The Severity of Carotid Calcifications, but Not Fibroblast Growth Factor 23, Is Associated with Mortality in Hemodialysis: A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Düsing, P.; Zietzer, A.; Goody, P.R.; Hosen, M.R.; Kurts, C.; Nickenig, G.; Jansen, F. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Bellasi, A.; Di Lullo, L.; Russo, D.; Ciarcia, R.; Magnocavalo, M.; Lavalle, C.; Di Iorio, B.R. Predictive value of measures of vascular calcification burden and progression for risk of death in incident to dialysis patients. J. Clin. Med. 2021, 10, 376. [Google Scholar] [CrossRef]
- Singh, A.; Tandon, S.; Tandon, C. An update on vascular calcification and potential therapeutics. Mol. Biol. Rep. 2021, 48, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Prié, D. FGF23 and cardiovascular risk. Ann. Endocrinol. 2021, 82, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Schuchhardt, J.; Bauer, C.; Brinker, M.; Kleinjung, F.; Vaitsiakhovich, T. Risk prediction modeling for cardiorenal clinical outcomes in patients with non-diabetic CKD using US nationwide real-world data. BMC Nephrol. 2025, 26, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Tang, R.N.; Wang, L.T.; Liu, B.C. Role of crosstalk between endothelial cells and smooth muscle cells in vascular calcification in chronic kidney disease. Cell Prolif. 2021, 54, e12980. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Araujo, N.; Marreiros, C.; Simes, D. The interplay between mineral metabolism, vascular calcification and inflammation in chronic kidney disease (CKD): Challenging old concepts with new facts. Aging 2019, 11, 4274–4299. [Google Scholar] [CrossRef] [PubMed]
- Hortells, L.; Sur, S.; St. Hilarie, C. Cell phenotype transitions in cardiovascular calcification. Front. Cardiovasc. Med. 2018, 5, 27. [Google Scholar] [CrossRef]
- Moldovan, D.; Moldovan, I.; Rusu, C.; Racasan, S.; Patiu, I.M.; Brumboiu, A.; Bondor, C.; Parvu, L.; Kacso, I.; Orasan, R.; et al. Vascular calcifications and renal osteodystrophy in chronic hemodialysis patients: What is the relationship between them? Int. Urol. Nephrol. 2011, 43, 1179–1186. [Google Scholar] [CrossRef]
- Ketteler, M.; Evenepoel, P.; Holden, R.M.; Isakova, T.; Jørgensen, H.S.; Komaba, H.; Wong, J. Chronic kidney disease–mineral and bone disorder: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2025, 107, 405–423. [Google Scholar] [CrossRef]
- Hannemann, A.; Nauck, M.; Völzke, H.; Weidner, H.; Platzbecker, U.; Hofbauer, L.C.; Baschant, U. Interactions of anemia, FGF-23, and bone in healthy adults—Results from the Study of Health in Pomerania (SHIP). J. Clin. Endocrinol. Metab. 2021, 106, e288–e299. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Leibrock, C.; Pandyra, A.A.; Stournaras, C.; Wagner, A.; Föller, M. Phosphate Homeostasis, Inflammation and the Regulation of FGF-23. Kidney Blood Press. Res. 2018, 43, 1742–1748. [Google Scholar] [CrossRef]
- Mendoza, J.M.; Isakova, T.; Cai, X.; Bayes, L.Y.; Faul, C.; Scialla, J.J.; Townsend, R.R. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 2017, 91, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Titan, S.M.; Zatzm, R.; Graciolli, F.G.; Dos Reis, L.M.; Barros, R.T.; Jorgetti, V.; Moysés, R.M. FGF-23 as a predictor of renal outcome in diabetic nephropathy. Clin. J. Am. Soc. Nephrol. 2011, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, D.; Moldovan, I.; Rusu, C.; Kacso, I.; Patiu, I.M.; Gherman-Caprioara, M. FGF-23, vascular calcification, and cardiovascular diseases in chronic hemodialysis patients. Int Urol. Nephrol. 2014, 46, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Maahs, E.; Schwartz, A.; Berezowitz, A.; Davis, S.; Guzman, R.J. An ultrasound-based femoral artery calcification score. J. Vasc. Surg. Cases Innov. Tech. 2024, 10, 101381. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Macías, J.G. Atherosclerosis, vascular calcification and osteoporosis. Med. Clínica 2025, 164, e13–e20. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Song, F.; Wang, L.; Fu, Q.; Cao, S.; Lu, Z. Carotid atherosclerosis detected by ultrasonography: A national Cross-Sectional study. J. Am. Heart Assoc. 2018, 7, e008701. [Google Scholar] [CrossRef] [PubMed]
- Mizuiri, S.; Nishizawa, Y.; Doi, T.; Yamashita, K.; Shigemoto, K.; Usui, K.; Masaki, T. Iron, coronary artery calcification, and mortality in patients undergoing hemodialysis. Ren. Fail. 2021, 43, 371–380. [Google Scholar] [CrossRef]
- Asicioglu, E.; Velioglu, A.; Arikan, H.; Koc, M.; Tuglular, S.; Ozener, C. Baseline carotid intima media thickness is associated with cardiovascular morbidity and mortality in peritoneal dialysis patients. Ther. Apher. Dial. 2021, 25, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Kusic Milicevic, J.; Vidakovic, R.; Markovic, R.; Andjelkovic Apostolovic, M.; Korac, M.; Trbojevic Stankovic, J.; Dragovic, G. Cardiovascular risk assessment and coronary artery calcification burden in asymptomatic patients in the initial years of haemodialysis. Ther. Apher. Dial. 2022, 6, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Liou, H.H.; Wu, C.K. Moderate-severe aortic arch calcification and high serum alkaline phosphatase co-modify the risk of cardiovascular events and mortality among chronic hemodialysis patients. Ren. Fail. 2025, 47, 2449572. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, O.M.; Mannstadt, M.; Isakova, T.; Rauh-Hain, J.A.; Tamez, H.; Shah, A.; Smith, K.; Lee, H.; Thadhani, R.; Jüppner, H.; et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N. Engl. J. Med. 2008, 359, 584–592. [Google Scholar] [CrossRef]
- Alderson, H.V.; Ritchie, J.P.; Middleton, R.; Larsson, A.; Larsson, T.E.; Kalra, P.A. FGF-23 and Osteoprotegerin but not Fetuin-A are associated with death and enhance risk prediction in non-dialysis chronic kidney disease stages 3–5. Nephrology 2016, 21, 566–573. [Google Scholar] [CrossRef]
- Salam, S.; Gallagher, O.; Gossiel, F.; Paggiosi, M.; Eastell, R.; Khwaja, A. Vascular calcification relationship to vascular biomarkers and bone metabolism in advanced chronic kidney disease. Bone 2021, 143, 115699. [Google Scholar] [CrossRef] [PubMed]
- Block, G.A.; Chertow, G.M.; Cooper, K.; Xing, S.; Fouqueray, B.; Halperin, M.; Danese, M.D. Fibroblast growth factor 23 as a risk factor for cardiovascular events and mortality in patients in the EVOLVE trial. Hemodial. Int. 2021, 25, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Coban, M.; Yilmaz, U.; Suleyman, D.; Asilturk, E.; Sozer, Y.; Bekir, E.R.; Ellidag, H.Y. Intact Fibroblast Growth Factor 23 and peripheral vascular complications in patients on hemodialysis. Dicle Tıp Dergisi 2020, 47, 66–73. [Google Scholar] [CrossRef]
- De Jong, M.A.; Eisenga, M.F.; van Ballegooijen, A.J.; Beulens, J.W.; Vervloet, M.G.; Navis, G.; De Borst, M.H. Fibroblast growth factor 23 and new-onset chronic kidney disease in the general population: The Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Nephrol. Dial. Transplant. 2021, 36, 121–128. [Google Scholar] [CrossRef]
- Olauson, H.; Qureshi, A.R.; Miyamoto, T.; Barany, P.; Heimburger, O.; Lindholm, B.; Stenvinkel, P.; Larsson, T.E. Relation between serum fibroblast growth factor-23 level and mortality in incident dialysis patients: Are gender and cardiovascular disease confounding the relationship? Nephrol. Dial. Transplant. 2010, 25, 3033–3038. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.; Kim, E.D.; Sozio, S.M.; Jaar, B.G.; Estrella, M.M.; Monroy-Trujillo, J.M.; Parekh, R.S. Calcification biomarkers, subclinical vascular disease, and mortality among multiethnic dialysis patients. Kidney Int. Rep. 2020, 5, 1729–1737. [Google Scholar] [CrossRef]
- Bouma-de Krijger, A.; de Roij van Zuijdewijn, C.L.; Nubé, M.J.; Grooteman, M.P.; Vervloet, M.G.; CONTRAST Study Group. Change in FGF23 concentration over time and its association with all-cause mortality in patients treated with haemodialysis or haemodiafiltration. Clin. Kidney J. 2021, 14, 891–897. [Google Scholar] [CrossRef]
- Palupi-Baroto, R.; Hermawan, K.; Murni, I.K.; Nurlita, T.; Prihastuti, Y.; Puspitawati, I.; Tandri, C.C.; Ambarsari, C.G. Carotid intima-media thickness, fibroblast growth factor 23, and mineral bone disorder in children with chronic kidney disease. BMC Nephrol. 2024, 25, 369. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, Y.; Hosoda, Y.; Horimoto, A.; Omae, K.; Ito, K.; Higuci, C.; Sakura, H.; Nitta, K.; Ogawa, T. Fibroblast growth factor 23 (FGF23) level is associated with ultrafiltration rate in patients on hemodialysis. Heart Vessel. 2020, 36, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Edmonston, D.; Grabner, A.; Wolf, M. FGF23 and klotho at the intersection of kidney and cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 11–24. [Google Scholar] [CrossRef]
- Kacso, I.M.; Potra, A.R.; Bondor, C.I.; Moldovan, D.; Rusu, C.; Patiu, I.M.; Kacso, G. Adiponectin predicts cardiovascular events in diabetes dialysis patients. Clin. Biochem. 2015, 48, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Kanda, E.; Lopes, M.B.; Tsuruya, K.; Hirakata, H.; Iseki, K.; Karaboyas, A.; Bieber, b.; Jacobson, S.J.; Dasgupta, I.; Robinson, B.M. The combination of malnutrition-inflammation and functional status limitations is associated with mortality in hemodialysis patients. Sci. Rep. 2021, 11, 1582. [Google Scholar] [CrossRef]
- Huang, J.; Hao, J.; Luo, H.; Chen, L.; Luo, H.; Liu, H.; Wang, P. Construction of a C-reactive protein-albumin-lymphocyte index-based prediction model for all-cause mortality in patients on maintenance hemodialysis. Ren. Fail. 2025, 47, 2444396. [Google Scholar] [CrossRef]
- Sánchez-Duffhues, G.; García de Vinuesa, A.; van de Pol, V.; Geerts, M.E.; de Vries, M.R.; Janson, S.G.; van Dam, H.; Lindeman, J.H.; Goumans, M.J.; Ten Dijke, P. Inflammation induces endothelial-to-mesenchymal transition and promotes vascular calcification through downregulation of BMPR2. J. Pathol. 2019, 247, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Donate-Correa, J.; Martín-Núñez, E.; Hernández-Carballo, C.; González-Luis, A.; Mora-Fernández, C.; Martín-Olivera, A.; Navarro-González, J.F. FGF23 as a potential pathophysiological factor in peripheral arterial disease associated with chronic kidney disease. Int. J. Mol. Sci. 2024, 25, 5457. [Google Scholar] [CrossRef] [PubMed]
- Izzo, C.; Secondulfo, C.; Bilancio, G.; Visco, V.; Virtuoso, N.; Migliarino, S.; Vecchione, C. Chronic kidney disease with mineral bone disorder and vascular calcification: An overview. Life 2024, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Xia, Z.; Qing, B.; Wang, W.; Chen, H.; Wang, J.; Yuan, Y. Systemic Inflammatory Response Index (SIRI) is associated with all-cause mortality and cardiovascular mortality in population with chronic kidney disease: Evidence from NHANES (2001–2018). Front. Immunol. 2024, 15, 1338025. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. Available online: https://kdigo.org/guidelines/ckd-evaluation-and-management/ (accessed on 22 January 2025). [CrossRef] [PubMed]
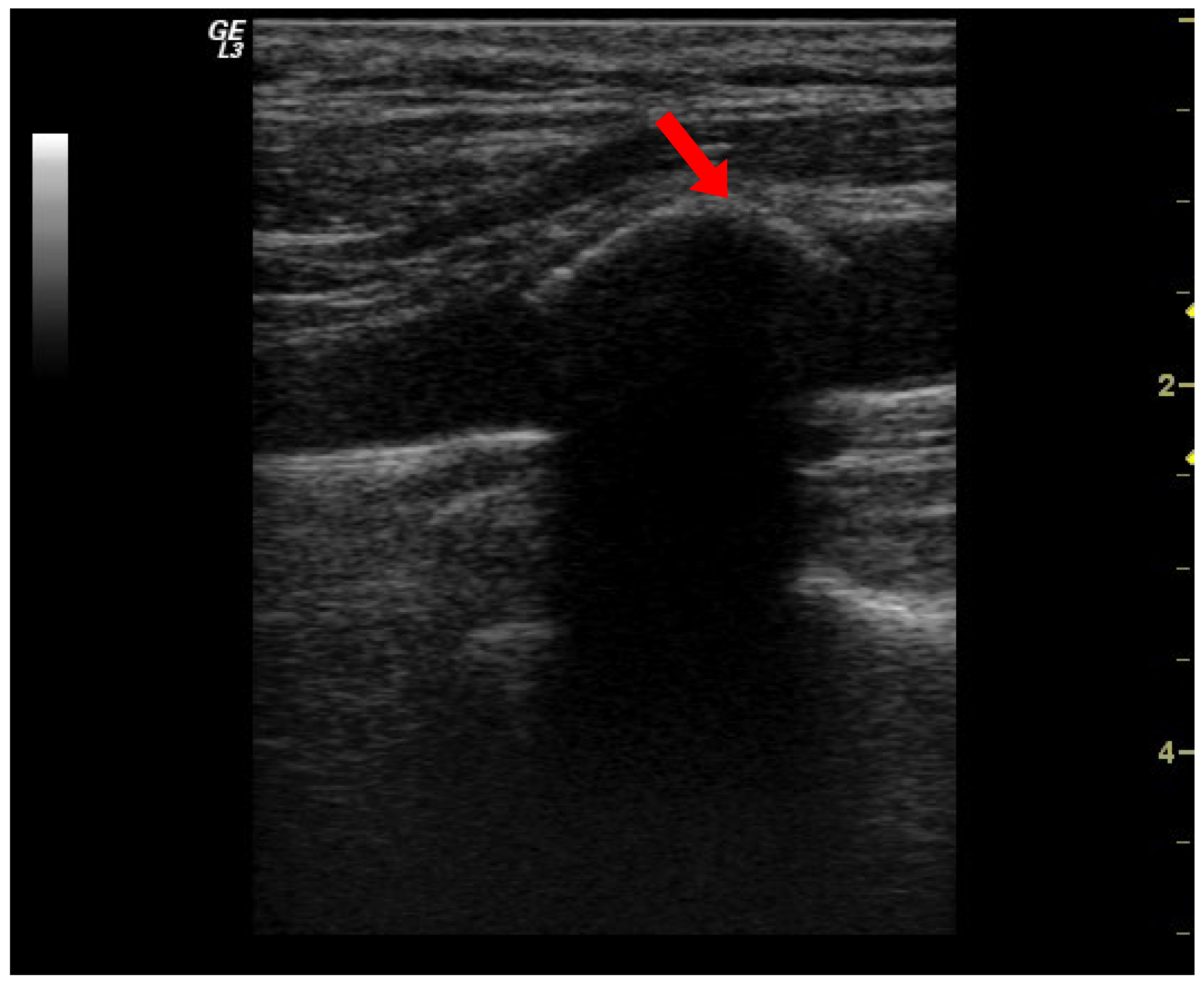

| Deceased (49 Patients) | Survivors (39 Patients) | p | |
|---|---|---|---|
| Age (years) | 63 (59–72) | 56 (46–64) | 0.003 |
| HD vintage (months) | 47 (29–64) | 49 (29.5–68) | 0.628 |
| Gender, males no. (%) | 27 (55.1) | 18 (46.2) | 0.404 |
| Diabetes, no. (%) | 17 (34.7) | 3 (7.7) | 0.003 |
| HTN, no. (%) | 38 (77.6) | 25 (64.1) | 0.165 |
| FGF-23 (pg/mL) | 42.30 (23.10–72.70) | 41.70 (20.95–70.90) | 0.592 |
| VC score | 4 (1–6) | 0 (0–2) | <0.001 |
| URR | 72.32 (69.57–79.45) | 77.33 (69.63–80.65) | 0.183 |
| spKt/V | 1.46(1.39–1.63) | 1.58 (1.40–1.66) | 0.183 |
| Bicarbonate (mEq/L) | 24 (22.7–25.6) | 22.9 (20.5–24.6) | 0.084 |
| K (mEq/L) | 4.48 ± 0.64 | 4.72 ± 0.55 | 0.067 |
| Ca (mg/dL) | 9.09 ± 0.65 | 9.07 ± 0.67 | 0.898 |
| P (mg/dL) | 4.34 ± 1.10 | 4.57 ± 1.54 | 0.427 |
| ALP (U/L) | 78.68 (64.13–99.56) | 67.75 (45.22–103.84) | 0.957 |
| iPTH (pg/mL) | 212.25 (144.75–547.65) | 273.4 (160.95–536) | 0.332 |
| Hb (g/dL) | 11.3 (10.6–12) | 11.4 (10.5–12.35) | 0.072 |
| Ferritin (ng/mL) | 498.51 (303.45–791.35) | 586.22 (402.46–816.45) | 0.784 |
| CRP (mg/dL) | 0.80 (0.35–2.02) | 0.47 (0.28–1.04) | 0.035 |
| Albumin (g/dL) | 3.75 (3.57–3.94) | 3.96 (3.80–4.08) | 0.005 |
| Creatinine (mg/dL) | 8.20 ± 1.98 | 8.73 ± 2.53 | 0.265 |
| Ca in HD solution | 1.5 (1.25–1.5) | 1.5 (1.25–1.5) | 0.854 |
| Treatment with Ca salts, no. (%) | 30 (61.2) | 17 (43.6) | 0.099 |
| Sevelamer, no. (%) | 10 (20.4) | 10 (25.6) | 0.561 |
| Vitamin D Treatment, no. (%) | 10 (20.4) | 10 (25.6) | 0.561 |
| Cardiovascular diseases, no. (%) | 23 (46.9) | 4 (10.3) | <0.001 |
| All-Cause Mortality | Cardiovascular Mortality | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age (years) | 1.04 (1.01–1.06) | 0.002 | 1.03 (1.00–1.06) | 0.019 |
| HD vintage (months) | 0.99 (0.99–1.00) | 0.577 | 0.99 (0.99–1.00) | 0.785 |
| Gender (males) | 0.77 (0.43–1.35) | 0.361 | 0.67 (0.34–1.33) | 0.254 |
| Diabetes | 2.74 (1.50–5.01) | 0.001 | 2.56 (1.25–5.24) | 0.01 |
| HTN | 1.41 (0.72–2.77) | 0.307 | 1.11 (0.52–2.39) | 0.779 |
| FGF-23 (pg/mL) | 1.00 (0.99–1.00) | 0.676 | 1.00 (0.99–1.00) | 0.841 |
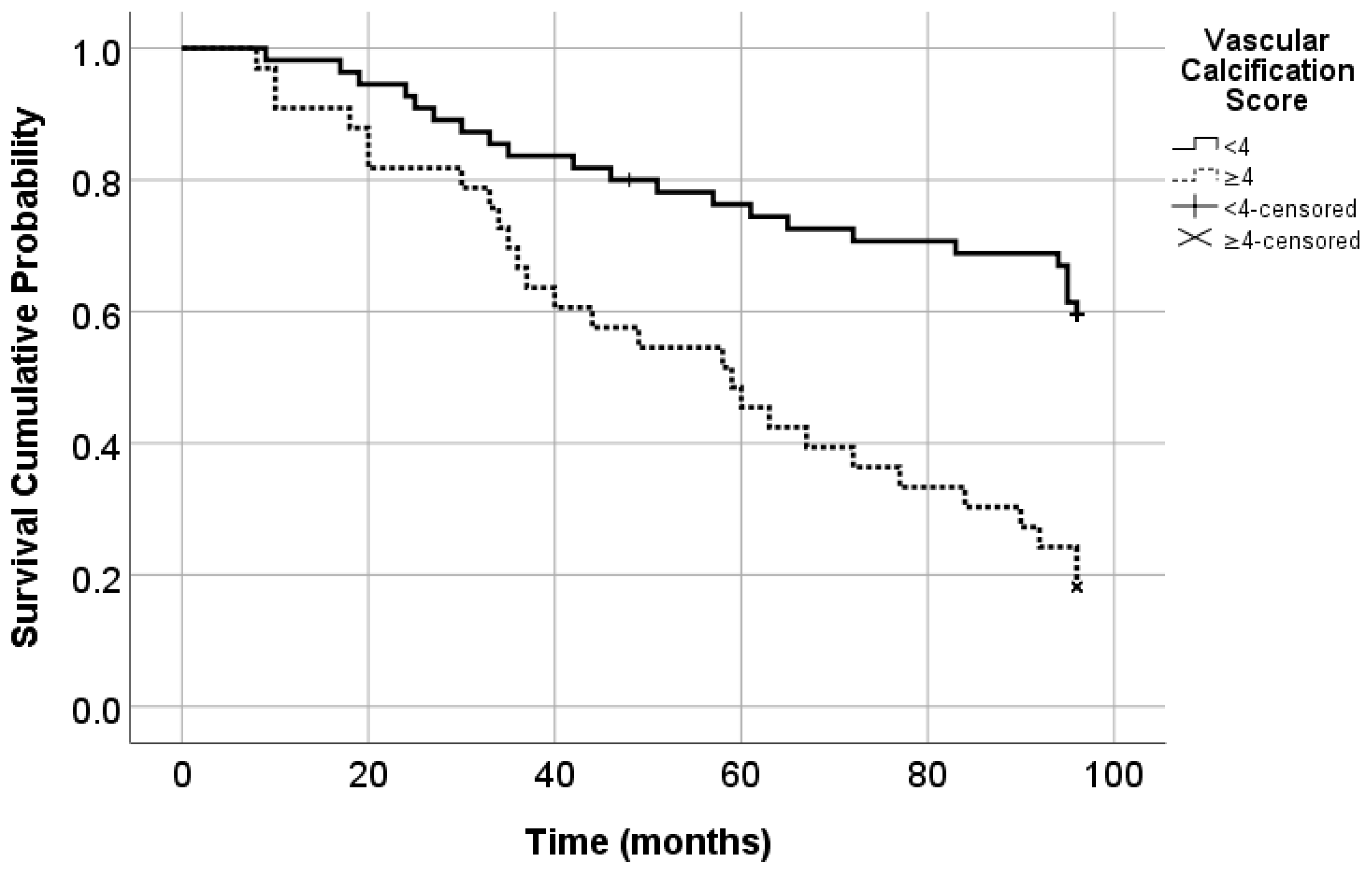
| Carotid VC score | 1.24 (1.11–1.39) | <0.001 | 1.22 (1.07–1.40) | 0.003 |
| URR | 0.98 (0.96–1.00) | 0.119 | 0.97 (0.95–0.99) | 0.026 |
| spKt/V | 0.48 (0.19–1.20) | 0.119 | 0.33 (0.12–0.87) | 0.026 |
| Bicarbonate (mEq/L) | 1.07 (0.98–1.16) | 0.092 | 1.05 (0.95–1.16) | 0.342 |
| K (mEq/L) | 0.65 (0.39–1.06) | 0.085 | 1.03 (0.58–1.81) | 0.917 |
| Ca (mg/dL) | 1.01 (0.64–1.60) | 0.948 | 1.27 (0.73–2.23) | 0.395 |
| P (mg/dL) | 0.88 (0.71–1.09) | 0.263 | 0.89 (0.69–1.16) | 0.411 |
| ALP (U/L) | 1.00 (0.99–1.00) | 0.945 | 1.00 (0.99–1.00) | 0.590 |
| iPTH (pg/mL) | 1.00 (0.99–1.00) | 0.210 | 1.00 (0.99–1.00) | 0.399 |
| Hb (g/dL) | 0.85 (0.70–1.04) | 0.117 | 0.89 (0.71–1.13) | 0.375 |
| Ferritin (ng/mL) | 1.00 (0.99–1.00) | 0.788 | 1.00 (1.00–1.00) | 0.514 |
| CRP (mg/dL) | 1.24 (1.10–1.38) | <0.001 | 1.27 (1.13–1.44) | <0.001 |
| Albumin (g/dL) | 0.41 (0.23–0.71) | 0.002 | 0.58 (0.27–1.24) | 0.162 |
| Creatinine (mg/dL) | 0.95 (0.84–1.06) | 0.371 | 1.03 (0.89–1.19) | 0.660 |
| Ca in HD solution | 1.03 (0.67–1.58) | 0.890 | 0.79 (0.45–1.30) | 0.330 |
| Treatment with Ca salts—patients (%) | 1.50 (0.84–2.68) | 0.164 | 1.17 (0.59–2.31) | 0.644 |
| Sevelamer—patients (%) | 0.69 (0.34–1.39) | 0.303 | 0.71 (0.31–1.64) | 0.430 |
| Vitamin D Treatment—patients (%) | 0.75 (0.37–1.50) | 0.419 | 0.76 (0.33–1.75) | 0.521 |
| Cardiovascular diseases | 2.84 (1.61–5.01) | <0.001 | 2.38 (1.20–4.72) | 0.013 |
| All-Cause Mortality | ||
|---|---|---|
| HR (95% CI) | p | |
| Age (years) | 1.02 (0.99–1.05) | 0.185 |
| FGF-23 (pg/mL) | 1.00 (0.99–1.00) | 0.090 |
| Diabetes | 2.35 (1.19–4.64) | 0.014 |
| VC score | 1.19 (1.01–1.39) | 0.031 |
| K | 0.94 (0.51–1.73) | 0.860 |
| Bicarbonate | 1.09 (0.97–1.23) | 0.122 |
| Albumin | 0.66 (0.31–1.43) | 0.297 |
| CRP | 1.28 (1.12–1.46) | <0.001 |
| Cardiovascular diseases | 1.34 (0.67–2.68) | 0.400 |
| Cardiovascular Mortality | ||
|---|---|---|
| HR (95% CI) | p | |
| Age (years) | 1.02 (0.98–1.06) | 0.221 |
| FGF-23 (pg/mL) | 1.00 (0.99–1.00) | 0.432 |
| Diabetes | 2.16 (1.03–4.55) | 0.041 |
| VC score | 1.22 (1.02–1.47) | 0.028 |
| CRP | 1.36 (1.18–1.56) | <0.001 |
| URR | 0.95 (0.93–0.99) | 0.009 |
| Cardiovascular diseases | 1.12 (0.51–2.44) | 0.762 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldovan, D. The Severity of Carotid Calcifications, but Not Fibroblast Growth Factor 23, Is Associated with Mortality in Hemodialysis: A Single Center Experience. Diseases 2025, 13, 73. https://doi.org/10.3390/diseases13030073
Moldovan D. The Severity of Carotid Calcifications, but Not Fibroblast Growth Factor 23, Is Associated with Mortality in Hemodialysis: A Single Center Experience. Diseases. 2025; 13(3):73. https://doi.org/10.3390/diseases13030073
Chicago/Turabian StyleMoldovan, Diana. 2025. "The Severity of Carotid Calcifications, but Not Fibroblast Growth Factor 23, Is Associated with Mortality in Hemodialysis: A Single Center Experience" Diseases 13, no. 3: 73. https://doi.org/10.3390/diseases13030073
APA StyleMoldovan, D. (2025). The Severity of Carotid Calcifications, but Not Fibroblast Growth Factor 23, Is Associated with Mortality in Hemodialysis: A Single Center Experience. Diseases, 13(3), 73. https://doi.org/10.3390/diseases13030073





