00), p-value: 0.000;
- BFB: OR: 5.34 (4.89–5.82), p-value: 0.000;
- RBB: OR: 2.63 (2.45–2.82), p-value: 0.000;
- Atrial fibrillation: 1.52 (1.47–1.67), p-value: 0.000.
5. Discussion
6. Conclusions
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogers, T.; Thourani, V.H.; Waksman, R. Transcatheter aortic valve replacement in intermediate- and low-risk patients. J. Am. Heart Assoc. 2018, 7, e007147. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, M.; Jabri, A.; Khalefa, B.B.; Al-Abdouh, A.; Madanat, L.; Albandak, M.; Alhuneafat, L.; Sukhon, F.; Shahrori, Z.; Mourid, M.R.; et al. Efficacy and safety of TAVR versus SAVR in patients with small aortic annuli: A systematic review and meta-analysis. Int. J. Cardiol. 2024, 411, 132243. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Vavalle, J.P. Transcatheter aortic valve replacement in intermediate and low risk patients—Clinical evidence. Ann. Cardiothorac. Surg. 2017, 6, 493–497. [Google Scholar] [CrossRef]
- Zou, Q.; Wei, Z.; Sun, S. Complications in transcatheter aortic valve replacement: A comprehensive analysis and management strategies. Curr. Probl. Cardiol. 2024, 49, 102478. [Google Scholar] [CrossRef] [PubMed]
- Mesnier, J.; Panagides, V.; Nuche, J.; Rodés-Cabau, J. Evolving indications of transcatheter aortic valve replacement—Where are we now, and where are we going. J. Clin. Med. 2022, 11, 3090. [Google Scholar] [CrossRef]
- Kermanshahchi, J.; Thind, B.; Davoodpour, G.; Hirsch, M.; Chen, J.; Reddy, A.J.; Yu, Z.; Falkenstein, B.E.; Javidi, D. Transcatheter aortic valve replacement (TAVR) versus surgical aortic valve replacement (SAVR): A review on the length of stay, cost, comorbidities, and procedural complications. Cureus 2024, 16, e54435. [Google Scholar] [CrossRef]
- Rouleau, S.G.; Brady, W.J.; Koyfman, A.; Long, B. Transcatheter aortic valve replacement complications: A narrative review for emergency clinicians. Am. J. Emerg. Med. 2022, 56, 77–86. [Google Scholar] [CrossRef]
- Eschenbach, L.K.; Erlebach, M.; Deutsch, M.; Ruge, H.; Bleiziffer, S.; Holzer, L.; Krane, M.; Voss, S.; Lange, R.; Burri, M. Stroke after transcatheter aortic valve replacement: A severe complication with low predictability. Catheter. Cardiovasc. Interv. 2022, 99, 1897–1905. [Google Scholar] [CrossRef]
- Wolfrum, M.; Handerer, I.J.; Moccetti, F.; Schmeisser, A.; Braun-Dullaeus, R.C.; Toggweiler, S. Cerebral embolic protection during transcatheter aortic valve replacement: A systematic review and meta-analysis of propensity score matched and randomized controlled trials using the Sentinel cerebral embolic protection device. BMC Cardiovasc. Disord. 2023, 23, 306. [Google Scholar] [CrossRef]
- Weber, M.; Sinning, J.M.; Hammerstingl, C.; Werner, N.; Grube, E.; Nickenig, G. Permanent pacemaker implantation after TAVR—Predictors and impact on outcomes. Interv. Cardiol. 2015, 10, 98–102. [Google Scholar] [CrossRef]
- Markham, R.; Sharma, R. A review of the Partner Trials. Interv. Cardiol. Clin. 2020, 9, 461–467. [Google Scholar] [CrossRef]
- Auffret, V.; Bakhti, A.; Leurent, G.; Bedossa, M.; Tomasi, J.; Soulami, R.B.; Verhoye, J.-P.; Donal, E.; Galli, E.; Loirat, A.; et al. Determinants and impact of heart failure readmission following transcatheter aortic valve replacement. Circ. Cardiovasc. Interv. 2020, 13, e008959. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71, Erratum in Circulation 2021, 143, e228; Erratum in Circulation 2021, 143, e784. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.V.; Manandhar, P.; Vemulapalli, S.; Kosinski, A.S.; Batchelor, W.B.; Thourani, V.H.; Mack, M.J.; Cohen, D.J. Trends in Transcatheter Aortic Valve Replacement Outcomes: Insights From the STS/ACC TVT Registry. JAMA Cardiol. 2024, 9, 1115–1123. [Google Scholar] [CrossRef]
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef]
- Mauri, V.; Abdel-Wahab, M.; Bleiziffer, S.; Veulemans, V.; Sedaghat, A.; Adam, M.; Nickenig, G.; Kelm, M.; Thiele, H.; Baldus, S.; et al. Temporal trends of TAVI treatment characteristics in high volume centers in Germany 2013–2020. Clin. Res. Cardiol. 2022, 111, 881–888. [Google Scholar] [CrossRef]
- Didier, R.; Le Breton, H.; Eltchaninoff, H.; Cayla, G.; Commeau, P.; Collet, J.-P.; Cuisset, T.; Dumonteil, N.; Verhoye, J.-P.; Beurtheret, S.; et al. Evolution of TAVI patients and techniques over the past decade: The French TAVI registries. Arch. Cardiovasc. Dis. 2022, 115, 206–213. [Google Scholar] [CrossRef]
- Graversen, P.L.; Butt, J.H.; Østergaard, L.; Jensen, A.D.; Warming, P.E.; Strange, J.E.; Møller, C.H.; Schou, M.; De Backer, O.; Køber, L.; et al. Changes in aortic valve replacement procedures in Denmark from 2008 to 2020. Heart 2023, 109, 557–563. [Google Scholar] [CrossRef]
- Stortecky, S.; Franzone, A.; Heg, D.; Tueller, D.; Noble, S.; Pilgrim, T.; Jeger, R.; Toggweiler, S.; Ferrari, E.; Nietlispach, F.; et al. Temporal trends in adoption and outcomes of transcatheter aortic valve implantation: A SwissTAVI Registry analysis. Eur. Heart J. Qual. Care Clin. Outcomes 2019, 5, 242–251. [Google Scholar] [CrossRef]
- Landes, U.; Barsheshet, A.; Finkelstein, A.; Guetta, V.; Assali, A.; Halkin, A.; Vaknin-Assa, H.; Segev, A.; Bental, T.; Ben-Shoshan, J.; et al. Temporal trends in transcatheter aortic valve implantation, 2008-2014: Patient characteristics, procedural issues, and clinical outcome. Clin. Cardiol. 2017, 40, 82–88. [Google Scholar] [CrossRef]
- Frydman, S.; Zahler, D.; Merdler, I.; Freund, O.; Shacham, Y.; Banai, S.; Finkelstein, A.; Steinvil, A. Temporal trends of transcatheter aortic valve implantation over 12 years: A high-volume single-center experience. J. Clin. Med. 2022, 11, 4962. [Google Scholar] [CrossRef]
- Davlouros, P.A.; Mplani, V.C.; Koniari, I.; Tsigkas, G.; Hahalis, G. Transcatheter aortic valve replacement and stroke: A comprehensive review. J. Geriatr. Cardiol. 2018, 15, 95. [Google Scholar] [CrossRef]
- Okuno, T.; Alaour, B.; Heg, D.; Tueller, D.; Pilgrim, T.; Muller, O.; Noble, S.; Jeger, R.; Reuthebuch, O.; Toggweiler, S.; et al. Long-term risk of stroke after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2023, 16, 2986. [Google Scholar] [CrossRef]
- Mastoris, I.; Schoos, M.M.; Dangas, G.D.; Mehran, R. Stroke after transcatheter aortic valve replacement: Incidence, risk factors, prognosis, and preventive strategies. Clin. Cardiol. 2014, 37, 756. [Google Scholar] [CrossRef]
- Lilly, S.M.; Deshmukh, A.J.; Epstein, A.E.; Ricciardi, M.J.; Shreenivas, S.; Velagapudi, P.; Wyman, J.F. 2020 ACC expert consensus decision pathway on management of conduction disturbances in patients undergoing transcatheter aortic valve replacement. J. Am. Coll. Cardiol. 2020, 76, 2391–2411. [Google Scholar] [CrossRef]
- Nazif, T.M.; Dizon, J.M.; Hahn, R.T.; Xu, K.; Babaliaros, V.; Douglas, P.S.; El-Chami, M.F.; Herrmann, H.C.; Mack, M.; Makkar, R.R.; et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2015, 8, 60–69. [Google Scholar] [CrossRef]
- Kiani, S.; Kamioka, N.; Black, G.B.; Lu, M.L.R.; Lisko, J.C.; Rao, B.; Mengistu, A.; Gleason, P.T.; Stewart, J.P.; Caughron, H.; et al. Development of a risk score to predict new pacemaker implantation after transcatheter aortic valve replacement. JACC Cardiovasc. Interv. 2019, 12, 2133–2142. [Google Scholar] [CrossRef]
- Auffret, V.; Puri, R.; Urena, M.; Chamandi, C.; Rodriguez-Gabella, T.; Philippon, F.; Rodés-Cabau, J. Conduction disturbances after transcatheter aortic valve replacement: Current status and future perspectives. Circulation 2017, 136, 1049–1069. [Google Scholar] [CrossRef]
- Franzone, A.; Pilgrim, T.; Arnold, N.; Heg, D.; Langhammer, B.; Piccolo, R.; Roost, E.; Praz, F.; Räber, L.; Valgimigli, M.; et al. Rates and predictors of hospital readmission after transcatheter aortic valve implantation. Eur. Heart J. 2017, 38, 2211–2217. [Google Scholar] [CrossRef] [PubMed]
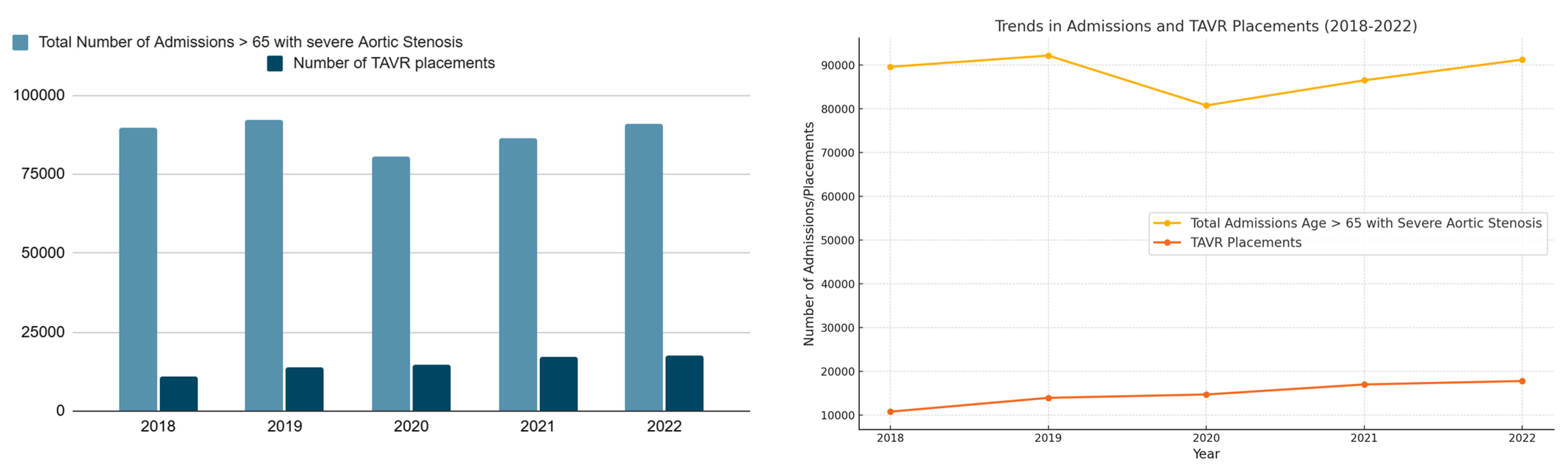
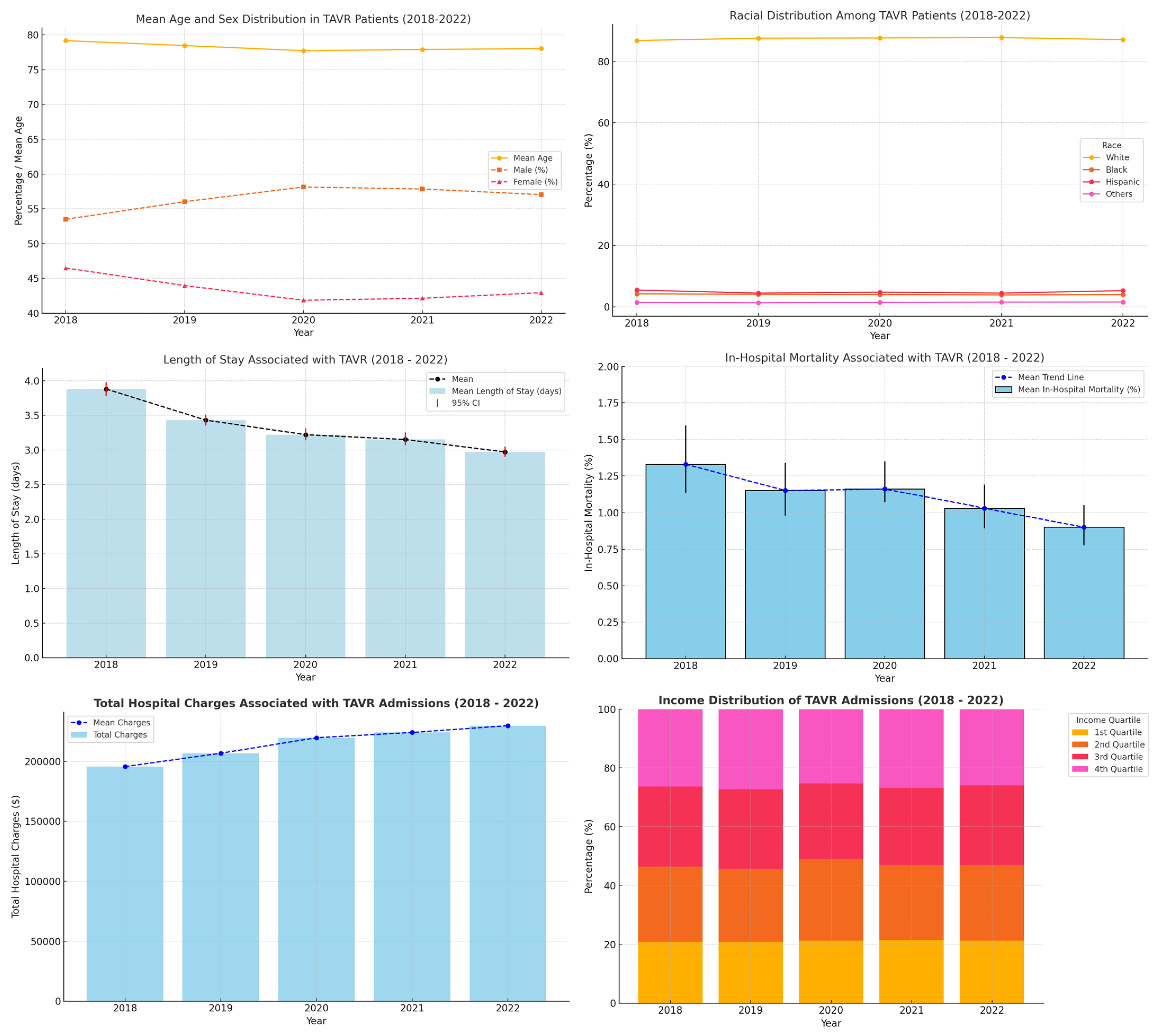

| 2018 | 2019 | 2020 | 2021 | 2022 | ||
|---|---|---|---|---|---|---|
| Total number of admissions with aortic stenosis (Age > 65) | 89,548 | 92,111 | 80,727 | 86,466 | 91,190 | |
| Total number of TAVR placements | 10,788 | 13,944 | 14,708 | 17,001 | 17,784 | |
| Mean age | 79.18 (79.02–79.34) | 78.47 (78.32–78.61) | 77.74 (77.60–77.88) | 77.91 (77.79–78.04) | 78.04 (77.92–78.16) | |
| Sex (%) | Male: | 53.50 (5717/10,788) | 56.03 (7808/13,944) | 58.14 (8530/14,708) | 57.85 (9690/17,001) | 57.05 (10,136/17,784) |
| Female: | 46.50 (4962/10,788) | 43.97 (5995/13,944) | 41.86 (6030/14,708) | 42.15 (7140/17,001) | 42.95 (7469/17,784) | |
| Mean hospital length of stay (days) | 3.88 (3.78–3.98) | 3.43 (3.35–3.51) | 3.22 (3.14–3.31) | 3.15 (3.07–3.25) | 2.97 (2.90–3.050) | |
| Mean of total hospital charges (USD) | 19,5643.8 | 20,6781.4 | 219,738.0 | 224,105.6 | 229,794.6 | |
| In-hospital mortality (%) | 1.33% (1.134–1.596%) (143/10,788) | 1.15% (0.98–1.34%) (160/13,944) | 1.16% (1.07–1.35%) (170/14,708) | 1.03% (0.893–1.19%) (175/17,001) | 0.90% (0.776–1.05%) (160/17,784) | |
| Race (%) | White | 86.83 (9277) | 87.59 (12,131) | 87.68 (12,795) | 87.80 (14,790) | 87.11 (15,472) |
| Black | 4.16 (448) | 4.06 (566) | 3.96 (582) | 3.81 (647) | 3.92 (697) | |
| Hispanic | 5.44 (586) | 4.43 (617) | 4.74 (697) | 4.43 (731) | 5.25 (933) | |
| Others | 1.38 | 1.25 | 1.40 | 1.49 | 1.52 | |
| AP DRG Mortality Index category (%) | Minor likelihood of dying | 7.43 | 15.18 | 16.28 | 18.41 | 19.74 |
| Moderate likelihood of dying | 44.61 | 48.31 | 47.51 | 48.71 | 48.89 | |
| Major likelihood of dying | 37.97 | 28.28 | 32.34 | 25.26 | 24.21 | |
| Extreme likelihood of dying | 9.98 | 8.23 | 10.23 | 7.62 | 7.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varughese, V.J.; Nagesh, V.K.; Tran, H.H.-V.; Yessin, O.; Jha, H.; Mason, A.; Thu, A.; Weissman, S.; Atoot, A. Trends and Outcomes of TAVR: An Analysis Using the National Inpatient Sample and Readmissions Database. Diseases 2025, 13, 149. https://doi.org/10.3390/diseases13050149
Varughese VJ, Nagesh VK, Tran HH-V, Yessin O, Jha H, Mason A, Thu A, Weissman S, Atoot A. Trends and Outcomes of TAVR: An Analysis Using the National Inpatient Sample and Readmissions Database. Diseases. 2025; 13(5):149. https://doi.org/10.3390/diseases13050149
Chicago/Turabian StyleVarughese, Vivek Joseph, Vignesh Krishnan Nagesh, Hadrian Hoang-Vu Tran, Olivia Yessin, Harsh Jha, Ashley Mason, Audrey Thu, Simcha Weissman, and Adam Atoot. 2025. "Trends and Outcomes of TAVR: An Analysis Using the National Inpatient Sample and Readmissions Database" Diseases 13, no. 5: 149. https://doi.org/10.3390/diseases13050149
APA StyleVarughese, V. J., Nagesh, V. K., Tran, H. H.-V., Yessin, O., Jha, H., Mason, A., Thu, A., Weissman, S., & Atoot, A. (2025). Trends and Outcomes of TAVR: An Analysis Using the National Inpatient Sample and Readmissions Database. Diseases, 13(5), 149. https://doi.org/10.3390/diseases13050149





