Correlation of Inflammatory Biomarkers and IgG4 Antibodies with Malaria in Cameroon’s Buea Municipality Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Site and Study Population
2.3. Sample Collection
2.4. Measurement of AGP and CRP Inflammatory Biomarkers
2.5. Measurement of IgG4 Levels Using the Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Data Analysis
2.7. Ethical Considerations
3. Results
3.1. Demographic Characteristics of Study Participants
3.2. Relationship Between Anemia and Malaria Status
3.3. Comparing the Levels of AGP, CRP, and IgG4 in Malaria-Positive and Malaria-Negative Children
3.4. Assessing the Association Between the Levels of AGP and CRP Biomarkers and IgG4 and Malaria
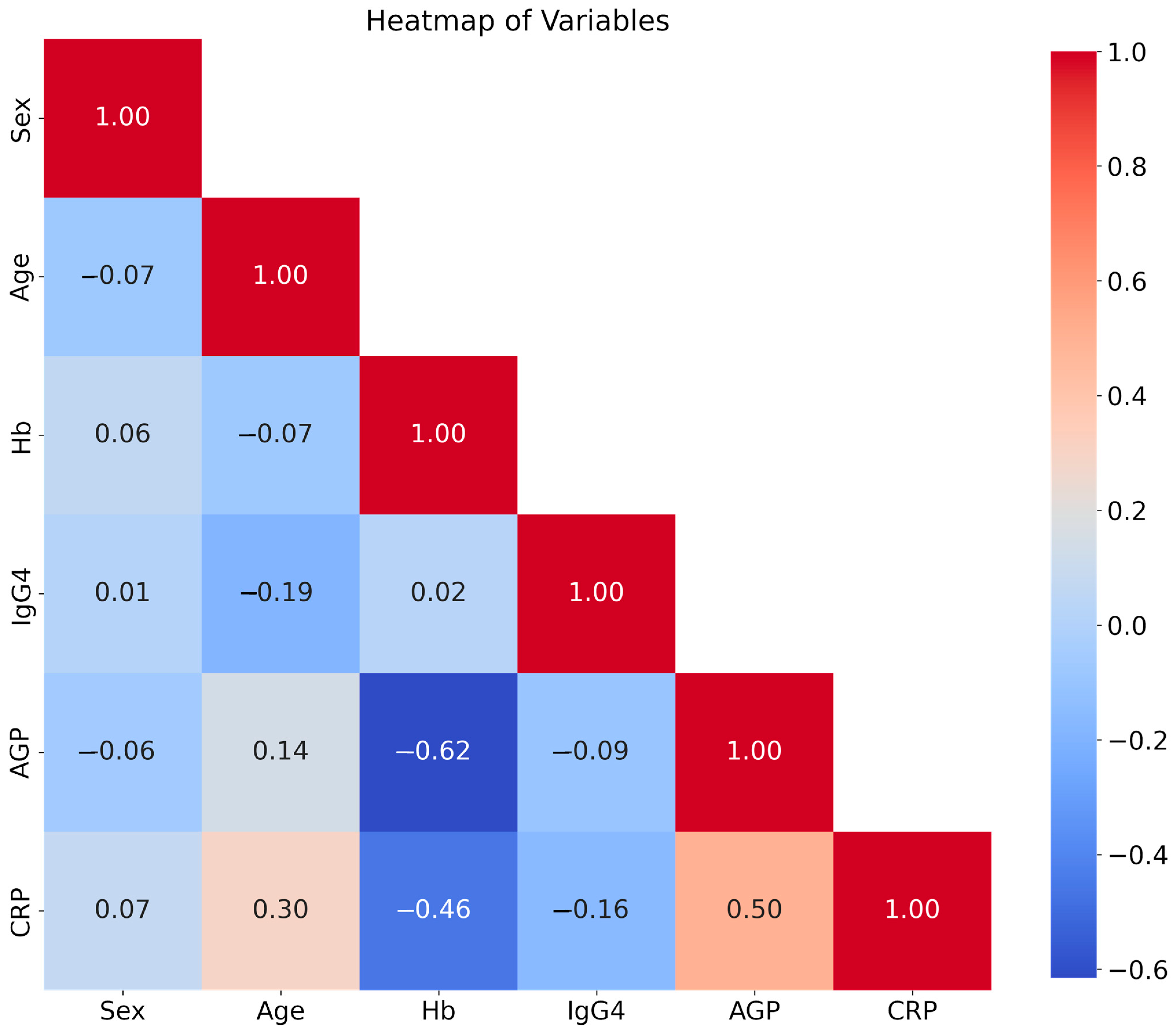
3.5. Correlation Heatmap of the Study Independent Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Garrison, A.; Boivin, M.J.; Fiévet, N.; Zoumenou, R.; Alao, J.M.; Massougbodji, A.; Cot, M.; Bodeau-Livinec, F. The Effects of Malaria in Pregnancy on Neurocognitive Development in Children at 1 and 6 Years of Age in Benin: A Prospective Mother–Child Cohort. Clin. Infect. Dis. 2022, 74, 766–775. [Google Scholar] [PubMed]
- World Health Organization. Mobilizing Communities to Help Prevent and Control Malaria in Cameroon. 2022. Available online: https://www.who.int/news-room/feature-stories/detail/mobilizing-communities-to-help-prevent-and-control-malaria-in-cameroon (accessed on 15 December 2024).
- World Health Organization. World Malaria Report 2016; World Health Organization: Geneva, Switzerland, 2016; p. 186. [Google Scholar]
- Berhe, A.D.; Doritchamou, J.Y.A.; Duffy, P.E. Malaria in pregnancy: Adverse pregnancy outcomes and the future of prevention. Front. Trop. Dis. 2023, 4, 1229735. [Google Scholar]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I. Role of Cytokines in Immunomodulation During Malaria Clearance. 2024. Available online: https://journals.lww.com/10.1097/MS9.0000000000002019 (accessed on 24 December 2024).
- Brindle, E.; Lillis, L.; Barney, R.; Bansil, P.; Hess, S.Y.; Wessells, K.R.; Ouédraogo, C.T.; Arredondo, F.; Barker, M.K.; Craft, N.E.; et al. A multicenter analytical performance evaluation of a multiplexed immunoarray for the simultaneous measurement of biomarkers of micronutrient deficiency, inflammation and malarial antigenemia. PLoS ONE 2021, 16, e0259509. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, F.; Al-Quraishy, S.; Dkhil, M.A. Liver-inherent immune system: Its role in blood-stage malaria. Front. Microbiol. 2014, 5, 559. [Google Scholar]
- Rubin, L.P.; Ross, A.C.; Stephensen, C.B.; Bohn, T.; Tanumihardjo, S.A. Metabolic Effects of Inflammation on Vitamin A and Carotenoids in Humans and Animal Models. Adv. Nutr. 2017, 8, 197–212. [Google Scholar] [CrossRef]
- Zandstra, J.; Jongerius, I.; Kuijpers, T.W. Future Biomarkers for Infection and Inflammation in Febrile Children. Front. Immunol. 2021, 12, 631308. [Google Scholar]
- Gonzales, S.J.; Reyes, R.A.; Braddom, A.E.; Batugedara, G.; Bol, S.; Bunnik, E.M. Naturally Acquired Humoral Immunity Against Plasmodium falciparum Malaria. Front. Immunol. 2020, 11, 594653. [Google Scholar]
- Oyong, D.A.; Loughland, J.R.; Soon, M.S.; Chan, J.-A.; Andrew, D.; Wines, B.D.; Hogarth, P.M.; Olver, S.D.; Collinge, A.D.; Varelias, A.; et al. Adults with Plasmodium falciparum malaria have higher magnitude and quality of circulating T-follicular helper cells compared to children. eBioMedicine 2022, 75, 103784. [Google Scholar]
- Gannon, B.M.; Glesby, M.J.; Finkelstein, J.L.; Raj, T.; Erickson, D.; Mehta, S. A point-of-care assay for alpha-1-acid glycoprotein as a diagnostic tool for rapid, mobile-based determination of inflammation. Curr. Res. Biotechnol. 2019, 1, 41–48. [Google Scholar]
- Niehues, T. C-reactive protein and other biomarkers—The sense and non-sense of using inflammation biomarkers for the diagnosis of severe bacterial infection. LymphoSign J. 2018, 5, 35–47. [Google Scholar] [CrossRef]
- Dobaño, C.; Santano, R.; Vidal, M.; Jiménez, A.; Jairoce, C.; Ubillos, I.; Dosoo, D.; Aguilar, R.; Williams, N.A.; Díez-Padrisa, N.; et al. Differential Patterns of IgG Subclass Responses to Plasmodium falciparum Antigens in Relation to Malaria Protection and RTS,S Vaccination. Front. Immunol. 2019, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Dinga, J.N.; Anu, E.F.; Feumba, R.D.; Qin, H.; Ayah, F.; Ayiseh, R.B.; Shey, R.A.; Gamua, S.D.; Tufon, A.K.; Manyam, R.; et al. Micronutrient Biomarkers and Their Association with Malaria Infection in Children in Buea Health District, Cameroon. Trop. Med. Infect. Dis. 2024, 9, 303. [Google Scholar] [CrossRef] [PubMed]
- Dinga, J.N.; Gamua, S.D.; Titanji, V.P.K. Enhanced acquired antibodies to a chimeric Plasmodium falciparum antigen; UB05-09 is associated with protective immunity against malaria. Parasite Immunol. 2017, 39, e12445. [Google Scholar] [CrossRef]
- Dinga, J.N.; Gamua, S.D.; Ghogomu, S.M.; Titanji, V.P.K. Preclinical efficacy and immunogenicity assessment to show that a chimeric Plasmodium falciparum UB05-09 antigen could be a malaria vaccine candidate. Parasite Immunol. 2018, 40, e12514. [Google Scholar] [CrossRef]
- Addo, O.Y.; Yu, E.X.; Williams, A.M.; Young, M.F.; Sharma, A.J.; Mei, Z.; Kassebaum, N.J.; Jefferds, M.E.D.; Suchdev, P.S. Evaluation of Hemoglobin Cutoff Levels to Define Anemia Among Healthy Individuals. JAMA Netw. Open 2021, 4, e2119123. [Google Scholar] [CrossRef]
- Fertrin, K.Y. Diagnosis and management of iron deficiency in chronic inflammatory conditions (CIC): Is too little iron making your patient sick? Hematology 2020, 2020, 478–486. [Google Scholar] [CrossRef]
- Ntenda, P.A.M.; Chirambo, A.C.; Nkoka, O.; El-Meidany, W.M.; Goupeyou-Youmsi, J. Implication of asymptomatic and clinical Plasmodium falciparum infections on biomarkers of iron status among school-aged children in Malawi. Malar. J. 2022, 21, 278. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Pulido, H.; Stanczyk, N.M.; De Moraes, C.M.; Mescher, M.C. A unique volatile signature distinguishes malaria infection from other conditions that cause similar symptoms. Sci. Rep. 2021, 11, 13928. [Google Scholar] [CrossRef]
- National Statistical Office (NSO), Community Health Sciences Unit (CHSU) [Malawi], Centre for Disease Control and Prevention (CDC), Emory University. Malawi Micronutrient Survey Key Indicators Report 2015-16. Atlanta, GA, USA; 2016. Available online: https://dhsprogram.com/pubs/PDF/FR319/FR319m.pdf (accessed on 15 December 2024).
- Foote, E.M.; Suchdev, P.S.; Williams, T.N.; Sadumah, I.; Sullivan, K.M.; Oremo, J.; Ruth, L.J. Determinants of Anemia among Preschool Children in Rural, Western Kenya. Am. J. Trop. Med. Hyg. 2013, 88, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Coban, C.; Lee, M.S.J.; Ishii, K.J. Tissue-specific immunopathology during malaria infection. Nat. Rev. Immunol. 2018, 18, 266–278. [Google Scholar] [CrossRef]
- Flannery, E.L.; Kangwanrangsan, N.; Chuenchob, V.; Roobsoong, W.; Fishbaugher, M.; Zhou, K.; Billman, Z.P.; Martinson, T.; Olsen, T.M.; Schäfer, C.; et al. Plasmodium vivax latent liver infection is characterized by persistent hypnozoites, hypnozoite-derived schizonts, and time-dependent efficacy of primaquine. Mol. Ther.-Methods Clin. Dev. 2022, 26, 427–440. [Google Scholar]
- Obeagu, E.I.; Okoroiwu, G.I.A.; Ubosi, N.I.; Obeagu, G.U.; Onohuean, H.; Muhammad, T.; Adias, T.C. Revolution in malaria detection: Unveiling current breakthroughs and tomorrow’s possibilities in biomarker innovation. Ann. Med. Surg. 2024, 86, 5859–5876. [Google Scholar]
- Alfaki, D.A.; Hussein, M.; Hassan, M.; Eloraish, A.G.; Elbasheir, M.M. Inflammatory immune mediators and Plasmodium falciparum infection: A cross-sectional study among Sudanese patients with severe and uncomplicated malaria. Explor. Immunol. 2023, 3, 406–415. [Google Scholar]
- Li, J.; Docile, H.J.; Fisher, D.; Pronyuk, K.; Zhao, L. Current Status of Malaria Control and Elimination in Africa: Epidemiology, Diagnosis, Treatment, Progress and Challenges. J. Epidemiol. Glob. Health 2024, 14, 561–579. [Google Scholar] [PubMed]
- Mbugi, E.V.; Hartog, G.D.; Veenemans, J.; Chilongola, J.O.; Verhoef, H.; Savelkoul, H.F.J. Nutrient Deficiencies and Potential Alteration in Plasma Levels of Naturally Acquired Malaria-Specific Antibody Responses in Tanzanian Children. Front. Nutr. 2022, 9, 872710. [Google Scholar]
- Feng, G.; Kurtovic, L.; Agius, P.A.; Aitken, E.H.; Sacarlal, J.; Wines, B.D.; Hogarth, P.M.; Rogerson, S.J.; Fowkes, F.J.I.; Dobaño, C.; et al. Induction, decay, and determinants of functional antibodies following vaccination with the RTS,S malaria vaccine in young children. BMC Med. 2022, 20, 289. [Google Scholar] [CrossRef]
- Dobbs, K.R.; Jagannathan, P.; Dechavanne, C. Editorial: Immune tolerance and human malaria. Front. Immunol. 2024, 15, 1450480. [Google Scholar]
- Rogers, K.J.; Vijay, R.; Butler, N.S. Anti-malarial humoral immunity: The long and short of it. Microbes Infect. 2021, 23, 104807. [Google Scholar]

| Malaria Status | ||||
|---|---|---|---|---|
| Variable | Category | Negative (%) | Positive (%) | p-Value |
| Fever | No | 37 (46.3) | 0 (0) | <0.001 |
| Yes | 0 (0) | 43 (53.8) | ||
| Hemoglobin level | Anemic | 16 (20) | 27 (33.7) | 0.053 |
| Non-Anemic | 21 (26.3) | 16 (20) | ||
| Biomarker | Malaria | Mean | SD | p-Value |
|---|---|---|---|---|
| AGP (g/L) | Negative | 0.28 | 0.23 | <0.001 |
| Positive | 0.55 | 0.37 | ||
| CRP (mg/L) | Negative | 3.02 | 7.20 | <0.001 |
| Positive | 28.61 | 20.20 | ||
| IgG4 (OD value) | Negative | 0.51 | 0.52 | <0.03 |
| Positive | 0.29 | 0.36 |
| Variable | Odds Ratio | 95% Confidence Interval | p-Value | Beta Coefficient |
|---|---|---|---|---|
| Sex | 1.123 | [0.465, 2.709] | 0.796 | 0.1160 |
| Age | 1.037 | [1.005, 1.069] | 0.022 | 0.0362 |
| Hemoglobin | 0.766 | [0.555, 1.057] | 0.104 | −0.2672 |
| IgG4 | 0.304 | [0.101, 0.918] | 0.035 | −1.1898 |
| AGP | 46.964 | [3.939, 559.958] | 0.002 | 3.8494 |
| CRP | 1.207 | [1.097, 1.328] | 0.0001 | 0.1883 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinga, J.N.; Ayah, F.; Anu, E.F.; Qin, H.; Gamua, S.D.; Tufon, A.K.; Amougou, M.E.; Manyam, R. Correlation of Inflammatory Biomarkers and IgG4 Antibodies with Malaria in Cameroon’s Buea Municipality Children. Diseases 2025, 13, 123. https://doi.org/10.3390/diseases13040123
Dinga JN, Ayah F, Anu EF, Qin H, Gamua SD, Tufon AK, Amougou ME, Manyam R. Correlation of Inflammatory Biomarkers and IgG4 Antibodies with Malaria in Cameroon’s Buea Municipality Children. Diseases. 2025; 13(4):123. https://doi.org/10.3390/diseases13040123
Chicago/Turabian StyleDinga, Jerome Nyhalah, Flora Ayah, Emmanuel Fondungallah Anu, Haowen Qin, Stanley Dobgima Gamua, Anthony Kukwah Tufon, Magloire Essissima Amougou, and Rameshbabu Manyam. 2025. "Correlation of Inflammatory Biomarkers and IgG4 Antibodies with Malaria in Cameroon’s Buea Municipality Children" Diseases 13, no. 4: 123. https://doi.org/10.3390/diseases13040123
APA StyleDinga, J. N., Ayah, F., Anu, E. F., Qin, H., Gamua, S. D., Tufon, A. K., Amougou, M. E., & Manyam, R. (2025). Correlation of Inflammatory Biomarkers and IgG4 Antibodies with Malaria in Cameroon’s Buea Municipality Children. Diseases, 13(4), 123. https://doi.org/10.3390/diseases13040123






