Abstract
Background: Almost all patients undergoing dialysis develop renal anemia and receive medicines such as erythropoietin and iron preparations. However, the conventional intravenous treatment with saccharated ferric oxide (SFO) is insufficient for these patients when they have incurable and persistent iron deficiency anemia due to secondary amyloidosis. Therefore, we administered 500 mg of ferric carboxymaltose (FCM) to such a patient with Crohn’s disease. Case presentation: A 56-year-old man on maintenance hemodialysis had secondary amyloidosis due to Crohn’s disease. Additionally, he was anemic and received 40 mg of SFO weekly; however, his hemoglobin (Hb) level remained low at 7 g/dL. Therefore, 500 mg of FCM was administered bimonthly from the first to the fourth dose, and the Hb level temporarily increased compared to that after the previous SFO administration. Since bimonthly administration did not adequately maintain the Hb level, FCM was administered monthly from the 5th to 12th dose, which stabilized the Hb level at 10–12 g/dL. No side effects, such as hypophosphatemia, were observed. Conclusions: A single dose of 500 mg FCM administered once every 1–2 months stabilizes the Hb level and contributes to efficient iron utilization in patients with incurable anemia undergoing hemodialysis.
1. Background
Although the proportion of patients with secondary amyloidosis undergoing maintenance hemodialysis is low, their prognosis is generally poor due to frequent occurrence of multiorgan damage including renal failure [1,2]. Secondary amyloidosis often develops after chronic inflammatory diseases such as rheumatoid arthritis and tuberculosis; however, Crohn’s disease is another important cause [3,4,5]. Inflammatory bowel diseases such as Crohn’s disease tend to cause iron-deficiency anemia, and such patients, with chronic kidney diseases, should be treated with intravenous iron preparations and erythropoietin [6].
Here, we report the case of a patient with secondary amyloidosis due to Crohn’s disease who was undergoing maintenance hemodialysis. The patient received 40 mg of SFO intravenously for anemia every week. However, since his Hb level gradually decreased, he received intravenous injections of FCM, which dramatically improved his condition as his Hb level increased.
2. Case Presentation
2.1. Patient
A 56-year-old Japanese man was diagnosed with Crohn’s disease at the age of 22 and was undergoing follow-ups with his family doctor. However, at 39 years, he had worsening abdominal pain, and he was diagnosed with amyloidosis based on a biopsy of the small intestinal mucosa. In the same year, amyloidosis led to renal dysfunction. At 41 years, he experienced anal bleeding and developed end-stage renal disease requiring hemodialysis. Since the age of 53 years, he has presented intermittent melena. Additionally, despite continuously receiving weekly intravenous injections of darbepoetin at 60 µg and SFO at 40 mg, his anemia worsened to a Hb level of 7.1 g/dL. Moreover, there was a decrease in the serum iron level (18 µg/dL), TSAT (4.9%), and serum ferritin level (23.2 ng/mL). Blood counts and biochemical test results are shown in Table 1.

Table 1.
Results of blood examination before the administration of FCM.
After the patient started dialysis, he underwent monthly chest X-rays and electrocardiograms and annual echocardiograms. However, the cardiothoraic ratio was less than 50%, the electrocardiogram did not reveal cardiomegaly, and there was no evidence of arrhythmia or ischemic heart disease. Furthermore, the echocardiogram did not reveal any valvular heart disease or wall motion abnormalities.
2.2. Clinical Course
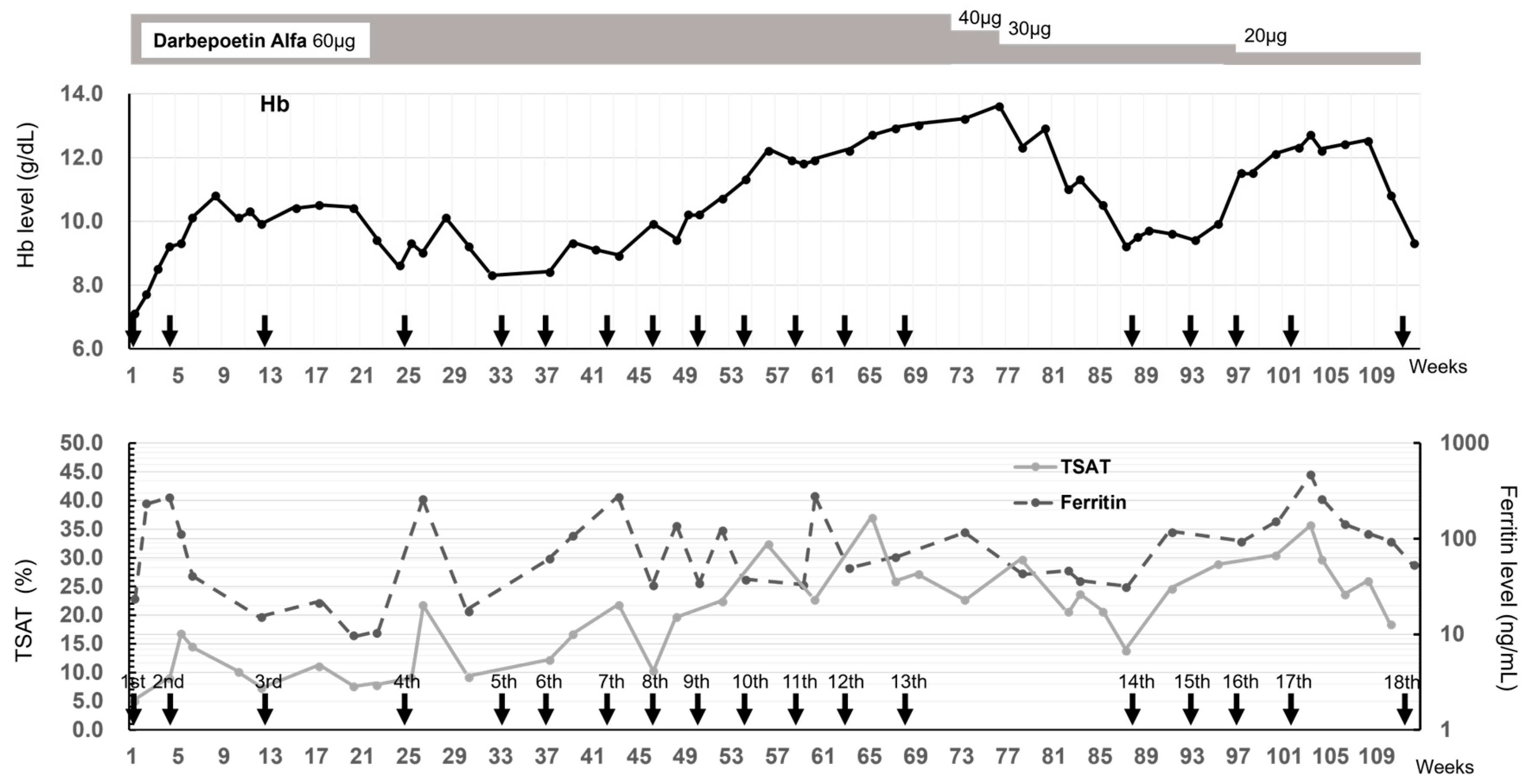
Given the low levels of all anemia-related blood parameters and severe iron-deficiency anemia, blood transfusions were administered several times. But the effects were temporary, and it was deemed difficult to continue the transfusion because irregular antibodies were detected. Finally, intravenous injections of 500 mg of FCM were initiated at the Department of Gastroenterology, Fukuoka University Hospital, while hemodialysis was performed three times a week at the Sanko Clinic (Figure 1). Follow-up blood tests after the first administration of FCM showed that the Hb level gradually increased from 7 g/dL to 9 g/dL, which temporarily exceeded 10 g/dL after the second FCM administration. However, when the FCM administration interval was increased to ≥8 weeks, the TSAT remained low at 10% until the 24th week before the 4th dose of FCM, the serum ferritin levels fluctuated greatly and were unstable, and the Hb level reduced to approximately 8 g/dL. Accordingly, the FCM administration interval was shortened to 4 weeks, and the Hb level increased again. After the 13th administration, the Hb level was high at 13.6 g/dL; therefore, the FCM withdrawal period was 20 weeks, and the darbepoetin dose could be reduced from 60 to 40 µg/week. Additionally, the serum ferritin level increased to approximately 100 ng/mL and never fell below 30 ng/mL; TSAT exceeded 20% and decreased after 17 weeks. After the 14th dose, favorable Hb levels were achieved with regular FCM administration. Unfortunately, the patient developed severe COVID-19 complicated by pneumonia and died 15 days after the 18th administration of FCM.
Figure 1.
Time course of Hb level, TSAT, and ferritin level after FCM administration. The 18 administrations of FCM are indicated by arrows. The change in Hb is indicated by the solid line in the upper figure. The changes in TSAT and ferritin are indicated by the solid and dashed lines in the lower figure, respectively.
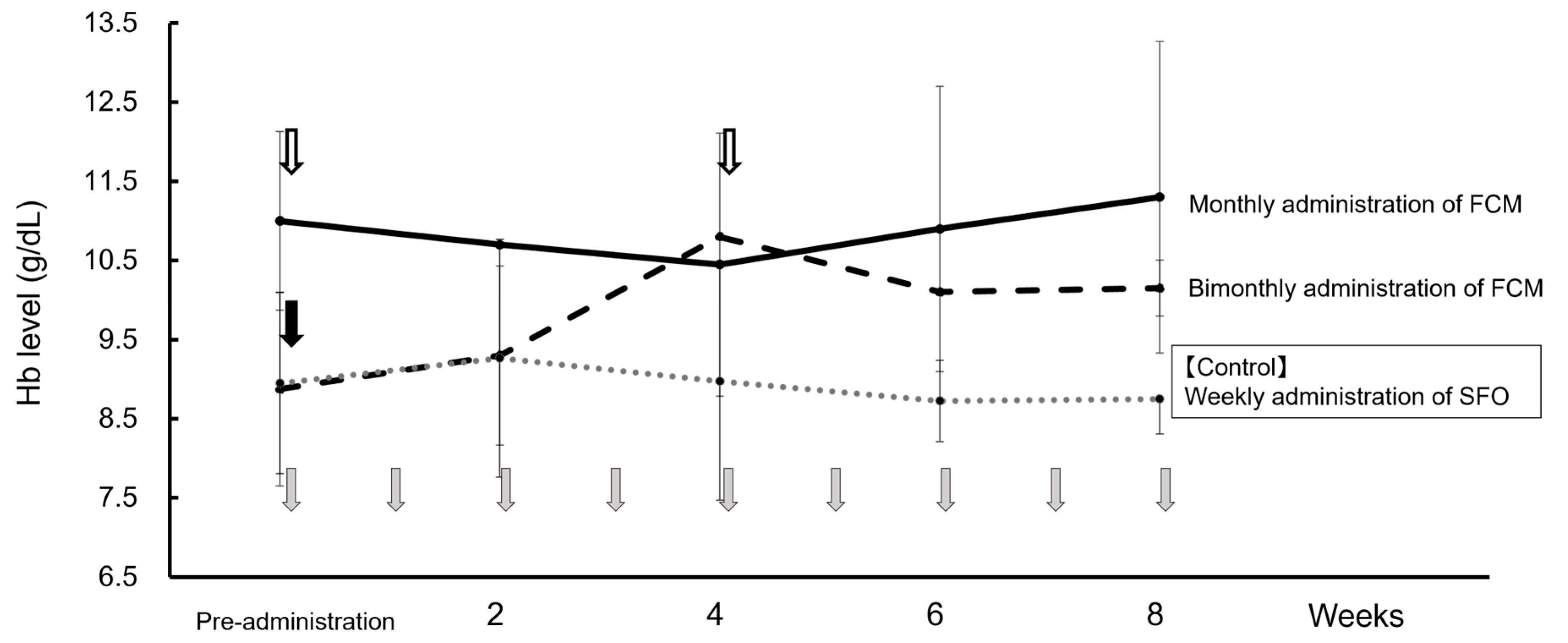
Before FCM administration, SFO was intravenously injected once per week. Therefore, using the weekly average Hb value with this administration method as a control, Figure 2 shows the changes in weekly average Hb values for bimonthly (doses 1–4) and monthly FCM administrations (doses 5–12). When FCM was administered bimonthly (56 days on average), the Hb level gradually increased from the 2nd week onwards compared to when SFO was administered, with a downward trend being observed from the 4th week onwards. Contrastingly, when FCM was administered monthly (31 days on average), the Hb levels remained stable at 10–12 g/dL each week. Serum ferritin levels temporarily increased by >300 ng/mL one week after FCM administration, but the high value did not persist. Furthermore, serum phosphorus levels did not decrease with FCM administration, liver function test values were within the normal range, and side effects such as headaches were not observed.
Figure 2.
Changes in the average Hb level at dosing intervals of FCM and SFO. First to fourth: bimonthly administration of FCM ( dashed line), fifth–twelfth: monthly administration of FCM (
dashed line), fifth–twelfth: monthly administration of FCM ( solid line), and weekly administration of SFO as a control drug before FCM administration (
solid line), and weekly administration of SFO as a control drug before FCM administration ( dotted line). Data are shown as mean ± standard.
dotted line). Data are shown as mean ± standard.
 dashed line), fifth–twelfth: monthly administration of FCM (
dashed line), fifth–twelfth: monthly administration of FCM ( solid line), and weekly administration of SFO as a control drug before FCM administration (
solid line), and weekly administration of SFO as a control drug before FCM administration ( dotted line). Data are shown as mean ± standard.
dotted line). Data are shown as mean ± standard.
3. Discussion
Regardless of the cause, secondary amyloidosis is often characterized by cardiac amyloidosis and must be differentiated from hypertrophic cardiomyopathy [7,8] and hypertensive heart disease [9]. However, in this case, as described in the case presentation, there were no cardiovascular abnormalities, and although the patient ultimately died of pneumonia due to COVID-19 infection, cardiovascular disorders were not the main cause of death. On the other hand, gastrointestinal amyloidosis due to Crohn’s disease induced refractory and inflammatory intestinal bleeding, and as a result, iron deficiency anemia was persistent and severe.
In dialysis patients, hematopoietic function is also reduced as a result of renal failure, and to improve such renal anemia, it has been common to administer ESAs intravenously, and recently oral administration of HIF-PHIs has also been put to practical use. On the other hand, dialysis patients are often iron deficient due to blood loss into dialysis lines and filters, frequent laboratory testing, and gastrointestinal bleeding, and they lose an average of 1 to 2 g of iron per year [10,11].
Therefore, for ESAs or HIF-PHIs to be maximally effective, it is essential to maintain an iron supply in amounts necessary for Hb synthesis and to compensate for losses. Oral iron administration is not prescribed for the treatment of iron deficiency in patients on dialysis because of incomplete effect, and the KDIGO practice guideline for anemia in chronic kidney disease (CKD) suggests using intravenous iron rather than oral iron. With iron gluconate and iron sucrose, the maximum single dose is usually 125 mg or 200 mg, for a total of 1000 mg per consecutive hemodialysis treatment, and a recent study recommend the 1500mg total cumulative dose of FCM [12]. In Japan, it is recommended that intravenous iron be slowly administered at 40 mg to patients undergoing hemodialysis at the end of a dialysis session once per week by setting 13 administrations as one cycle while confirming improvement in anemia and evaluating the iron status to ensure that serum ferritin levels are <300 ng/mL [13]. According to this guideline, we administered SFO as gastrointestinal bleeding due to Crohn’s disease continued, however, iron stores in the body tended to be insufficient, and Hb was always less than 8 g/dL. In cases of severe anemia, blood transfusion is an option, but in the case of dialysis patients, it is likely to cause hemolytic transfusion reactions, risk of infection, fluid and iron overload, and hyperkalemia, and the improvement of anemia is only temporary [14,15]. Therefore, the use of blood transfusions was limited.
Recently, a 500 mg intravenous formulation of FCM was launched for severe iron deficiency anemia. FCM is a type I polynuclear iron (III) hydroxide carbohydrate complex designed to mimic physiologically occurring ferritin proteins [16]. Serum iron and ferritin reach their maximum blood concentrations 15–30 min and 48–120 h after FCM administration, respectively. Thereafter, total serum iron levels fall below the limit of quantification in most of the patients within 60–96 h, and the urinary excretion rate of FCM is negligible [16].
FCM has been widely used in gynecological diseases and inflammatory bowel diseases that cause iron deficiency anemia; however, there are no recommendations regarding the use of FCM in patients undergoing dialysis in the guidelines of renal anemia by the KDIGO Conference [17] or the Japan Society for Dialysis Therapy [13]. Further, there have been no reported cases regarding the use of FCM in the United States and Japan.
Contrastingly, numerous clinical studies in Europe have demonstrated the efficacy and safety of treatment with FCM (Table 2). Covic et al. [18] conducted a multicenter open-label clinical study on the use of FCM in 163 patients undergoing hemodialysis. They found that intravenously injecting 100–200 mg of FCM two or three times a week for 6 weeks increased the Hb level from 9.1 ± 1.30 g/dL to 10.3 ± 1.63 g/dL in 100 patients, with serious side effects in 7.4%. Hofman et al. [19] reported that, in 221 stable patients undergoing hemodialysis, switching from iron sucrose to FCM at a 1:1 ratio improved the iron status parameters, reduced the dose of ESAs, and increased Hb levels. A similar efficacy was reported after switching from ferric gluconate to FCM, which resulted in a reduction in the administered iron doses [20,21,22].

Table 2.
Studies of FCM treatments in adult dialysis patients.
Therefore, in our presented case, FCM was considered the only way to improve refractory iron deficiency anemia and thus was administered at a dose of 500 mg per administration. Even in Europe, the dose for patients undergoing dialysis is usually ≤200 mg per administration. However, Portolés-Pérezi et al. [23] demonstrated that iron indicators were safely improved when 500 mg of FCM was intravenously infused at once in patients undergoing home dialysis. Diebold and Kistler [24] concluded that intravenous FCM infusion resulted in dose-dependent ferritin elevation of extended duration. Further, they recommended temporal coordination of blood sampling for iron status evaluation and maintaining an intravenous iron dosing schedule. Accordingly, we considered that FCM administration could be used to improve low Hb levels while ensuring safety by performing appropriate evaluations even during hemodialysis, with consideration of the administration interval.
Secondary amyloidosis is associated with prolonged chronic inflammation in patients with Crohn’s disease, which results in the excessive production of various cytokines. Chronically increased levels of cytokines, such as IL-6, result in increased hepcidin production in the liver, which in turn induces ACD by suppressing iron absorption from the gastrointestinal tract and iron recycling via macrophages [25,26]. Therefore, in this case, it is possible that ACD was a contributing factor to the severe anemia rather than just simple renal or hemorrhagic anemia.
A stable Hb level was obtained while gradually reducing the darbepoetin dose by administering 500 mg of FCM once every 1–2 months. Additionally, hypophosphatemia is a known side effect of FCM [27]; however, the patient did not show low serum phosphorus levels or other side effects.
The patient developed COVID-19 two weeks after the 18th administration of FCM and died of respiratory failure due to pneumonia. The relationship between the exacerbation of bacterial or virus infection and iron overload status is known. However, laboratory findings on day 7 of the 18th FCM administration showed a Hb level of 9.9 g/dL; serum iron level, 77 μg/dL; serum ferritin level, 348 ng/mL; and TSAT, 31.3%. Therefore, FCM administration may not play a causal role in COVID-19 infection.
4. Conclusions
The guidelines for renal anemia in patients with CKD recommend administering intravenous iron to patients undergoing dialysis at a dose of 40 mg per weekly to avoid iron overload. However, in patients with incurable persistent iron deficiency anemia, such as those with secondary amyloidosis due to Crohn’s disease, a single dose of 500 mg FCM once every 1–2 months may stabilize Hb and contribute towards efficient iron utilization.
However, the limitation of the current study is the case report. In particular, various factors with inflammatory systemic diseases can act as biases. Further trials administrating FCM for many hemodialysis patients with secondary amyloidosis should help strengthen our findings.
Author Contributions
M.U., A.F., Y.S., K.U. and T.S. were involved in the hemodialysis treatment. F.H. was the leading instructor in the treatment of anemia. M.U. and T.S. were the major contributors to the writing of the manuscript, and K.M. reviewed it. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Fukuoka University (protocol code No. 2017M070 and date of approval 19 July 2017).
Informed Consent Statement
The patient died as presented in the text, but consent for publication was obtained from his family.
Data Availability Statement
The data presented in this study are available to request to the corresponding author.
Acknowledgments
We thank the patient and all the staff involved.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Hb | hemoglobin |
| SFO | saccharated ferric oxide |
| FCM | ferric carboxymaltose |
| TSAT | transferrin saturation |
| COVID-19 | coronavirus disease 2019 |
| ESA | erythropoiesis stimulating agent |
| HIF-PHI | hypoxia-inducible factor prolyl hydroxylase inhibitor |
| CKD | chronic kidney disease |
| ACD | anemia of chronic disease |
References
- Lachmann, H.J.; Goodman, H.J.B.; Gilbertson, J.A.; Gallimore, J.R.; Sabin, C.A.; Gillmore, J.D.; Hawkins, P.N. Natural history and outcome in systemic AA amyloidosis. N. Engl. J. Med. 2007, 356, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Unsal, A.; Sokmen, M.; Kaptanogullari, O.H.; Alkim, C.; Kabukcuoglu, F.; Ozagari, A.; Bor, E. Renal involvement in AA amyloidosis: Clinical outcomes and survival. Kidney Blood Press Res. 2013, 37, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Barahona-Correa, J.E.; Morales, S.D.; Andrade-Pérez, R.; Hani, A. Renal amyloidosis and Crohn disease. Ochsner. J. 2021, 21, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Bunker, D.; Gorevic, P. AA amyloidosis: Mount Sinai experience, 1997–2012. Mt. Sinai J. Med. 2012, 79, 749–756. [Google Scholar] [CrossRef]
- Corica, D.; Romano, C. Renal involvement in inflammatory bowel diseases. J. Crohns. Colitis. 2016, 10, 226–235. [Google Scholar] [CrossRef]
- Avni, T.; Bieber, A.; Steinmetz, T.; Leibovici, L.; Gafter-Gvili, A. Treatment of anemia in inflammatory bowel disease- Systematic review and meta-analysis. PLoS ONE 2013, 8, e75540. [Google Scholar] [CrossRef]
- Pelliccia, F.; Alfieri, O.; Calabrò, P.; Cecchi, F.; Ferrazzi, P.; Gragnano, F.; Kaski, J.P.; Limongelli, G.; Maron, M.; Rapezzi, C.; et al. Multidisciplinary evaluation and management of obstructive hypertrophic cardiomyopathy in 2020: Towards the HCM Heart Team. Int. J. Cardiol. 2020, 304, 86–92. [Google Scholar] [CrossRef]
- Wang, J.; Marzolf, A.; Zhang, J.C.L.; Owens, A.; Han, Y. Cardiac amyloidosis masked as hypertrophic cardiomyopathy: A case report. Cardiol. Res. 2016, 7, 178–180. [Google Scholar] [CrossRef][Green Version]
- Gallo-Fernández, I.; López-Aguilera, J.; González-Manzanares, R.; Pericet-Rodriguez, C.; Carmona-Rico, M.J.; Perea-Armijo, J.; Castillo-Domínguez, J.C.; Anguita-Sánchez, M. Clinical differences between transthyretin cardiac amyloidosis and hypertensive heart disease. Med. Clin. 2024, 162, 205–212. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kuragano, T.; Kaibe, S.; Nagasawa, Y.; Hasuike, Y. Should we reconsider iron administration based on prevailing ferritin and hepcidin concentrations? Clin. Exp. Nephrol. 2012, 16, 819–826. [Google Scholar] [CrossRef]
- Eschbach, W.; Cook, J.D.; Scribner, B.H.; Finch, C.A. Iron balance in hemodialysis patients. Ann. Intern Med. 1977, 87, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.A.; Myers, J.; Goodnough, L.T. Intravenous iron therapy in patients with iron deficiency anemia: Dosingng considerations. Anemia 2015, 2015, 763576. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Nishi, S.; Tomo, T.; Masakane, I.; Saito, K.; Nangaku, M.; Hattori, M.; Suzuki, T.; Morita, S.; Ashida, A.; et al. Japanese society for dialysis therapy: Guidelines for renal anemia in chronic kidney disease. Ren. Replace Ther. 2017, 3, 36. [Google Scholar] [CrossRef]
- Tanhehco, Y.C.; Berns, J.S. Red blood cell transfusion risks in patients with end-stage renal disease. Semin. Dial. 2012, 25, 539–544. [Google Scholar] [CrossRef]
- Schlarmann, J.; Schurek, H.J.; Neumann, K.H.; Neumann Eckert, G. Chloride-induced increase of plasma potassium after transfusion of erythrocytes in dialysis patients. Nephron 1984, 37, 240–245. [Google Scholar] [CrossRef]
- Geisser, P.; Banké-Bochita, J. Pharmacokinetics, safety and tolerability of intravenous ferric carboxymaltose: A dose-escalation study in volunteers with mild iron-deficiency anaemia. Arzneim. Forsch. 2010, 60, 362–372. [Google Scholar] [CrossRef]
- Babitt, J.L.; Eisenga, M.F.; Haase, V.H.; Kshirsagar, V.; Levin, A.; Locatelli, F.; Małyszko, J.; Swinkels, D.W.; Tarng, D.C.; Cheung, M.; et al. Controversies in optimal anemia management: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2021, 99, 1280–1295. [Google Scholar] [CrossRef]
- Covic, A.; Mircescu, G. The safety and efficacy of intravenous ferric carboxymaltose in anaemic patients undergoing haemodialysis: A multi-centre, open-label, clinical study. Nephrol. Dial. Transplant. 2010, 25, 2722–2730. [Google Scholar] [CrossRef]
- Hofman, J.M.G.; Eisenga, M.F.; Diepenbroek, A.; Nolte, I.M.; van Dam, B.; Westerhuis, R.; Bakker, S.J.L.; Franssen, C.F.M.; Gaillard, C.A.J.M. Switching iron sucrose to ferric carboxymaltose associates to better control of iron status in hemodialysis patients. BMC Nephrol. 2018, 19, 242. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Pasqualetti, P.; Tocco, T.C.D.; Campo, S.; Rovito, S.; Bucca, M.; Ragusa, A.; Monardo, P. Ferric carboxymaltose versus ferric gluconate in hemodialysis patients: Reduction of erythropoietin dose in 4 years of follow-up. Kidney Res. Clin. Pract. 2020, 39, 334–343. [Google Scholar] [CrossRef]
- Gobbi, L.; Scaparrotta, G.; Rigato, M.; Cattarin, L.; Qassim, L.; Carraro, G.; Rossi, B.; Calò, L.A. Intravenous ferric carboxymaltose for iron deficiency anemia in dialysis patients: Effect of a new protocol adopted for a hemodialysis limited assistance center. Ther. Apher. Dial. 2020, 24, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Conti, P.; Berto, P.; Molinaro, S.; Baldini, F.; Egan, C.G.; Panichi, V. Efficacy, safety and pharmacoeconomic analysis of intravenous ferric carboxymaltose in anemic hemodialysis patients unresponsive to ferric gluconate treatment: A multicenter retrospective study. J. Clin. Med. 2022, 11, 5284. [Google Scholar] [CrossRef] [PubMed]
- Portolés-Pérez, J.; Durá-Gúrpide, B.; Merino-Rivas, J.L.; Martín-Rodriguez, L.; Hevia-Ojanguren, C.; Burguera-Vion, V.; Yuste-Lozano, C.; Sánchez-García, L.; Rodriguez-Palomares, J.R.; Paraiso, V.; et al. Effectiveness and safety of ferric carboxymaltose therapy in peritoneal dialysis patients: An observational study. Clin. Kidney J. 2021, 14, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Diebold, M.; Kistler, A.D. Evaluation of iron stores in hemodialysis patients on maintenance ferric carboxymaltose dosing. BMC Nephrol. 2019, 20, 76. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Andrews, N.C. Anemia of inflammation: The cytokine-hepcidin link. J. Clin. Investig. 2004, 113, 1251–1253. [Google Scholar] [CrossRef]
- Fang, W.; Kenny, R.; Rizvi, Q.U.A.; McMahon, L.P.; Garg, M. Hypophosphataemia after ferric carboxymaltose in unrelated to symptoms, intestinal inflammation or vitamin D status. BMC Gastroenterol. 2020, 20, 183. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).