The Genetic and Biological Basis of Pseudoarthrosis in Fractures: Current Understanding and Future Directions
Abstract
1. Introduction
2. Biological and Molecular Factors in Non-Union Fractures
3. The Vascularization in Non-Union Fracture Formation
Blood Flow Angiogenesis and Angiogenetic Factors
4. Genetic Factors in Non-Union Fractures
| Genes Investigated | Study Group | Results | Polymorphisms of Interest | Authors |
|---|---|---|---|---|
| ADAM8, CALY, ECHS1, FUOM, MIR202, MIR202HG, PRAP1, TUBGCP2, ZNF511, AMPD3, TRACR1, ACHE, MIR6875, MUC3A, MUC12, MUC17, SERPINE1, SLC12A9, SRRT, TRIM56, TRIP6, USFP1, ACAT1, ATM, C11orf65, EXPH5, KDELC2, NPAT, ASTN2, RBMS3 | (cohort study) 1760 Northern Europeans with upper or lower fractures—131 non-union | CALY gene SNP was one of the most strongly associated with non-union risk TACR1 gene may influence pain reception and healing process | CALY (rs2298122) | [118] |
| NOS2 | 1229 Han Chinese patients with long bone fractures—346 patients with non-union vs. 883 union group | An NOS2 SNP was associated with increased risk of non-union (only in tibial diaphysis fracture subgroup) | T allele of NOS2 (rs2297514) | [112] |
| CYR61 | 250 patients with non-union vs. 250 healthy individuals | CYR61 heterozygous genotype affects mRNA expression and may be a risk factor that increases chances for non-union | Heterozygous TG genotype and G allele | [117] |
| CSF1, IL1B, IL6, IL11, TNFSF11, TNFRSF11B, IFN1α, TNF, COL2A1, COL1A1, TGFB1, TGFB2, TGFB3, BMP2, BMP4, BMP5, BMP6, BMP7, BMP8A, MSTN, GDF10, MMP9, MMP13, VEGFA, VEGFC, ANGPT1, PTN, NOS2, ADRB2, PTGS2 | 62 patients with long bone fractures—33 non-union vs. 29 union group | Variations in IL1B and NOS2 genes may contribute to non-union risk SNPs of MMP13 and BMP6 could be protective against non-union | IL1B (rs2853550) NOS2 (rs2297514) and (rs2248814) MMP13 (rs3819089) BMP6 (rs270393) | [113] |
| FAM5C, BMP4, FGF3, FGF1O, FGFR1 | 167 patients with long bone fractures—66 non-union vs. 101 union group | BMP4 haplotype and an FGFR1 SNP associated with non-union risk FAM5C SNP associated with uneventful healing | BMP4 GTAA haplotype FGFR1 (rs13317) FAM5C (rs1342913) | [111] |
| TLR2, TLR4, CCR2, CD14, CRP, IL-6, IL-1ra, TGF-β | 108 patients with non-union (34 with viable bacterial strains) vs. 122 union group (20 with viable bacterial strains) | Some polymorphisms in TLR 4 and TGF-β may lead to impaired pathogen recognition, prolonged pathogen existence in the fracture site, and higher risk of delayed healing | TLR 4 gene 1/W (Asp299Gly) TGF-β gene codon 10 mutant T and T/C allele | [116] |
| IGF-1, BMP-2, BMP-4, BMP-7, IL1b, IL-2, IL-3, IL-8, MMP-9, MMP-13, PDGF-A, TNF-α | 50 patients with non-union (21 femoral and 29 tibial) vs. 44 union group | PDGF polymorphisms seem to be a risk factor for non-union MMP-13 is highly associated with uneventful healing | PDGF-A CCG haplotype MMP-13 (rs2252070) | [114] |
| BMP-2, BMP-7, NOGGIN, SMAD6 | 109 patients with long bone fractures—62 patients with non-union vs. 47 union group | Two genotypes (of NOGGIN and SMAD6) were found to be associated with non-union risk | G/G genotype of rs1472857 of NOGGIN T/T genotype of rs2053423 of SMAD6 | [110] |
5. Diagnostic and Predictive Biomarkers
6. Therapeutic Implications
6.1. Current Treatment Strategies
6.2. Gene Therapy
6.3. Stem Cell Therapy and Tissue Engineering
6.4. BMPs and Other Growth Factors
7. Discussion
| Future Direction | Description | Bibliography |
|---|---|---|
| Personalized medicine | Tailoring medical treatment based on an individual’s genetic profile to optimize bone healing outcomes. | [121] |
| Genetic testing | Identifying patients at risk of delayed healing or non-unions due to genetic variants (e.g., BMP2, VEGF polymorphisms). | [113] |
| Genome-Wide Association Studies (GWAS) | Identifying genetic variants linked to bone healing and pseudoarthrosis through large-scale analyses. | [185] |
| Next-Generation Sequencing (NGS) | Providing detailed genetic profiles to guide personalized treatment plans. | [186] |
| CRISPR/Cas9 and gene editing | Potentially correcting genetic defects that impair bone repair, enhancing endogenous growth factors. | [186] |
| 3D bioprinting | Creating patient-specific bone grafts using genetic and anatomical data for improved implant integration. | [187] |
| Nanotechnology and smart biomaterials | Delivering growth factors and improving implant integration through advanced, targeted therapies. | [190] |
| Stem cell therapies (e.g., MSCs, iPSCs) | Utilizing patient-derived cells to promote osteogenesis and angiogenesis in non-union fractures. | [200] |
| AI-driven medicine | Optimizing treatment plans and real-time monitoring of fracture healing using artificial intelligence. | [189] |
| Epigenetics and miRNA studies | Exploring environmental impacts (e.g., nutrition, smoking) on gene expression and their effect on healing. | [194] |
| Combination therapies | Integrating mechanical stimulation (e.g., electromagnetic fields) with biological therapies for enhanced healing. | [195] |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal stromal cell |
| BMP | Bone morphogenetic protein |
| MMP | Matrix metalloproteinase |
| MiRNAs | MicroRNAs |
| SOST | Sclerostin |
| DDK1 | Dickkopf-1 |
| PDGFs | Platelet-derived growth factors |
| HIFs | Hypoxia-inducible factors |
| TGF-β | Transforming growth factor-beta |
| PlGF-1 | Placental growth factor-1 |
| ECM | Extracellular matrix |
| NF1 | Neurofibromatosis type 1 |
| IL-1 | Interleukin 1 |
| CXCR4 | Chemokine receptor type 4 |
| RUNX2 | Runt-related transcription factor 2 |
| OCN | Osteocalcin |
| OPG | Osteoprotegerin |
| SNPs | Single-nucleotide polymorphisms |
| ABGs | Autologous bone grafts |
| PEMF | Pulsed electromagnetic field |
| ESCs | Embryonic stem cells |
| iPSCs | Induced pluripotent stem cells |
| TNF-α | Tumor necrosis factor-alpha |
| GWAS | Genome-Wide Association Studies |
| NGS | Next-Generation Sequencing |
References
- Abbas, S.; Chokotho, L.; Nyamulani, N.; Oliver, V.L. The Burden of Long Bone Fracture and Health System Response in Malawi: A Scoping Review. Injury 2024, 55, 111243. [Google Scholar] [CrossRef]
- Nicholson, J.; Makaram, N.; Simpson, A.; Keating, J. Fracture Nonunion in Long Bones: A Literature Review of Risk Factors and Surgical Management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S. Fracture Non-Union: A Review of Clinical Challenges and Future Research Needs. Malays. Orthop. J. 2019, 13, 1–10. [Google Scholar] [CrossRef]
- Bowers, K.M.; Anderson, D.E. Delayed Union and Nonunion: Current Concepts, Prevention, and Correction: A Review. Bioengineering 2024, 11, 525. [Google Scholar] [CrossRef]
- Takahara, S.; Niikura, T.; Lee, S.Y.; Iwakura, T.; Okumachi, E.; Kuroda, R.; Kurosaka, M. Human Pseudoarthrosis Tissue Contains Cells with Osteogenic Potential. Injury 2016, 47, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Gómez-Barrena, E.; Baldini, N. Association between Bone Turnover Markers and Fracture Healing in Long Bone Non-Union: A Systematic Review. J. Clin. Med. 2024, 13, 2333. [Google Scholar] [CrossRef]
- Chitwood, J.R.; Chakraborty, N.; Hammamieh, R.; Moe, S.M.; Chen, N.X.; Kacena, M.A.; Natoli, R.M. Predicting Fracture Healing with Blood Biomarkers: The Potential to Assess Patient Risk of Fracture Nonunion. Biomarkers 2021, 26, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Tiwari, V. Congenital Tibial Pseudarthrosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sheen, J.R.; Mabrouk, A.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Dudley, A.C.; Griffioen, A.W. Pathological Angiogenesis: Mechanisms and Therapeutic Strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef]
- Basile, G.; Fozzato, S.; Petrucci, Q.A.; Gallina, M.; Bianco Prevot, L.; Accetta, R.; Zaami, S. Treatment of Femoral Shaft Pseudarthrosis, Case Series and Medico-Legal Implications. J. Clin. Med. 2022, 11, 7407. [Google Scholar] [CrossRef]
- Vanderkarr, M.F.; Ruppenkamp, J.W.; Vanderkarr, M.; Holy, C.E.; Blauth, M. Risk Factors and Healthcare Costs Associated with Long Bone Fracture Non-Union: A Retrospective US Claims Database Analysis. J. Orthop. Surg. Res. 2023, 18, 745. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Lana, J.F.; Onishi, K.; Buford, D.; Peng, J.; Mahmood, A.; Fonseca, L.F.; van Zundert, A.; Podesta, L. Angiogenesis and Tissue Repair Depend on Platelet Dosing and Bioformulation Strategies Following Orthobiological Platelet-Rich Plasma Procedures: A Narrative Review. Biomedicines 2023, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Menger, M.M.; Laschke, M.W.; Nussler, A.K.; Menger, M.D.; Histing, T. The Vascularization Paradox of Non-Union Formation. Angiogenesis 2022, 25, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Panteli, M.; Vun, J.S.H.; Pountos, I.; Howard, A.J.; Jones, E.; Giannoudis, P.V. Biological and Molecular Profile of Fracture Non-Union Tissue: A Systematic Review and an Update on Current Insights. J. Cell. Mol. Med. 2022, 26, 601–623. [Google Scholar] [CrossRef]
- Aluganti Narasimhulu, C.; Singla, D.K. The Role of Bone Morphogenetic Protein 7 (BMP-7) in Inflammation in Heart Diseases. Cells 2020, 9, 280. [Google Scholar] [CrossRef]
- Proia, P.; Rossi, C.; Alioto, A.; Amato, A.; Polizzotto, C.; Pagliaro, A.; Kuliś, S.; Baldassano, S. MiRNAs Expression Modulates Osteogenesis in Response to Exercise and Nutrition. Genes 2023, 14, 1667. [Google Scholar] [CrossRef]
- Bravo Vázquez, L.A.; Moreno Becerril, M.Y.; Mora Hernández, E.O.; de León Carmona, G.G.; Aguirre Padilla, M.E.; Chakraborty, S.; Bandyopadhyay, A.; Paul, S. The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential. Molecules 2021, 27, 211. [Google Scholar] [CrossRef]
- Zhang, M.; Appelboom, G.; Ratliff, J.K.; Soltys, S.G.; Adler, J.R.; Park, J.; Chang, S.D. Radiographic Rate and Clinical Impact of Pseudarthrosis in Spine Radiosurgery for Metastatic Spinal Disease. Cureus 2018, 10, e3631. [Google Scholar] [CrossRef] [PubMed]
- Dincel, A.S.; Jørgensen, N.R.; IOF-IFCC Joint Committee on Bone Metabolism (C-BM). New Emerging Biomarkers for Bone Disease: Sclerostin and Dickkopf-1 (DKK1). Calcif. Tissue Int. 2023, 112, 243–257. [Google Scholar] [CrossRef]
- Panteli, M.; Pountos, I.; Jones, E.; Giannoudis, P.V. Biological and Molecular Profile of Fracture Non-Union Tissue: Current Insights. J. Cell. Mol. Med. 2015, 19, 685–713. [Google Scholar] [CrossRef]
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bönig, H.; Henrich, D.; Marzi, I. Autologous Cell-Based Therapy for Treatment of Large Bone Defects: From Bench to Bedside. Eur. J. Trauma Emerg. Surg. 2018, 44, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Nasadiuk, K.; Kolanowski, T.; Kowalewski, C.; Wozniak, K.; Oldak, T.; Rozwadowska, N. Harnessing Mesenchymal Stromal Cells for Advanced Wound Healing: A Comprehensive Review of Mechanisms and Applications. Int. J. Mol. Sci. 2025, 26, 199. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. The Roles of Vascular Endothelial Growth Factor in Bone Repair and Regeneration. Bone 2016, 91, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, M.; Sikora, M.; Stec, A.; Zaremba, M.; Maciejewski, C.; Pawlik, K.; Rudnicka, L. Hypoxia-Inducible Factor-1α (HIF-1α) as a Biomarker for Changes in Microcirculation in Individuals with Systemic Sclerosis. Dermatol. Ther. 2023, 13, 1549–1560. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Pneumaticos, S.; Giannoudis, P.V. Fracture Non-Union: Can Biomarkers Predict Outcome? Injury 2013, 44, 1725–1732. [Google Scholar] [CrossRef]
- Jensen, S.S.; Jensen, N.M.; Gundtoft, P.H.; Kold, S.; Zura, R.; Viberg, B. Risk Factors for Nonunion Following Surgically Managed, Traumatic, Diaphyseal Fractures: A Systematic Review and Meta-Analysis. EFORT Open Rev. 2022, 7, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Filipowska, J.; Tomaszewski, K.A.; Niedźwiedzki, Ł.; Walocha, J.A.; Niedźwiedzki, T. The Role of Vasculature in Bone Development, Regeneration and Proper Systemic Functioning. Angiogenesis 2017, 20, 291–302. [Google Scholar] [CrossRef]
- Shineh, G.; Patel, K.; Mobaraki, M.; Tayebi, L. Functional Approaches in Promoting Vascularization and Angiogenesis in Bone Critical-Sized Defects via Delivery of Cells, Growth Factors, Drugs, and Particles. J. Funct. Biomater. 2023, 14, 99. [Google Scholar] [CrossRef]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Goncharov, E.N.; Koval, O.A.; Nikolaevich Bezuglov, E.; Engelgard, M.; Igorevich, E.I.; Velentinovich Kotenko, K.; Encarnacion Ramirez, M.D.J.; Montemurro, N. Comparative Analysis of Stromal Vascular Fraction and Alternative Mechanisms in Bone Fracture Stimulation to Bridge the Gap between Nature and Technological Advancement: A Systematic Review. Biomedicines 2024, 12, 342. [Google Scholar] [CrossRef]
- Knight, M.N.; Hankenson, K.D. Mesenchymal Stem Cells in Bone Regeneration. Adv. Wound Care 2013, 2, 306–316. [Google Scholar] [CrossRef]
- Arellano, M.Y.G.; VanHeest, M.; Emmadi, S.; Abdul-Hafez, A.; Ibrahim, S.A.; Thiruvenkataramani, R.P.; Teleb, R.S.; Omar, H.; Kesaraju, T.; Mohamed, T.; et al. Role of Mesenchymal Stem/Stromal Cells (MSCs) and MSC-Derived Extracellular Vesicles (EVs) in Prevention of Telomere Length Shortening, Cellular Senescence, and Accelerated Biological Aging. Bioengineering 2024, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qiao, Z.; Pfeifer, R.; Pape, H.-C.; Mao, K.; Tang, H.; Meng, B.; Chen, S.; Liu, H. Modulation of Fracture Healing by Senescence-Associated Secretory Phenotype (SASP): A Narrative Review of the Current Literature. Eur. J. Med. Res. 2024, 29, 38. [Google Scholar] [CrossRef]
- Shen, F.; Xiao, H.; Shi, Q. Mesenchymal Stem Cells Derived from the Fibrotic Tissue of Atrophic Nonunion or the Bone Marrow of Iliac Crest: A Donor-Matched Comparison. Regen. Ther. 2023, 24, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- May, R.D.; Frauchiger, D.A.; Albers, C.E.; Tekari, A.; Benneker, L.M.; Klenke, F.M.; Hofstetter, W.; Gantenbein, B. Application of Cytokines of the Bone Morphogenetic Protein (BMP) Family in Spinal Fusion—Effects on the Bone, Intervertebral Disc and Mesenchymal Stromal Cells. Curr. Stem Cell Res. Ther. 2019, 14, 618–643. [Google Scholar] [CrossRef]
- Lewis, C.J.; Mardaryev, A.N.; Poterlowicz, K.; Sharova, T.Y.; Aziz, A.; Sharpe, D.T.; Botchkareva, N.V.; Sharov, A.A. Bone Morphogenetic Protein Signalling Suppresses Wound-Induced Skin Repair by Inhibiting Keratinocyte Proliferation and Migration. J. Investig. Dermatol. 2014, 134, 827–837. [Google Scholar] [CrossRef]
- Nair, V.; Patil, V.S.; Todkar, A.; Shah, M.; Devarmani, S. Bone Morphogenetic Proteins: A Promising Approach for Enhancing Fracture Healing. Cureus 2024, 16, e66619. [Google Scholar] [CrossRef]
- Wu, M.; Wu, S.; Chen, W.; Li, Y.-P. The Roles and Regulatory Mechanisms of TGF-β and BMP Signaling in Bone and Cartilage Development, Homeostasis and Disease. Cell Res. 2024, 34, 101–123. [Google Scholar] [CrossRef]
- Morikawa, M.; Derynck, R.; Miyazono, K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb. Perspect. Biol. 2016, 8, a021873. [Google Scholar] [CrossRef]
- MacFarlane, E.G.; Haupt, J.; Dietz, H.C.; Shore, E.M. TGF-β Family Signaling in Connective Tissue and Skeletal Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a022269. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.-P. Wnt/β-Catenin Signaling Components and Mechanisms in Bone Formation, Homeostasis, and Disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Wei, E.; Hu, M.; Wu, L.; Pan, X.; Zhu, Q.; Liu, H.; Liu, Y. TGF-β Signaling Regulates Differentiation of MSCs in Bone Metabolism: Disputes among Viewpoints. Stem Cell Res. Ther. 2024, 15, 156. [Google Scholar] [CrossRef]
- Kišonaitė, M.; Wang, X.; Hyvönen, M. Structure of Gremlin-1 and Analysis of Its Interaction with BMP-2. Biochem. J. 2016, 473, 1593–1604. [Google Scholar] [CrossRef]
- Mörsdorf, D.; Knabl, P.; Genikhovich, G. Highly Conserved and Extremely Evolvable: BMP Signalling in Secondary Axis Patterning of Cnidaria and Bilateria. Dev. Genes. Evol. 2024, 234, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Glister, C.; Regan, S.L.; Samir, M.; Knight, P. Gremlin, Noggin, Chordin and Follistatin Differentially Modulate BMP Induced Suppression of Androgen Secretion by Bovine Ovarian Theca Cells. J. Mol. Endocrinol. 2019, 62, 15–25. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Sig Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Florio, M.; Kostenuik, P.J.; Stolina, M.; Asuncion, F.J.; Grisanti, M.; Ke, H.Z.; Ominsky, M.S. Dual Inhibition of the Wnt Inhibitors DKK1 and Sclerostin Promotes Fracture Healing and Increases the Density and Strength of Uninjured Bone: An Experimental Study in Nonhuman Primates. J. Bone Jt. Surg. Am. 2023, 105, 1145–1155. [Google Scholar] [CrossRef]
- Starlinger, J.; Santol, J.; Kaiser, G.; Sarahrudi, K. Close Negative Correlation of Local and Circulating Dickkopf-1 and Sclerostin Levels during Human Fracture Healing. Sci. Rep. 2024, 14, 6524. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.Z.; Richards, W.G.; Li, X.; Ominsky, M.S. Sclerostin and Dickkopf-1 as Therapeutic Targets in Bone Diseases. Endocr. Rev. 2012, 33, 747–783. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Wang, F.-S.; Lian, W.-S.; Yang, F.-S.; Chen, J.-W.; Huang, P.-H.; Liao, C.-Y.; Kuo, S.-J. Dickkopf-1 (DKK1) Blockade Mitigates Osteogenesis Imperfecta (OI) Related Bone Disease. Mol. Med. 2024, 30, 66. [Google Scholar] [CrossRef] [PubMed]
- Breinbauer, R.; Mäling, M.; Ehnert, S.; Blumenstock, G.; Schwarz, T.; Jazewitsch, J.; Erne, F.; Reumann, M.K.; Rollmann, M.F.; Braun, B.J.; et al. B7-1 and PlGF-1 Are Two Possible New Biomarkers to Identify Fracture-Associated Trauma Patients at Higher Risk of Developing Complications: A Cohort Study. BMC Musculoskelet. Disord. 2024, 25, 677. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Almalki, S.G.; Agrawal, D.K. Effects of Matrix Metalloproteinases on the Fate of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2016, 7, 129. [Google Scholar] [CrossRef]
- Zhou, W.; Yan, K.; Xi, Q. BMP Signaling in Cancer Stemness and Differentiation. Cell Regen. 2023, 12, 37. [Google Scholar] [CrossRef]
- Hassan, M.Q.; Tye, C.E.; Stein, G.S.; Lian, J.B. Non-Coding RNAs: Epigenetic Regulators of Bone Development and Homeostasis. Bone 2015, 81, 746–756. [Google Scholar] [CrossRef]
- Sikora, M.; Marycz, K.; Smieszek, A. Small and Long Non-Coding RNAs as Functional Regulators of Bone Homeostasis, Acting Alone or Cooperatively. Mol. Ther.—Nucleic Acids 2020, 21, 792–803. [Google Scholar] [CrossRef]
- Groven, R.V.M.; Peniche Silva, C.J.; Balmayor, E.R.; van der Horst, B.N.J.; Poeze, M.; Blokhuis, T.J.; van Griensven, M. Specific microRNAs Are Associated with Fracture Healing Phases, Patient Age and Multi-Trauma. J. Orthop. Translat 2022, 37, 1–11. [Google Scholar] [CrossRef]
- Yao, J.; Xin, R.; Zhao, C.; Yu, C. MicroRNAs in Osteoblast Differentiation and Fracture Healing: From Pathogenesis to Therapeutic Implication. Injury 2024, 55, 111410. [Google Scholar] [CrossRef]
- Goodman, R.S.; Jung, S.; Balko, J.M.; Johnson, D.B. Biomarkers of Immune Checkpoint Inhibitor Response and Toxicity: Challenges and Opportunities. Immunol. Rev. 2023, 318, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Juan, C.; Shelton, J.M.; Paria, N.; Oxendine, I.; Wassell, M.; Kidane, Y.H.; Cornelia, R.; Jeffery, E.C.; Podeszwa, D.A.; et al. Spatial Transcriptomics Implicates Impaired BMP Signaling in NF1 Fracture Pseudarthrosis in Murine and Patient Tissues. JCI Insight 2024, 9, e176802. [Google Scholar] [CrossRef] [PubMed]
- Kaspiris, A.; Savvidou, O.D.; Vasiliadis, E.S.; Hadjimichael, A.C.; Melissaridou, D.; Iliopoulou-Kosmadaki, S.; Iliopoulos, I.D.; Papadimitriou, E.; Chronopoulos, E. Current Aspects on the Pathophysiology of Bone Metabolic Defects during Progression of Scoliosis in Neurofibromatosis Type 1. J. Clin. Med. 2022, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zheng, Y.; Liu, Y.; Yan, A.; Hu, Z.; Yang, Y.; Xiang, S.; Li, L.; Chen, W.; Peng, Y.; et al. Identification and Characterization of NF1 and Non-NF1 Congenital Pseudarthrosis of the Tibia Based on Germline NF1 Variants: Genetic and Clinical Analysis of 75 Patients. Orphanet J. Rare Dis. 2019, 14, 221. [Google Scholar] [CrossRef]
- Saul, D.; Menger, M.M.; Ehnert, S.; Nüssler, A.K.; Histing, T.; Laschke, M.W. Bone Healing Gone Wrong: Pathological Fracture Healing and Non-Unions—Overview of Basic and Clinical Aspects and Systematic Review of Risk Factors. Bioengineering 2023, 10, 85. [Google Scholar] [CrossRef]
- Frade, B.B.; Dias, R.B.; Gemini Piperni, S.; Bonfim, D.C. The Role of Macrophages in Fracture Healing: A Narrative Review of the Recent Updates and Therapeutic Perspectives. Stem Cell Investig. 2023, 10, 4. [Google Scholar] [CrossRef]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Front. Immunol. 2021, 12, 778078. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Weeks, J.; Moreno, J.R.; He, B.; Xue, T.; Rainbolt, J.; Morita, Y.; Shu, Y.; Liu, Y.; et al. Reduced Angiogenesis and Delayed Endochondral Ossification in CD163−/− Mice Highlights a Role of M2 Macrophages during Bone Fracture Repair. J. Orthop. Res. 2023, 41, 2384–2393. [Google Scholar] [CrossRef]
- Yellowley, C. CXCL12/CXCR4 Signaling and Other Recruitment and Homing Pathways in Fracture Repair. Bonekey Rep. 2013, 2, 300. [Google Scholar] [CrossRef]
- Schulze, F.; Lang, A.; Schoon, J.; Wassilew, G.I.; Reichert, J. Scaffold Guided Bone Regeneration for the Treatment of Large Segmental Defects in Long Bones. Biomedicines 2023, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Zeineddin, A.; Wu, F.; Dong, J.-F.; Huang, H.; Zou, L.; Chao, W.; Dorman, B.; Kozar, R.A. Trauma-Derived Extracellular Vesicles Are Sufficient to Induce Endothelial Dysfunction and Coagulopathy. Shock 2022, 58, 38–44. [Google Scholar] [CrossRef]
- Zieba, J.T.; Chen, Y.-T.; Lee, B.H.; Bae, Y. Notch Signaling in Skeletal Development, Homeostasis and Pathogenesis. Biomolecules 2020, 10, 332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, W.; Masson, A.; Li, Y.-P. Cell Signaling and Transcriptional Regulation of Osteoblast Lineage Commitment, Differentiation, Bone Formation, and Homeostasis. Cell Discov. 2024, 10, 1–39. [Google Scholar] [CrossRef]
- Mollentze, J.; Durandt, C.; Pepper, M.S. An In Vitro and In Vivo Comparison of Osteogenic Differentiation of Human Mesenchymal Stromal/Stem Cells. Stem Cells Int. 2021, 2021, 9919361. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.-P. Signaling and Transcriptional Regulation in Osteoblast Commitment and Differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- ElHawary, H.; Baradaran, A.; Abi-Rafeh, J.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Bone Healing and Inflammation: Principles of Fracture and Repair. Semin. Plast. Surg. 2021, 35, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Diban, F.; Lodovico, S.D.; Fermo, P.D.; D’Ercole, S.; D’Arcangelo, S.; Giulio, M.D.; Cellini, L. Biofilms in Chronic Wound Infections: Innovative Antimicrobial Approaches Using the In Vitro Lubbock Chronic Wound Biofilm Model. Int. J. Mol. Sci. 2023, 24, 1004. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Tjempakasari, A.; Suroto, H.; Santoso, D. Mesenchymal Stem Cell Senescence and Osteogenesis. Medicina 2021, 58, 61. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-Like Growth Factor-1 as a Possible Alternative to Bone Morphogenetic Protein-7 to Induce Osteogenic Differentiation of Human Mesenchymal Stem Cells in Vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef]
- Prahasanti, C.; Perdana, S. The Roles of Insulin Growth Factors-1 (IGF-1) in Bone Graft to Increase Osteogenesis. Res. J. Pharm. Technol. 2022, 15, 1737–1742. [Google Scholar] [CrossRef]
- Chaverri, D.; Vivas, D.; Gallardo-Villares, S.; Granell-Escobar, F.; Pinto, J.A.; Vives, J. A Pilot Study of Circulating Levels of TGF-Β1 and TGF-Β2 as Biomarkers of Bone Healing in Patients with Non-Hypertrophic Pseudoarthrosis of Long Bones. Bone Rep. 2021, 16, 101157. [Google Scholar] [CrossRef]
- Jiménez-Ortega, R.F.; Ortega-Meléndez, A.I.; Patiño, N.; Rivera-Paredez, B.; Hidalgo-Bravo, A.; Velázquez-Cruz, R. The Involvement of microRNAs in Bone Remodeling Signaling Pathways and Their Role in the Development of Osteoporosis. Biology 2024, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- El-Jawhari, J.J.; Kleftouris, G.; El-Sherbiny, Y.; Saleeb, H.; West, R.M.; Jones, E.; Giannoudis, P.V. Defective Proliferation and Osteogenic Potential with Altered Immunoregulatory Phenotype of Native Bone Marrow-Multipotential Stromal Cells in Atrophic Fracture Non-Union. Sci. Rep. 2019, 9, 17340. [Google Scholar] [CrossRef]
- Santolini, E.; Goumenos, S.D.; Giannoudi, M.; Sanguineti, F.; Stella, M.; Giannoudis, P.V. Femoral and Tibial Blood Supply: A Trigger for Non-Union? Injury 2014, 45, 1665–1673. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in Bone Regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Van Gastel, N.; Carmeliet, G. Bringing New Life to Damaged Bone: The Importance of Angiogenesis in Bone Repair and Regeneration. Bone 2015, 70, 19–27. [Google Scholar] [CrossRef]
- Elliott, D.S.; Newman, K.J.H.; Forward, D.P.; Hahn, D.M.; Ollivere, B.; Kojima, K.; Handley, R.; Rossiter, N.D.; Wixted, J.J.; Smith, R.M.; et al. A Unified Theory of Bone Healing and Nonunion: BHN Theory. Bone Jt. J. 2016, 98-B, 884–891. [Google Scholar] [CrossRef]
- Kanakaris, N.K.; Tosounidis, T.H.; Giannoudis, P.V. Surgical Management of Infected Non-Unions: An Update. Injury 2015, 46, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Miclau, K.R.; Brazina, S.A.; Bahney, C.S.; Hankenson, K.D.; Hunt, T.K.; Marcucio, R.S.; Miclau, T. Stimulating Fracture Healing in Ischemic Environments: Does Oxygen Direct Stem Cell Fate during Fracture Healing? Front. Cell Dev. Biol. 2017, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Hopf, H.W.; Gibson, J.J.; Angeles, A.P.; Constant, J.S.; Feng, J.J.; Rollins, M.D.; Zamirul Hussain, M.; Hunt, T.K. Hyperoxia and Angiogenesis. Wound Repair Regen. 2005, 13, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, R.E.; Silva, M.J. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013, 1, 311–322. [Google Scholar] [CrossRef]
- Glowacki, J. Angiogenesis in Fracture Repair. Clin. Orthop. Relat. Res. 1998, 355S, S82–S89. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Schiller, M.; Zeuschner, D.; Bixel, M.G.; Milia, C.; Gamrekelashvili, J.; Limbourg, A.; Medvinsky, A.; Santoro, M.M.; et al. Blood Flow Controls Bone Vascular Function and Osteogenesis. Nat. Commun. 2016, 7, 13601. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Soultanis, K.; Soucacos, P.N. Vascular Anatomy and Microcirculation of Skeletal Zones Vulnerable to Osteonecrosis: Vascularization of the Femoral Head. Orthop. Clin. N. Am. 2004, 35, 285–291. [Google Scholar] [CrossRef]
- Soucacos, P. Osteonecrosis of the Human Skeleton. Orthop. Clin. N. Am. 2004, 35, xiii–xv. [Google Scholar] [CrossRef]
- Schlundt, C.; Bucher, C.H.; Tsitsilonis, S.; Schell, H.; Duda, G.N.; Schmidt-Bleek, K. Clinical and Research Approaches to Treat Non-Union Fracture. Curr. Osteoporos. Rep. 2018, 16, 155–168. [Google Scholar] [CrossRef]
- Carlier, A.; Geris, L.; Bentley, K.; Carmeliet, G.; Carmeliet, P.; Van Oosterwyck, H. MOSAIC: A Multiscale Model of Osteogenesis and Sprouting Angiogenesis with Lateral Inhibition of Endothelial Cells. PLoS Comput. Biol. 2012, 8, e1002724. [Google Scholar] [CrossRef]
- Jakobsson, L.; Franco, C.A.; Bentley, K.; Collins, R.T.; Ponsioen, B.; Aspalter, I.M.; Rosewell, I.; Busse, M.; Thurston, G.; Medvinsky, A.; et al. Endothelial Cells Dynamically Compete for the Tip Cell Position during Angiogenic Sprouting. Nat. Cell Biol. 2010, 12, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef]
- Deckers, M.M.L.; Van Bezooijen, R.L.; Van Der Horst, G.; Hoogendam, J.; Van Der Bent, C.; Papapoulos, S.E.; Löwik, C.W.G.M. Bone Morphogenetic Proteins Stimulate Angiogenesis through Osteoblast-Derived Vascular Endothelial Growth Factor A. Endocrinology 2002, 143, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Rodan, S.B.; Wesolowski, G.; Thomas, K.A.; Yoon, K.; Rodan, G.A. Effects of Acidic and Basic Fibroblast Growth Factors on Osteoblastic Cells. Connect. Tissue Res. 1989, 20, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, H.; Bundgaard, K.G.; Christensen, K.S.; Lind, M.; Hansen, E.S.; Hvid, I. Effects of Locally Applied Vascular Endothelial Growth Factor (VEGF) and VEGF-inhibitor to the Rabbit Tibia during Distraction Osteogenesis. J. Orthop. Res. 2003, 21, 335–340. [Google Scholar] [CrossRef]
- Haubruck, P.; Kammerer, A.; Korff, S.; Apitz, P.; Xiao, K.; Büchler, A.; Biglari, B.; Zimmermann, G.; Daniel, V.; Schmidmaier, G.; et al. The Treatment of Nonunions with Application of BMP-7 Increases the Expression Pattern for Angiogenic and Inflammable Cytokines: A Matched Pair Analysis. J. Inflamm. Res. 2016, 9, 155–165. [Google Scholar] [CrossRef]
- Dimitriou, R.; Kanakaris, N.; Soucacos, P.N.; Giannoudis, P.V. Genetic Predisposition to Non-Union: Evidence Today. Injury 2013, 44, S50–S53. [Google Scholar] [CrossRef]
- Dimitriou, R.; Carr, I.M.; West, R.M.; Markham, A.F.; Giannoudis, P.V. Genetic Predisposition to Fracture Non-Union: A Case Control Study of a Preliminary Single Nucleotide Polymorphisms Analysis of the BMP Pathway. BMC Musculoskelet. Disord. 2011, 12, 44. [Google Scholar] [CrossRef]
- Guimarães, J.M.; Guimarães, I.C.d.V.; Duarte, M.E.L.; Vieira, T.; Vianna, V.F.; Fernandes, M.B.C.; Vieira, A.R.; Casado, P.L. Polymorphisms in BMP4 and FGFR1 Genes Are Associated with Fracture Non-Union. J. Orthop. Res. 2013, 31, 1971–1979. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, K.; Zhu, Y.; Wang, Z.; Li, Z.; Zhang, J. Genetic Polymorphisms of NOS2 and Predisposition to Fracture Non-Union: A Case Control Study Based on Han Chinese Population. PLoS ONE 2018, 13, e0193673. [Google Scholar] [CrossRef]
- Sathyendra, V.; Donahue, H.J.; Vrana, K.E.; Berg, A.; Fryzel, D.; Gandhi, J.; Reid, J.S. Single Nucleotide Polymorphisms in Osteogenic Genes in Atrophic Delayed Fracture-Healing: A Preliminary Investigation. J. Bone Jt. Surg. Am. 2014, 96, 1242–1248. [Google Scholar] [CrossRef] [PubMed]
- Zeckey, C.; Hildebrand, F.; Glaubitz, L.-M.; Jürgens, S.; Ludwig, T.; Andruszkow, H.; Hüfner, T.; Krettek, C.; Stuhrmann, M. Are Polymorphisms of Molecules Involved in Bone Healing Correlated to Aseptic Femoral and Tibial Shaft Non-Unions? J. Orthop. Res. 2011, 29, 1724–1731. [Google Scholar] [CrossRef]
- Yan, T.; Li, J.; Zhou, X.; Yang, Z.; Zhang, Y.; Zhang, J.; Xu, N.; Huang, Y.; Yang, H. Genetic Determinants of Fracture Non-Union: A Systematic Review from the Literature. Gene 2020, 751, 144766. [Google Scholar] [CrossRef]
- Szczęsny, G.; Olszewski, W.L.; Zagozda, M.; Rutkowska, J.; Czapnik, Z.; Swoboda-Kopeć, E.; Górecki, A. Genetic Factors Responsible for Long Bone Fractures Non-Union. Arch. Orthop. Trauma Surg. 2011, 131, 275–281. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, S.R.; Singh, A.; Kumar, V.; Walliullah, S.; Rizvi, N.; Yadav, M.; Ahmad, M.K.; Mahdi, A.A. Study of Cysteine-Rich Protein 61 Genetic Polymorphism in Predisposition to Fracture Nonunion: A Case Control. Genet. Res. Int. 2015, 2015, 754872. [Google Scholar] [CrossRef]
- McCoy, T.H.; Fragomen, A.T.; Hart, K.L.; Pellegrini, A.M.; Raskin, K.A.; Perlis, R.H. Genomewide Association Study of Fracture Nonunion Using Electronic Health Records. JBMR Plus 2019, 3, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Ali, M.; Al-Omar, H.K.; AlTabash, K.W.; AlOmran, A.K.; AlDakheel, D.A.; AlSayed, H.N. Genetic Influence of Fracture Nonunion (FNU): A Systematic Review. Pharmacogenom. Pers. Med. 2023, 16, 569–575. [Google Scholar] [CrossRef]
- Zimmermann, G.; Schmeckenbecher, K.H.K.; Boeuf, S.; Weiss, S.; Bock, R.; Moghaddam, A.; Richter, W. Differential Gene Expression Analysis in Fracture Callus of Patients with Regular and Failed Bone Healing. Injury 2012, 43, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Tran, B.N.H.; Nguyen, N.D.; Nguyen, V.X.; Center, J.R.; Eisman, J.A.; Nguyen, T.V. Genetic Profiling and Individualized Prognosis of Fracture. J. Bone Miner. Res. 2011, 26, 414–419. [Google Scholar] [CrossRef]
- Xiao, D.; Fang, L.; Liu, Z.; He, Y.; Ying, J.; Qin, H.; Lu, A.; Shi, M.; Li, T.; Zhang, B.; et al. DNA Methylation–Mediated Rbpjk Suppression Protects against Fracture Nonunion Caused by Systemic Inflammation. J. Clin. Investig. 2024, 134. [Google Scholar] [CrossRef]
- Mills, L.A.; Aitken, S.A.; Simpson, A.H.R.W. The Risk of Non-Union per Fracture: Current Myths and Revised Figures from a Population of over 4 Million Adults. Acta Orthop. 2017, 88, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Copuroglu, C.; Calori, G.M.; Giannoudis, P.V. Fracture Non-Union: Who Is at Risk? Injury 2013, 44, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Christensen, F.B.; Laursen, M.; Høy, K.; Hansen, E.S.; Bünger, C. Smoking as a Predictor of Negative Outcome in Lumbar Spinal Fusion. Spine 2001, 26, 2623–2628. [Google Scholar] [CrossRef]
- Hernigou, J.; Schuind, F. Smoking as a Predictor of Negative Outcome in Diaphyseal Fracture Healing. Int. Orthop. 2013, 37, 883–887. [Google Scholar] [CrossRef]
- Elmali, N.; Ertem, K.; Ozen, S.; Inan, M.; Baysal, T.; Güner, G.; Bora, A. Fracture Healing and Bone Mass in Rats Fed on Liquid Diet Containing Ethanol. Alcohol. Clin. Exp. Res. 2002, 26, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Zura, R.; Xiong, Z.; Einhorn, T.; Watson, J.T.; Ostrum, R.F.; Prayson, M.J.; Della Rocca, G.J.; Mehta, S.; McKinley, T.; Wang, Z.; et al. Epidemiology of Fracture Nonunion in 18 Human Bones. JAMA Surg. 2016, 151, e162775. [Google Scholar] [CrossRef]
- Schmal, H.; Brix, M.; Bue, M.; Ekman, A.; Ferreira, N.; Gottlieb, H.; Kold, S.; Taylor, A.; Tengberg, P.T.; Ban, I.; et al. Nonunion—Consensus from the 4th Annual Meeting of the Danish Orthopaedic Trauma Society. EFORT Open Rev. 2020, 5, 46. [Google Scholar] [CrossRef]
- Chaverri, D.; Vives, J. Toward the Clinical Use of Circulating Biomarkers Predictive of Bone Union. Biomark. Med. 2017, 11, 1125–1133. [Google Scholar] [CrossRef]
- Kempf, I.; Grosse, A.; Rigaut, P. The Treatment of Noninfected Pseudarthrosis of the Femur and Tibia with Locked Intramedullary Nailing. Clin. Orthop. Relat. Res. 1986, 212, 142. [Google Scholar] [CrossRef]
- Granchi, D.; Gómez-Barrena, E.; Rojewski, M.; Rosset, P.; Layrolle, P.; Spazzoli, B.; Donati, D.M.; Ciapetti, G. Changes of Bone Turnover Markers in Long Bone Nonunions Treated with a Regenerative Approach. Stem Cells Int. 2017, 2017, 3674045. [Google Scholar] [CrossRef]
- Kumar, M.; Shelke, D.; Shah, S. Prognostic Potential of Markers of Bone Turnover in Delayed-Healing Tibial Diaphyseal Fractures. Eur. J. Trauma Emerg. Surg. 2019, 45, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.; Klitscher, D.; Georg, T.; Frank, J.; Marzi, I.; Herrmann, W. Different Kinetics of Bone Markers in Normal and Delayed Fracture Healing of Long Bones. Clin. Chem. 2002, 48, 2263–2266. [Google Scholar]
- Marchelli, D.; Piodi, L.P.; Corradini, C.; Parravicini, L.; Verdoia, C.; Ulivieri, F.M. Increased Serum OPG in Atrophic Nonunion Shaft Fractures. J. Orthop. Traumatol. 2009, 10, 55–58. [Google Scholar] [CrossRef]
- Granchi, D.; Ciapetti, G.; Gómez-Barrena, E.; Rojewski, M.; Rosset, P.; Layrolle, P.; Spazzoli, B.; Donati, D.M.; Baldini, N. Biomarkers of Bone Healing Induced by a Regenerative Approach Based on Expanded Bone Marrow–Derived Mesenchymal Stromal Cells. Cytotherapy 2019, 21, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, A.; Müller, U.; Roth, H.J.; Wentzensen, A.; Grützner, P.A.; Zimmermann, G. TRACP 5b and CTX as Osteological Markers of Delayed Fracture Healing. Injury 2011, 42, 758–764. [Google Scholar] [CrossRef]
- Burska, A.N.; Giannoudis, P.V.; Tan, B.H.; Ilas, D.; Jones, E.; Ponchel, F. Dynamics of Early Signalling Events during Fracture Healing and Potential Serum Biomarkers of Fracture Non-Union in Humans. J. Clin. Med. 2020, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Y.; Yu, Y.; Zhao, Y.; Zhang, D.; Yu, A. Identification of Up-Regulated ANXA3 Resulting in Fracture Non-Union in Patients With T2DM. Front. Endocrinol. 2022, 13, 890941. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Zimmerman, G.; Marcucio, R. Biological Perspectives of Delayed Fracture Healing. Injury 2014, 45, S8–S15. [Google Scholar] [CrossRef]
- Zimmermann, G.; Henle, P.; Küsswetter, M.; Moghaddam, A.; Wentzensen, A.; Richter, W.; Weiss, S. TGF-Beta1 as a Marker of Delayed Fracture Healing. Bone 2005, 36, 779–785. [Google Scholar] [CrossRef]
- Xu, J.; Liu, J.; Gan, Y.; Dai, K.; Zhao, J.; Huang, M.; Huang, Y.; Zhuang, Y.; Zhang, X. High-Dose TGF-Β1 Impairs Mesenchymal Stem Cell-Mediated Bone Regeneration via Bmp2 Inhibition. J. Bone Miner. Res. 2020, 35, 167–180. [Google Scholar] [CrossRef]
- Granchi, D.; Devescovi, V.; Pratelli, L.; Verri, E.; Magnani, M.; Donzelli, O.; Baldini, N. Serum Levels of Fibroblast Growth Factor 2 in Children with Orthopedic Diseases: Potential Role in Predicting Bone Healing. J. Orthop. Res. 2013, 31, 249–256. [Google Scholar] [CrossRef]
- Goebel, S.; Lienau, J.; Rammoser, U.; Seefried, L.; Wintgens, K.F.; Seufert, J.; Duda, G.; Jakob, F.; Ebert, R. FGF23 Is a Putative Marker for Bone Healing and Regeneration. J. Orthop. Res. 2009, 27, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Yang, K.D.; Ko, J.-Y.; Huang, C.-C.; Huang, H.-Y.; Wang, F.-S. The Effects of Shockwave on Bone Healing and Systemic Concentrations of Nitric Oxide (NO), TGF-Beta1, VEGF and BMP-2 in Long Bone Non-Unions. Nitric Oxide 2009, 20, 298–303. [Google Scholar] [CrossRef]
- Peng, H.; Lu, S.-L.; Bai, Y.; Fang, X.; Huang, H.; Zhuang, X.-Q. MiR-133a Inhibits Fracture Healing via Targeting RUNX2/BMP2. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Jian, G.; Xie, D.; Kuang, X.; Zheng, P.; Liu, H.; Dong, X. Identification and Validation of miR-29b-3p and LIN7A as Important Diagnostic Markers for Bone Non-Union by WGCNA. J. Cell. Mol. Med. 2024, 28, e18522. [Google Scholar] [CrossRef]
- Wei, J.; Chen, H.; Fu, Y.; Zhang, B.; Zhang, L.; Tao, S.; Lin, F. Experimental Study of Expression Profile and Specific Role of Human microRNAs in Regulating Atrophic Bone Nonunion. Medicine 2020, 99, e21653. [Google Scholar] [CrossRef]
- Breulmann, F.L.; Hatt, L.P.; Schmitz, B.; Wehrle, E.; Richards, R.G.; Della Bella, E.; Stoddart, M.J. Prognostic and Therapeutic Potential of microRNAs for Fracture Healing Processes and Non-Union Fractures: A Systematic Review. Clin. Transl. Med. 2023, 13, e1161. [Google Scholar] [CrossRef] [PubMed]
- Waki, T.; Lee, S.Y.; Niikura, T.; Iwakura, T.; Dogaki, Y.; Okumachi, E.; Kuroda, R.; Kurosaka, M. Profiling microRNA Expression in Fracture Nonunions: Potential Role of microRNAs in Nonunion Formation Studied in a Rat Model. Bone Jt. J. 2015, 97-B, 1144–1151. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Frey, S.P.; Zellweger, R. Bone Morphogenetic Proteins in Clinical Applications. ANZ J. Surg. 2007, 77, 626–631. [Google Scholar] [CrossRef]
- Olson, S.; Hahn, D. Surgical Treatment of Non-Unions: A Case for Internal Fixation. Injury 2006, 37, 681–690. [Google Scholar] [CrossRef]
- Pederson, W.C.; Person, D.W. Long Bone Reconstruction with Vascularized Bone Grafts. Orthop. Clin. N. Am. 2007, 38, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Miclau, T. Autologous Iliac Crest Bone Graft: Should It Still Be the Gold Standard for Treating Nonunions? Injury 2007, 38, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.A.; Benirschke, S.K. Treatment of Tibial Malunions and Nonunions with Reamed Intramedullary Nails. Orthop. Clin. N. Am. 1990, 21, 715–724. [Google Scholar] [CrossRef]
- Jauregui, J.J.; Bor, N.; Thakral, R.; Standard, S.C.; Paley, D.; Herzenberg, J.E. Life- and Limb-Threatening Infections Following the Use of an External Fixator. Bone Jt. J. 2015, 97-B, 1296–1300. [Google Scholar] [CrossRef]
- Cox, G.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Reamer-Irrigator-Aspirator Indications and Clinical Results: A Systematic Review. Int. Orthop. (SICOT) 2011, 35, 951–956. [Google Scholar] [CrossRef]
- Tsang, S.T.J.; Mills, L.A.; Frantzias, J.; Baren, J.P.; Keating, J.F.; Simpson, A.H.R.W. Exchange Nailing for Nonunion of Diaphyseal Fractures of the Tibia: Our Results and an Analysis of the Risk Factors for Failure. Bone Jt. J. 2016, 98-B, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Busse, J.W.; Bhandari, M.; Kulkarni, A.V.; Tunks, E. The Effect of Low-Intensity Pulsed Ultrasound Therapy on Time to Fracture Healing: A Meta-Analysis. CMAJ 2002, 166, 437. [Google Scholar] [PubMed]
- Griffin, M.; Bayat, A. Electrical Stimulation in Bone Healing: Critical Analysis by Evaluating Levels of Evidence. Eplasty 2011, 11, e34. [Google Scholar]
- Schofer, M.D.; Block, J.E.; Aigner, J.; Schmelz, A. Improved Healing Response in Delayed Unions of the Tibia with Low-Intensity Pulsed Ultrasound: Results of a Randomized Sham-Controlled Trial. BMC Musculoskelet. Disord. 2010, 11, 229. [Google Scholar] [CrossRef]
- Evans, C.H. Gene Therapy for Bone Healing. Expert. Rev. Mol. Med. 2010, 12, e18. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone Regeneration: Current Concepts and Future Directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, B.C.; Zhang, J.J.; Xu, C.; Zhang, L.C.; Kang, J.Y.; Zhao, H. Treatment of Rabbit Femoral Defect by Firearm With BMP-4 Gene Combined With TGF-Β1. J. Trauma Inj. Infect. Crit. Care 2009, 66, 450–456. [Google Scholar] [CrossRef]
- Chan, A.; Tsourkas, A. Intracellular Protein Delivery: Approaches, Challenges, and Clinical Applications. BME Front. 2024, 5, 0035. [Google Scholar] [CrossRef]
- Young, L.S.; Searle, P.F.; Onion, D.; Mautner, V. Viral Gene Therapy Strategies: From Basic Science to Clinical Application. J. Pathol. 2006, 208, 299–318. [Google Scholar] [CrossRef]
- Kurian, K.M. Retroviral Vectors. Mol. Pathol. 2000, 53, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, C.; Philippe, S.; Mallet, J.; Serguera, C. Non-Integrating Lentiviral Vectors. Curr. Gene Ther. 2008, 8, 430–437. [Google Scholar] [CrossRef]
- Douglas, J.T. Adenoviral Vectors for Gene Therapy. Mol. Biotechnol. 2007, 36, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Glover, D.J.; Lipps, H.J.; Jans, D.A. Towards Safe, Non-Viral Therapeutic Gene Expression in Humans. Nat. Rev. Genet. 2005, 6, 299–310. [Google Scholar] [CrossRef]
- Evans, C.; Liu, F.-J.; Glatt, V.; Hoyland, J.; Kirker-Head, C.; Walsh, A.; Betz, O.; Wells, J.; Betz, V.; Porter, R.M.; et al. Use of Genetically Modified Muscle and Fat Grafts to Repair Defects in Bone and Cartilage. Eur. Cells Mater. 2009, 18, 96–111. [Google Scholar] [CrossRef]
- Pountos, I.; Georgouli, T.; Kontakis, G.; Giannoudis, P.V. Efficacy of Minimally Invasive Techniques for Enhancement of Fracture Healing: Evidence Today. Int. Orthop. (SICOT) 2010, 34, 3–12. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O.C. Bridging the Regeneration Gap: Stem Cells, Biomaterials and Clinical Translation in Bone Tissue Engineering. Arch. Biochem. Biophys. 2008, 473, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, J.; Leyh, B. Paracrine Effects of Stem Cells in Wound Healing and Cancer Progression. Int. J. Oncol. 2014, 44, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.G.; Brady, D.J.; Healy, K.M.; Henry, K.A.; Ogunsola, A.S.; Ma, X. Stem Cells and Acellular Preparations in Bone Regeneration/Fracture Healing: Current Therapies and Future Directions. Cells 2024, 13, 1045. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef]
- Ismail, H.D.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Kusnadi, Y.; Merlina, M.; Yulisa, N.D. Mesenchymal Stem Cell Implantation in Atrophic Nonunion of the Long Bones: A Translational Study. Bone Jt. Res. 2016, 5, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Su, Y.; Li, J.; Liu, X.; Liu, Y.; Zhao, L.; Li, L.; Zhang, L. Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells: Whether They Can Become New Stars of Cell Therapy. Stem Cell Res. Ther. 2024, 15, 367. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Z.; Ge, C.; Krebsbach, P.; Franceschi, R.T. Healing Cranial Defects with AdRunx2-Transduced Marrow Stromal Cells. J. Dent. Res. 2007, 86, 1207–1211. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current Concepts of Molecular Aspects of Bone Healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Al-Hamed, F.S.; Abu-Nada, L.; Rodan, R.; Sarrigiannidis, S.; Ramirez-Garcialuna, J.L.; Moussa, H.; Elkashty, O.; Gao, Q.; Basiri, T.; Baca, L.; et al. Differences in Platelet-rich Plasma Composition Influence Bone Healing. J. Clin. Periodontol. 2021, 48, 1613–1623. [Google Scholar] [CrossRef]
- Chen, F.; Ma, Z.; Dong, G.; Wu, Z. Composite Glycidyl Methacrylated Dextran (Dex-GMA)/Gelatin Nanoparticles for Localized Protein Delivery. Acta Pharmacol. Sin. 2009, 30, 485–493. [Google Scholar] [CrossRef]
- Dimitriou, R.; Giannoudis, P.V. The Genetic Profile of Bone Repair. Clin. Cases Miner. Bone Metab. 2013, 10, 19–21. [Google Scholar] [CrossRef] [PubMed]
- Reumann, M.K.; Nair, T.; Strachna, O.; Boskey, A.L.; Mayer-Kuckuk, P. Production of VEGF Receptor 1 and 2 mRNA and Protein during Endochondral Bone Repair Is Differential and Healing Phase Specific. J. Appl. Physiol. 2010, 109, 1930–1938. [Google Scholar] [CrossRef]
- Trenkmann, M. GWAS Cracks Fracture Risk. Nat. Rev. Rheumatol. 2019, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, Y.; Zhang, T.; Wang, H.; Hou, Z.; Zhang, Y.; Cui, W.; Chen, W. “Genetic Scissors” CRISPR/Cas9 Genome Editing Cutting-Edge Biocarrier Technology for Bone and Cartilage Repair. Bioact. Mater. 2023, 22, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D Bioprinting Methods and Techniques: Applications on Artificial Blood Vessel Fabrication. Acta Cardiol. Sin. 2019, 35, 284. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture Healing: Mechanisms and Interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone Regenerative Medicine: Classic Options, Novel Strategies, and Future Directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Advancements and Frontiers in Nano-Based 3D and 4D Scaffolds for Bone and Cartilage Tissue Engineering. Int. J. Nanomed. 2019, 14, 4333–4351. [Google Scholar] [CrossRef]
- Tafat, W.; Budka, M.; McDonald, D.; Wainwright, T.W. Artificial Intelligence in Orthopaedic Surgery: A Comprehensive Review of Current Innovations and Future Directions. Comput. Struct. Biotechnol. Rep. 2024, 1, 100006. [Google Scholar] [CrossRef]
- Liu, H.; Chen, H.; Han, Q.; Sun, B.; Liu, Y.; Zhang, A.; Fan, D.; Xia, P.; Wang, J. Recent Advancement in Vascularized Tissue-Engineered Bone Based on Materials Design and Modification. Mater. Today Bio 2023, 23, 100858. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Li, R.; Bai, H.; Zhu, Z.; Zhu, L.; Zhu, C.; Che, Z.; Liu, H.; Wang, J.; et al. Collagen-Based Biomaterials for Bone Tissue Engineering. Mater. Des. 2021, 210, 110049. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Wang, D.; Yi, Y.; Liu, Z.; Wu, M.; Wu, Y.; Zhang, Q. Landscape of the Epigenetic Regulation in Wound Healing. Front. Physiol. 2022, 13, 949498. [Google Scholar] [CrossRef]
- Ball, J.R.; Shelby, T.; Hernandez, F.; Mayfield, C.K.; Lieberman, J.R. Delivery of Growth Factors to Enhance Bone Repair. Bioengineering 2023, 10, 1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, L.; Theopold, J.; Schleifenbaum, S.; Heyde, C.-E.; Osterhoff, G. Methods for Bone Quality Assessment in Human Bone Tissue: A Systematic Review. J. Orthop. Surg. Res. 2022, 17, 174. [Google Scholar] [CrossRef]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2018, 2495848. [Google Scholar] [CrossRef]
- Mousaei Ghasroldasht, M.; Seok, J.; Park, H.-S.; Liakath Ali, F.B.; Al-Hendy, A. Stem Cell Therapy: From Idea to Clinical Practice. Int. J. Mol. Sci. 2022, 23, 2850. [Google Scholar] [CrossRef] [PubMed]
- Bowles-Welch, A.C.; Jimenez, A.C.; Stevens, H.Y.; Frey Rubio, D.A.; Kippner, L.E.; Yeago, C.; Roy, K. Mesenchymal Stromal Cells for Bone Trauma, Defects, and Disease: Considerations for Manufacturing, Clinical Translation, and Effective Treatments. Bone Rep. 2023, 18, 101656. [Google Scholar] [CrossRef]
- Smolinska, V.; Csobonyeiova, M.; Zamborsky, R.; Danisovic, L. Stem Cells and Their Derivatives: An Implication for the Regeneration of Nonunion Fractures. Cell Transplant. 2023, 32, 09636897231183530. [Google Scholar] [CrossRef]
- Veith, A.P.; Henderson, K.; Spencer, A.; Sligar, A.D.; Baker, A.B. Therapeutic Strategies for Enhancing Angiogenesis in Wound Healing. Adv. Drug Deliv. Rev. 2019, 146, 97–125. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, E.; Lu, L.; Zhang, H.; Yang, X.; Hao, Y. Effectiveness of Low-Intensity Pulsed Ultrasound on Osteoarthritis: Molecular Mechanism and Tissue Engineering. Front. Med. 2024, 11, 1292473. [Google Scholar] [CrossRef]
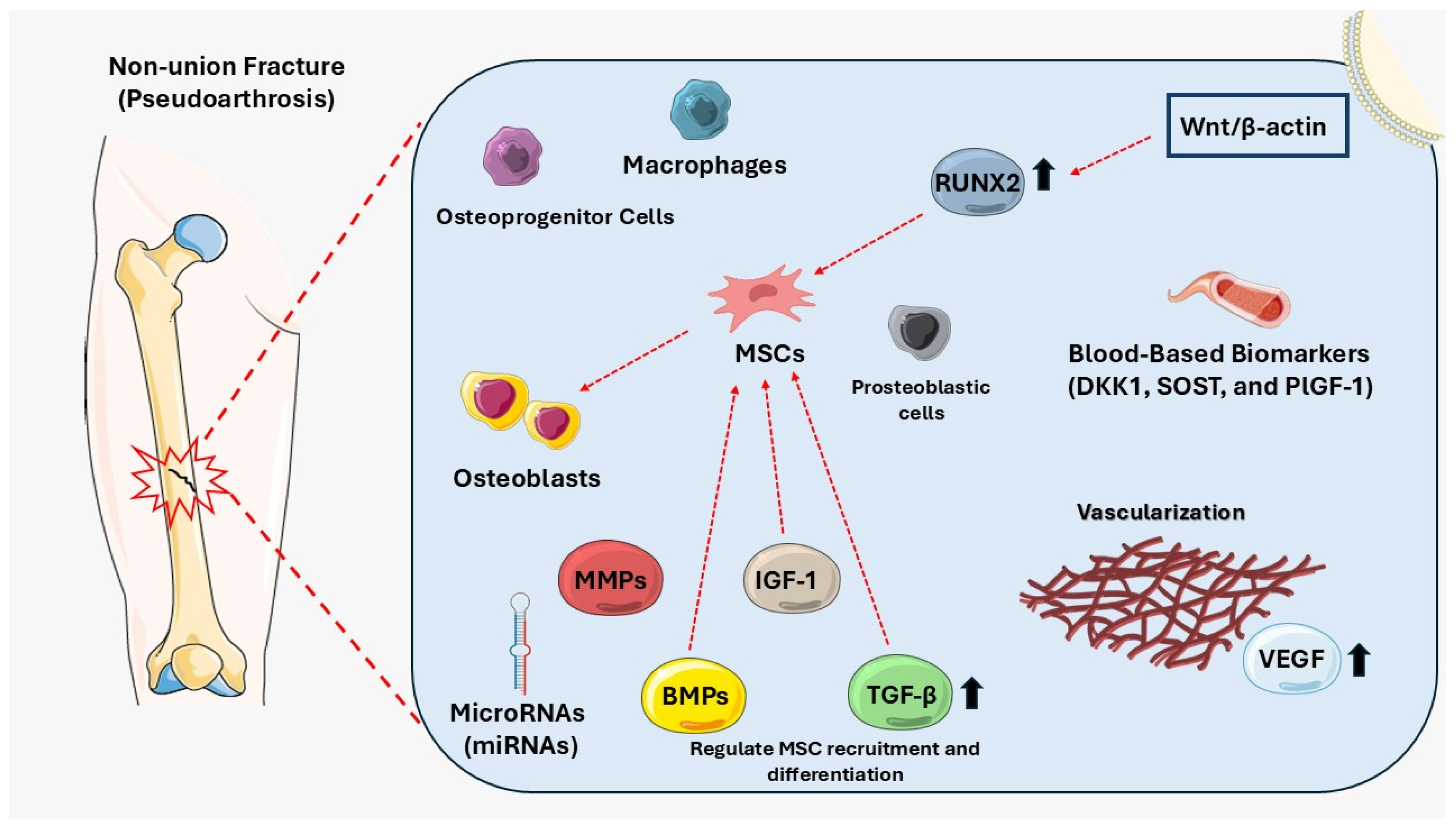
| Biological Factor | Role in Non-Union (Pseudoarthrosis) | References |
|---|---|---|
| Vascularization | Facilitates oxygen, nutrient, and signaling molecule delivery for osteogenesis. Impaired angiogenesis, often due to VEGF deficiency, results in poor vascular supply and delayed healing. | [25] |
| Mesenchymal stem cells (MSCs) | Essential for osteoblast differentiation and bone repair. MSC dysfunction or senescence reduces regenerative capacity and creates an inflammatory environment, impairing healing. | [34,82] |
| Bone morphogenetic proteins (BMPs) | Promote MSC differentiation into osteoblasts. Reduced BMP levels or increased inhibitors (e.g., Noggin, Gremlin) disrupt bone repair and contribute to non-union. | [17,46,50] |
| Insulin-like growth factor-1 (IGF-1) | Enhances osteoblast proliferation, bone matrix synthesis, and MSC differentiation, promoting bone regeneration. Moreover, IGF-1 upregulates osteocalcin and osteopontin while reducing osteoclast activity, improving fracture healing and reducing non-union risk. | [83,84] |
| Transforming growth factor-beta (TGF-β) | Regulates MSC recruitment and differentiation. Overactive TGF-β signaling might lead to fibrosis, impairing osteogenesis and contributing to fracture non-union. | [43,46,85] |
| Blood-based biomarkers | Biomarkers such as DKK1, SOST, and PlGF-1 indicate imbalances in bone formation and resorption. Elevated levels are associated with increased non-union risk. | [21] |
| Matrix metalloproteinases (MMPs) | Degrade extracellular matrix during healing. Overactive MMPs (e.g., MMP-7, MMP-12) disrupt BMP signaling and impair bone regeneration, leading to pseudoarthrosis. | [58] |
| MicroRNAs (miRNAs) | Regulate gene expression, essential for osteogenesis. Aberrant miRNA activity (e.g., hsa-miR-149, hsa-miR-221) suppresses bone-forming genes, hindering fracture repair. | [86] |
| Macrophages | M1 macrophages drive inflammation; M2 macrophages support repair and vascularization. Dysregulated macrophage activity delays healing and fosters non-union. | [87] |
| Osteoprogenitor cells | Contribute to bone formation. Decreased numbers or impaired differentiation due to aging or trauma correlate with delayed healing and non-union development. | [88] |
| Osteoblasts | Responsible for bone formation. Impaired osteoblast maturation, marked by decreased RUNX2 and OCN expression, delays fracture healing in pseudoarthrosis. | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsifaki, A.; Kalouda, G.; Maroulaki, S.; Foukas, A.; Armakolas, A. The Genetic and Biological Basis of Pseudoarthrosis in Fractures: Current Understanding and Future Directions. Diseases 2025, 13, 75. https://doi.org/10.3390/diseases13030075
Kotsifaki A, Kalouda G, Maroulaki S, Foukas A, Armakolas A. The Genetic and Biological Basis of Pseudoarthrosis in Fractures: Current Understanding and Future Directions. Diseases. 2025; 13(3):75. https://doi.org/10.3390/diseases13030075
Chicago/Turabian StyleKotsifaki, Amalia, Georgia Kalouda, Sousanna Maroulaki, Athanasios Foukas, and Athanasios Armakolas. 2025. "The Genetic and Biological Basis of Pseudoarthrosis in Fractures: Current Understanding and Future Directions" Diseases 13, no. 3: 75. https://doi.org/10.3390/diseases13030075
APA StyleKotsifaki, A., Kalouda, G., Maroulaki, S., Foukas, A., & Armakolas, A. (2025). The Genetic and Biological Basis of Pseudoarthrosis in Fractures: Current Understanding and Future Directions. Diseases, 13(3), 75. https://doi.org/10.3390/diseases13030075





