Effects of Accentuated Eccentric and Maximal Strength High-Resistance Training Programs with or Without a Curcumin-Based Formulation Supplement on Body Composition, Blood Pressure, and Metabolic Parameters in Older Adults
Abstract
1. Introduction
2. Materials and Methods
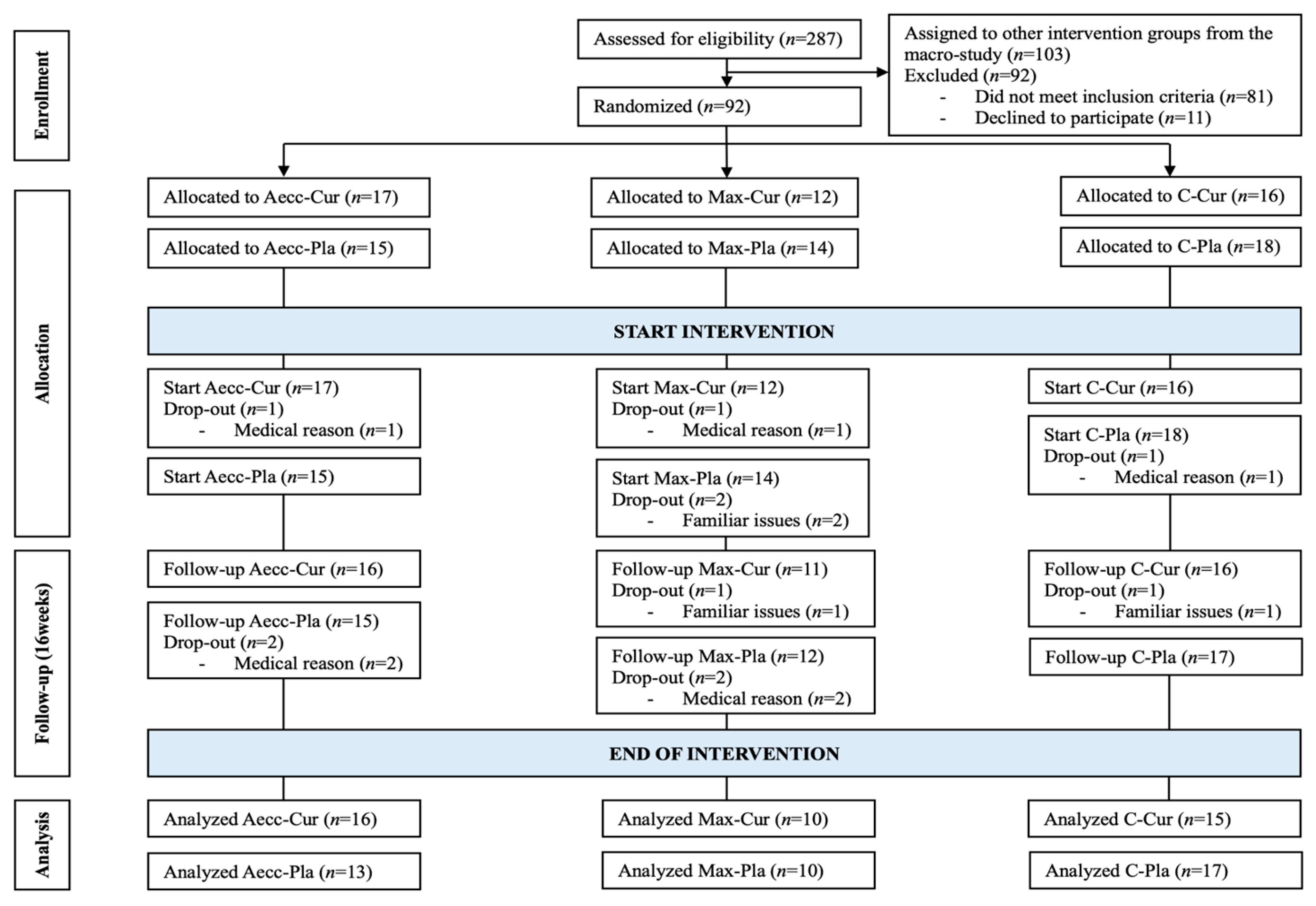
2.1. Participants
2.2. Study Design
2.3. Testing Procedures
2.4. Training Procedures
2.4.1. Accentuated Eccentric Elastic Band Program (Aecc)
2.4.2. Maximal Strength Elastic Band Program (Max)
2.5. Supplementation Procedures
2.6. Dietary and Physical Activity Control
2.7. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Blood Pressure
3.3. Metabolic Parameters
3.4. Clinical Relevance
4. Discussion
4.1. Body Composition
4.2. Blood Pressure
4.3. Metabolic Parameters
4.4. Synergistic Effects of Curcumin and RT
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1RM | One-repetition maximum |
| Aecc | Accentuated eccentric |
| ANCOVA | Analysis of covariance |
| ANOVA | Analysis of variance |
| BMI | Body mass index |
| C | Control group |
| Cur | Curcumin |
| ES | Effect sizes |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| Max | Maximal strength group |
| MCID | Minimal clinically important difference |
| MetS | Metabolic syndrome |
| MET | Metabolic equivalent of task |
| Pla | Placebo group |
| RPE | Rating of Perceived Exertion |
| RT | Resistance training |
References
- Zafar, U.; Khaliq, S.; Ahmad, H.U.; Manzoor, S.; Lone, K.P. Metabolic Syndrome: An Update on Diagnostic Criteria, Pathogenesis, and Genetic Links. Horm 2018, 17, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.; Palmer, J.; Valtos, J.; Iasiello, C.; Sowers, J. Metabolic Syndrome in the Elderly. Curr. Diab. Rep. 2006, 6, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Jensen, M.D. Metabolic Changes in Aging Humans: Current Evidence and Therapeutic Strategies. J. Clin. Investig. 2022, 132, e158451. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Hernández, J.A.; Rodríguez-Felix, F.; Juárez-Onofre, J.E.; Ruiz-Cruz, S.; Robles-García, M.A.; Borboa-Flores, J.; Wong-Corral, F.J.; Cinco-Moroyoqui, F.J.; Castro-Enríquez, D.D.; Del-Toro-Sánchez, C.L. Zein-Polysaccharide Nanoparticles as Matrices for Antioxidant Compounds: A Strategy for Prevention of Chronic Degenerative Diseases. Food Res. Int. 2018, 111, 451–471. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Ruiz-Cruz, S.; Juárez, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; López-Ahumada, G.A.; Rodríguez-Félix, F. Gallic Acid-Loaded Zein Nanoparticles by Electrospraying Process. J. Food Sci. 2019, 84, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C. Metabolic Syndrome and Health-Related Quality of Life among U.S. Adults. Ann. Epidemiol. 2008, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Leon, A.S.; Wilmore, J.H.; Skinner, J.S.; Rao, D.C.; Rankinen, T.; Bouchard, C. Targeting the Metabolic Syndrome with Exercise: Evidence from the HERITAGE Family Study. Med. Sci. Sports Exerc. 2003, 35, 1703–1709. [Google Scholar] [CrossRef]
- Stewart, K.J.; Bacher, A.C.; Turner, K.; Lim, J.G.; Hees, P.S.; Shapiro, E.P.; Tayback, M.; Ouyang, P. Exercise and Risk Factors Associated with Metabolic Syndrome in Older Adults. Am. J. Prev. Med. 2005, 28, 9–18. [Google Scholar] [CrossRef]
- Den Boer, A.T.; Herraets, I.J.T.; Stegen, J.; Roumen, C.; Corpeleijn, E.; Schaper, N.C.; Feskens, E.; Blaak, E.E. Prevention of the Metabolic Syndrome in IGT Subjects in a Lifestyle Intervention: Results from the SLIM Study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Saez-Berlanga, Á.; Castillo, D.; Alfredo Martínez, J.; Gene-Morales, J. Effects of Supplementation with Curcuminoids and Physical Exercise on Conditions Derived from Metabolic Syndrome in Women: A Systematic Review with a Secondary Analysis of Interactions. Nutr. Clin. Med. 2024, 18, 24–40. [Google Scholar] [CrossRef]
- Villanueva, M.G.; He, J.; Schroeder, E.T. Periodized Resistance Training with and without Supplementation Improve Body Composition and Performance in Older Men. Eur. J. Appl. Physiol. 2014, 114, 891. [Google Scholar] [CrossRef] [PubMed]
- Tomeleri, C.M.; Ribeiro, A.S.; Souza, M.F.; Schiavoni, D.; Schoenfeld, B.J.; Venturini, D.; Barbosa, D.S.; Landucci, K.; Sardinha, L.B.; Cyrino, E.S. Resistance Training Improves Inflammatory Level, Lipid and Glycemic Profiles in Obese Older Women: A Randomized Controlled Trial. Exp. Gerontol. 2016, 84, 80–87. [Google Scholar] [CrossRef]
- Frontera, W.R.; Bigard, X. The Benefits of Strength Training in the Elderly. Sci. Sports 2002, 17, 109–116. [Google Scholar] [CrossRef]
- Ihalainen, J.K.; Inglis, A.; Mäkinen, T.; Newton, R.U.; Kainulainen, H.; Kyröläinen, H.; Walker, S. Strength Training Improves Metabolic Health Markers in Older Individual Regardless of Training Frequency. Front. Physiol. 2019, 10, 435558. [Google Scholar] [CrossRef] [PubMed]
- Geirsdottir, O.G.; Arnarson, A.; Briem, K.; Ramel, A.; Jonsson, P.V.; Thorsdottir, I. Effect of 12-Week Resistance Exercise Program on Body Composition, Muscle Strength, Physical Function, and Glucose Metabolism in Healthy, Insulin-Resistant, and Diabetic Elderly Icelanders. J. Gerontol. Ser. A 2012, 67, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Kalapotharakos, V.I.; Michalopoulou, M.; Tokmakidis, S.; Godolias, G.; Strimpakos, N.; Karteroliotis, K. Effects of a Resistance Exercise Programme on the Performance of Inactive Older Adults. Int. J. Ther. Rehabil. 2013, 11, 318–323. [Google Scholar] [CrossRef]
- Berger, R.A. Optimum Repetitions for the Development of Strength. Res. Q. Am. Assoc. Health Phys. Educ. Recreat. 1962, 33, 334–338. [Google Scholar] [CrossRef]
- Romero-Arenas, S.; Blazevich, A.J.; Martínez-Pascual, M.; Pérez-Gómez, J.; Luque, A.J.; López-Román, F.J.; Alcaraz, P.E. Effects of High-Resistance Circuit Training in an Elderly Population. Exp. Gerontol. 2013, 48, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Khodadad Kashi, S.; Mirzazadeh, Z.S.; Saatchian, V. A Systematic Review and Meta-Analysis of Resistance Training on Quality of Life, Depression, Muscle Strength, and Functional Exercise Capacity in Older Adults Aged 60 Years or More. Biol. Res. Nurs. 2023, 25, 88–106. [Google Scholar] [CrossRef]
- Wagle, J.P.; Taber, C.B.; Cunanan, A.J.; Bingham, G.E.; Carroll, K.M.; DeWeese, B.H.; Sato, K.; Stone, M.H. Accentuated Eccentric Loading for Training and Performance: A Review. Sport. Med. 2017, 47, 2473–2495. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Cantwell, C.J.; Campbell, B.A.; Schroeder, Z.S.; Marshall, L.K.; Taber, C.B. Braking and Propulsion Phase Characteristics of Traditional and Accentuated Eccentric Loaded Back Squats. J. Hum. Kinet. 2024, 91, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Wagle, J.P.; Cunanan, A.J.; Carroll, K.M.; Sams, M.L.; Wetmore, A.; Bingham, G.E.; Taber, C.B.; Deweese, B.H.; Sato, K.; Stuart, C.A.; et al. Accentuated Eccentric Loading and Cluster Set Configurations in the Back Squat: A Kinetic and Kinematic Analysis. J. Strength Cond. Res. 2021, 35, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.D.; Sundstrup, E.; Andersen, C.H.; Aagaard, P.; Andersen, L.L. Muscle Activity during Leg Strengthening Exercise Using Free Weights and Elastic Resistance: Effects of Ballistic vs Controlled Contractions. Hum. Mov. Sci. 2013, 32, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Furtado, G.E.; Teixeira, A.M.; Flandez, J.; Naclerio, F. Concurrent and Construct Validation of a New Scale for Rating Perceived Exertion during Elastic Resistance Training in The Elderly. J. Sports Sci. Med. 2020, 19, 175. [Google Scholar]
- Lopes, J.S.S.; Machado, A.F.; Micheletti, J.K.; de Almeida, A.C.; Cavina, A.P.; Pastre, C.M. Effects of Training with Elastic Resistance versus Conventional Resistance on Muscular Strength: A Systematic Review and Meta-Analysis. SAGE Open Med. 2019, 7, 2050312119831116. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Mena, R.; Calatayud, J.; Gargallo, P.; Flández, J.; Page, P. Effects of Strength Training with Variable Elastic Resistance across the Lifespan: A Systematic Review. Cult. Cienc. Deport. 2020, 15, 147–164. [Google Scholar] [CrossRef]
- Colado, J.C.; Triplett, N.T. Effects of a Short-Term Resistance Program Using Elastic Bands versus Weight Machines for Sedentary Middle-Aged Women. J. Strength Cond. Res. 2008, 22, 1441–1448. [Google Scholar] [CrossRef]
- Gene-Morales, J.; Gené-Sampedro, A.; Salvador, R.; Colado, J.C. Adding the Load Just Above Sticking Point Using Elastic Bands Optimizes Squat Performance, Perceived Effort Rate, and Cardiovascular Responses. J. Sports Sci. Med. 2020, 19, 735. [Google Scholar]
- Gargallo, P.; Tamayo, E.; Jiménez-Martínez, P.; Juesas, A.; Casaña, J.; Benitez-Martinez, J.C.; Gene-Morales, J.; Fernandez-Garrido, J.; Saez, G.T.; Colado, J.C. Multicomponent and Power Training with Elastic Bands Improve Metabolic and Inflammatory Parameters, Body Composition and Anthropometry, and Physical Function in Older Women with Metabolic Syndrome: A 20-Week Randomized, Controlled Trial. Exp. Gerontol. 2024, 185, 112340. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Hurr, C.; Kim, S. Effects of Elastic Band Exercise on Functional Fitness and Blood Pressure Response in the Healthy Elderly. Int. J. Environ. Res. Public Health 2020, 17, 7144. [Google Scholar] [CrossRef]
- Stojanović, M.D.M.; Mikić, M.; Milošević, Z.; Vuković, J.; Jezdimirović, T.; Vučetić, V. Effects of Chair-Based, Low–Load Elastic Band Resistance Training on Functional Fitness and Metabolic Biomarkers in Older Women. J. Sport. Sci. Med. 2021, 20, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Patti, A.M.; Al-Rasadi, K.; Giglio, R.V.; Nikolic, D.; Mannina, C.; Castellino, G.; Chianetta, R.; Banach, M.; Cicero, A.F.G.; Lippi, G.; et al. Natural Approaches in Metabolic Syndrome Management. Arch. Med. Sci. 2018, 14, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Bogdanski, P.; Suliburska, J.; Szulinska, M.; Stepien, M.; Pupek-Musialik, D.; Jablecka, A. Green Tea Extract Reduces Blood Pressure, Inflammatory Biomarkers, and Oxidative Stress and Improves Parameters Associated with Insulin Resistance in Obese, Hypertensive Patients. Nutr. Res. 2012, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated Fatty Acids and Risk of Coronary Heart Disease: Modulation by Replacement Nutrients. Curr. Atheroscler. Rep. 2010, 12, 384–390. [Google Scholar] [CrossRef]
- Houston, M. The Role of Nutrition and Nutraceutical Supplements in the Treatment of Hypertension. World J. Cardiol. 2014, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Whigham, L.D.; Watras, A.C.; Schoeller, D.A. Efficacy of Conjugated Linoleic Acid for Reducing Fat Mass: A Meta-Analysis in Humans. Am. J. Clin. Nutr. 2007, 85, 1203–1211. [Google Scholar] [CrossRef]
- Tabrizi, R.; Tamtaji, O.R.; Lankarani, K.B.; Akbari, M.; Dadgostar, E.; Dabbaghmanesh, M.H.; Kolahdooz, F.; Shamshirian, A.; Momen-Heravi, M.; Asemi, Z. The Effects of Resveratrol Intake on Weight Loss: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Bressan, D.; Renaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential Role of Bioavailable Curcumin in Weight Loss and Omental Adipose Tissue Decrease: Preliminary Data of a Randomized, Controlled Trial in Overweight People with Metabolic Syndrome. Preliminary Study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar] [PubMed]
- Dehzad, M.J.; Ghalandari, H.; Amini, M.R.; Askarpour, M. Effects of Curcumin/Turmeric Supplementation on Lipid Profile: A GRADE-Assessed Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2023, 75, 102955. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Gancarz, M.; Kondracka, A.; Rusinek, R.; Oniszczuk, A. Curcumin and Weight Loss: Does It Work? Int. J. Mol. Sci. 2022, 23, 639. [Google Scholar] [CrossRef] [PubMed]
- Jabczyk, M.; Nowak, J.; Hudzik, B.; Zubelewicz-Szkodzińska, B. Curcumin in Metabolic Health and Disease. Nutrients 2021, 13, 4440. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.A.L.; De Magalhaes, C.O.D.; Dias, I.R.; De Oliveira, L.R.S.; Improta-Caria, A.C.; Cassilhas, R.C. Cross Talk Mechanisms of Aerobic Exercise Training on Obesity, Type 2 Diabetes, and Alzheimer’s Disease: The Role of Insulin Resistance. Rev. Assoc. Med. Bras. 2022, 68, 963–967. [Google Scholar] [CrossRef]
- Choi, Y.; Tanabe, Y.; Akazawa, N.; Zempo-Miyaki, A.; Maeda, S. Curcumin Supplementation Attenuates the Decrease in Endothelial Function Following Eccentric Exercise. J. Exerc. Nutr. Biochem. 2019, 23, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bittel, A.J.; Bittel, D.C.; Mittendorfer, B.; Patterson, B.W.; Okunade, A.L.; Yoshino, J.; Porter, L.C.; Abumrad, N.A.; Reeds, D.N.; Cade, W.T. A Single Bout of Resistance Exercise Improves Postprandial Lipid Metabolism in Overweight/Obese Men with Prediabetes. Diabetologia 2020, 63, 611–623. [Google Scholar] [CrossRef]
- Ferreira Mendes, B.; Improta-Caria, A.C.; Diniz e Magalhães, C.O.; Dias Peixoto, M.F.; Cardoso Cassilhas, R.; de Oliveira, E.M.; De Sousa, R.A.L. Resistance Training Reduces Blood Pressure: Putative Molecular Mechanisms. Curr. Hypertens. Rev. 2024, 20, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Dehzad, M.J.; Ghalandari, H.; Askarpour, M. Curcumin/Turmeric Supplementation Could Improve Blood Pressure and Endothelial Function: A Grade-Assessed Systematic Review and Dose-Response Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. ESPEN 2024, 59, 194–207. [Google Scholar] [CrossRef]
- Delong, D.M.; Delong, E.R.; Wood, P.D.; Lippel, K.; Rifkind, B.M. A Comparison of Methods for the Estimation of Plasma Low- and Very Low-Density Lipoprotein Cholesterol. The Lipid Research Clinics Prevalence Study. JAMA 1986, 256, 2372–2377. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Gené-Morales, J.; Jiménez-Martínez, P.; Flandez, J.; Ferri-Caruana, A.M.; Babiloni-Lopez, C. Rating of Perceived Exertion in the First Repetition Is Related to the Total Repetitions Performed in Elastic Bands Training. Mot. Control 2023, 27, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Vieira, E.R.; Palmer, R.C.; Chaves, P.H.M. Prevention of Falls in Older People Living in the Community. BMJ 2016, 353, i1419. [Google Scholar] [CrossRef] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of Curcumin on Cardiovascular Risk Factors in Obese and Overweight Adolescent Girls: A Randomized Clinical Trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and Efficacy of Curcumin versus Diclofenac in Knee Osteoarthritis: A Randomized Open-Label Parallel-Arm Study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, V.; Voci, S.M.; Mendes-Netto, R.S.; da Silva, D.G. The Relative Validity of a Food Record Using the Smartphone Application MyFitnessPal. Nutr. Diet. 2018, 75, 219–225. [Google Scholar] [CrossRef]
- Evenepoel, C.; Clevers, E.; Deroover, L.; van Loo, W.; Matthys, C.; Verbeke, K. Accuracy of Nutrient Calculations Using the Consumer-Focused Online App MyFitnessPal: Validation Study. J. Med. Internet Res. 2020, 22, e18237. [Google Scholar] [CrossRef] [PubMed]
- Van Breukelen, G.J.P.; Van Dijk, K.R.A. Use of Covariates in Randomized Controlled Trials. J. Int. Neuropsychol. Soc. 2007, 13, 903–904. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Altman, D.G. Statistics Notes: Analysing Controlled Trials with Baseline and Follow up Measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Corder, G.W.; Foreman, D.I. Non-Parametric Statistics: A Step-by-Step Approach; Wiley: Hoboken, NJ, USA, 2014; Volume 2, pp. 1–283. [Google Scholar]
- Quade, D. Rank Analysis of Covariance. J. Am. Stat. Assoc. 1967, 62, 1187–1200. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 0805802835. [Google Scholar]
- Beaton, D.E.; Boers, M.; Wells, G.A. Many Faces of the Minimal Clinically Important Difference (MCID): A Literature Review and Directions for Future Research. Curr. Opin. Rheumatol. 2002, 14, 109–114. [Google Scholar] [CrossRef]
- King, M.T. A Point of Minimal Important Difference (MID): A Critique of Terminology and Methods. Expert Rev. Pharmacoecon. Outcomes Res. 2011, 11, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Draak, T.H.P.; de Greef, B.T.A.; Faber, C.G.; Merkies, I.S.J. The Minimum Clinically Important Difference: Which Direction to Take. Eur. J. Neurol. 2019, 26, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K. Intention-to-Treat Concept: A Review. Perspect. Clin. Res. 2011, 2, 109. [Google Scholar] [CrossRef] [PubMed]
- Poobalan, A.; Aucott, L.; Smith, W.C.S.; Avenell, A.; Jung, R.; Broom, J.; Grant, A.M. Effects of Weight Loss in Overweight/Obese Individuals and Long-Term Lipid Outcomes—a Systematic Review. Obes. Rev. 2004, 5, 43–50. [Google Scholar] [CrossRef]
- Oh, Y.H.; Choi, S.; Lee, G.; Son, J.S.; Kim, K.H.; Park, S.M. Changes in Body Composition Are Associated with Metabolic Changes and the Risk of Metabolic Syndrome. J. Clin. Med. 2021, 10, 745. [Google Scholar] [CrossRef]
- Law, M.R.; Morris, J.K.; Wald, N.J. Use of Blood Pressure Lowering Drugs in the Prevention of Cardiovascular Disease: Meta-Analysis of 147 Randomised Trials in the Context of Expectations from Prospective Epidemiological Studies. BMJ 2009, 338, 1245. [Google Scholar] [CrossRef] [PubMed]
- Keutmann, S.; Zylla, S.; Dahl, M.; Friedrich, N.; Landgraf, R.; Heinemann, L.; Kallner, A.; Nauck, M.; Petersmann, A. Measurement Uncertainty Impacts Diagnosis of Diabetes Mellitus: Reliable Minimal Difference of Plasma Glucose Results. Diabetes Ther. 2020, 11, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Larsen, S.; Borch-Johnsen, K.; Nissinen, A.; Pekkanen, J.; Tuomilehto, J.; Jousilahti, P.; Lindstrøm, J.; Pyörälä, M.; Pyorala, K.; et al. Glucose Tolerance and Cardiovascular Mortality: Comparison of Fasting and 2-Hour Diagnostic Criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef]
- Odden, M.C.; Shlipak, M.G.; Tager, I.B. Serum Creatinine and Functional Limitation in Elderly Persons. J. Gerontol. Ser. A 2009, 64A, 370–376. [Google Scholar] [CrossRef]
- Marston, N.A.; Giugliano, R.P.; Im, K.A.; Silverman, M.G.; O’Donoghue, M.L.; Wiviott, S.D.; Ference, B.A.; Sabatine, M.S. Association Between Triglyceride Lowering and Reduction of Cardiovascular Risk Across Multiple Lipid-Lowering Therapeutic Classes: A Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Circulation 2019, 140, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Jimenez, A. Differential Effects of Aerobic Exercise, Resistance Training and Combined Exercise Modalities on Cholesterol and the Lipid Profile: Review, Synthesis and Recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Gordon, D.J.; Probstfield, J.L.; Garrison, R.J.; Neaton, J.D.; Castelli, W.P.; Knoke, J.D.; Jacobs, D.R.; Bangdiwala, S.; Tyroler, H.A. High-Density Lipoprotein Cholesterol and Cardiovascular Disease. Four Prospective American Studies. Circulation 1989, 79, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A.; Flack, J. Effect of Long-Term Exposure to Lower Low-Density Lipoprotein Cholesterol Beginning Early in Life on the Risk of Coronary Heart Disease: A Mendelian Randomization Analysis. J. Am. Coll. Cardiol. 2012, 60, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. The Mechanisms of Muscle Hypertrophy and Their Application to Resistance Training. J. Strength. Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of Resistance Training on Older Adults. Sport. Med. 2004, 34, 329–348. [Google Scholar] [CrossRef]
- Cavalcante, E.F.; Ribeiro, A.S.; Do Nascimento, M.A.; Silva, A.M.; Tomeleri, C.M.; Nabuco, H.C.G.; Pina, F.L.C.; Mayhew, J.L.; Da Silva-Grigoletto, M.E.; Da Silva, D.R.P.; et al. Effects of Different Resistance Training Frequencies on Fat in Overweight/Obese Older Women. Int. J. Sports Med. 2018, 39, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.B. The Effect of Time-under-Tension and Weight Lifting Cadence on Aerobic, Anaerobic, and Recovery Energy Expenditures: 3 Submaximal Sets. Appl. Physiol. Nutr. Metab. 2012, 37, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Progressive Resistance Exercise and Resting Blood Pressure. Hypertension 2000, 35, 838–843. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Fagard, R.H. Effect of Resistance Training on Resting Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. J. Hypertens. 2005, 23, 251–259. [Google Scholar] [CrossRef]
- Queiroz, A.C.C.; Kanegusuku, H.; Forjaz, C.L. de M. Effects of Resistance Training on Blood Pressure in the Elderly. Arq. Bras. Cardiol. 2010, 95, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Fecchio, R.Y.; de Sousa, J.C.S.; Oliveira-Silva, L.; da Silva Junior, N.D.; Pio-Abreu, A.; da Silva, G.V.; Drager, L.F.; Low, D.A.; Forjaz, C.L.M. Effects of Dynamic, Isometric and Combined Resistance Training on Blood Pressure and Its Mechanisms in Hypertensive Men. Hypertens. Res. 2023, 46, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, M.M.; Boardley, D.; Lambert, C.P.; Flynn, M.G. Effects of Endurance Training and Resistance Training on Plasma Lipoprotein Profiles in Elderly Women. J. Gerontol. Ser. A 2002, 57, B54–B60. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.; Carvalho, J.; Soares, J.M.C.; Marques, F.; Mota, J. Effects of Resistance and Multicomponent Exercise on Lipid Profiles of Older Women. Maturitas 2009, 63, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Beunders, R.; Beunders, R.; Bongers, C.C.W.G.; Bongers, C.C.W.G.; Pickkers, P.; Pickkers, P. The Effects of Physical Exercise on the Assessment of Kidney Function. J. Appl. Physiol. 2020, 128, 1459–1460. [Google Scholar] [CrossRef]
- Rule, A.D.; Larson, T.S.; Bergstralh, E.J.; Slezak, J.M.; Jacobsen, S.J.; Cosio, F.G. Using Serum Creatinine to Estimate Glomerular Filtration Rate: Accuracy in Good Health and in Chronic Kidney Disease. Ann. Intern. Med. 2004, 141, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Calvo, J.S.; Martínez, A.C.; García, A.C.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed]

| Variable | Aecc-Cur (n = 16) | Aecc-Pla (n = 13) | Max-Cur (n = 10) | Max-Pla (n = 10) | C-Cur (n = 15) | C-Pla (n = 17) |
|---|---|---|---|---|---|---|
| Age (years) | 68.1 ± 5.1 | 69.5 ± 4.0 | 67.0 ± 4.2 | 68.0 ± 5.2 | 69.5 ± 4.7 | 66.9 ± 4.4 |
| Weight (kg) | 73.1 ± 11.0 | 69.5 ± 12.0 | 73.2 ± 10.7 | 71.1 ± 11.1 | 70.7 ± 11.3 | 70.1 ± 11.7 |
| Height (cm) | 167.5 ± 6.7 | 163.2 ± 7.2 | 163.0 ± 7.7 | 165.9 ± 7.0 | 166.9 ± 4.7 | 162.7 ± 7.2 |
| Body mass index (kg/m2) | 26.2 ± 3.1 | 26.8 ± 2.9 | 26.3 ± 3.4 | 25.7 ± 3.4 | 25.3 ± 4.0 | 25.8 ± 3.4 |
| Body fat percentage (%) | 32.5 ± 6.3 | 34.4 ± 5.6 | 36.1 ± 4.9 | 33.8 ± 5.8 | 35.7 ± 5.1 | 33.4 ± 5.4 |
| Weekly physical activity (MET) | 830.0 ± 8.5 | 728.5 ± 10.0 | 781.2 ± 9.1 | 804.4 ± 8.8 | 709.2 ± 10.4 | 684.0 ± 12.5 |
| Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) | |
|---|---|---|---|---|---|---|---|---|---|
| Body fat mass (kg) | (1) Aecc-Cur | 23.2 ± 5.9 | 20.6 ± 6.0 | −11.4 | <0.001 | 0.42 | 1–2 | 0.015 | 0.09 |
| 1–3 | <0.001 | 0.67 | |||||||
| (2) Aecc-Pla | 22.8 ± 4.8 | 21.0 ± 4.0 | −8.0 | <0.001 | 0.40 | 1–5 | <0.001 | 0.51 | |
| 1–6 | <0.001 | 0.54 | |||||||
| (3) Max-Cur | 28.7 ± 5.7 | 24.6 ± 5.7 | −14.3 | <0.001 | 0.69 | 2–3 | <0.001 | 0.72 | |
| 2–4 | 0.015 | 0.58 | |||||||
| (4) Max-Pla | 27.8 ± 8.5 | 24.7 ± 8.2 | −11.1 | <0.001 | 0.35 | 2–5 | 0.004 | 0.48 | |
| 2–6 | <0.001 | 0.53 | |||||||
| (5) C-Pla | 23.7 ± 7.3 | 24.3 ± 8.1 | 2.4 | <0.001 | 0.05 | 3–4 | 0.013 | 0.03 | |
| 3–5 | <0.001 | 0.04 | |||||||
| (6) C-Cur | 23.3 ± 6.5 | 24.0 ± 6.4 | 3.1 | 0.003 | 0.11 | 3–6 | <0.001 | 0.11 | |
| 4–5 | <0.001 | 0.06 | |||||||
| 4–6 | <0.001 | 0.12 | |||||||
| Body muscle mass (kg) | (1) Aecc-Cur | 44.1 ± 8.1 | 44.9 ± 7.9 | 1.8 | <0.001 | 0.10 | 1–5 | <0.001 | 0.16 |
| 1–6 | 0.021 | 0.11 | |||||||
| (2) Aecc-Pla | 43.1 ± 9.9 | 43.8 ± 10.1 | 1.4 | 0.001 | 0.07 | 2–5 | <0.001 | 0.11 | |
| 2–6 | <0.001 | 0.09 | |||||||
| (3) Max-Cur | 43.6 ± 8.1 | 44.6 ± 8.3 | 2.3 | 0.001 | 0.12 | 3–5 | <0.001 | 0.18 | |
| 3–6 | 0.001 | 0.12 | |||||||
| (4) Max-Pla | 39.5 ± 7.3 | 40.2 ± 7.3 | 1.7 | 0.005 | 0.10 | 4–5 | 0.001 | 0.14 | |
| 4–6 | 0.038 | 0.10 | |||||||
| (5) C-Pla | 38.0 ± 6.7 | 37.8 ± 6.7 | −0.7 | 0.005 | 0.03 | ||||
| (6) C-Cur | 41.6 ± 13.1 | 41.3 ± 12.9 | −0.8 | 0.001 | 0.03 | ||||
| Group | Mean ± SD Pre-Test | Mean ± SD Post-Test | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) | |
|---|---|---|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | (1) Aecc-Cur | 133.5 ± 17.0 | 118.2 ± 17.4 | −11.4 | <0.001 | 0.93 | 1–5 | 0.017 | 0.99 |
| 1–6 | 0.029 | 0.76 | |||||||
| (2) Aecc-Pla | 130.5 ± 11.8 | 117.7 ± 12.0 | −9.8 | <0.001 | 1.07 | 2–5 | 0.017 | 1.08 | |
| 2–6 | 0.032 | 0.93 | |||||||
| (3) Max-Cur | 135.3 ± 13.1 | 120.2 ± 13.6 | −11.2 | <0.001 | 1.17 | 3–5 | 0.024 | 0.82 | |
| 3–6 | 0.041 | 0.69 | |||||||
| (4) Max-Pla | 130.5 ± 11.6 | 117.7 ± 10.4 | −9.9 | <0.001 | 1.17 | 4–5 | 0.018 | 1.14 | |
| 4–6 | 0.031 | 0.96 | |||||||
| (5) C-Pla | 126.5 ± 13.7 | 130.2 ± 11.4 | 2.9 | <0.001 | 0.29 | ||||
| (6) C-Cur | 128.3 ± 13.5 | 129.3 ± 12.9 | 0.7 | 0.461 | 0.08 | ||||
| Diastolic blood pressure (mmHg) | (1) Aecc-Cur | 82.3 ± 8.6 | 76.1 ± 7.9 | −7.6 | <0.001 | 0.75 | 3–5 | 0.018 | 1.09 |
| 3–6 | 0.036 | 0.81 | |||||||
| (2) Aecc-Pla | 80.3 ± 7.1 | 75.2 ± 7.5 | −6.3 | <0.001 | 0.71 | ||||
| (3) Max-Cur | 83.6 ± 7.5 | 74.9 ± 7.8 | −10.4 | <0.001 | 1.10 | ||||
| (4) Max-Pla | 80.2 ± 10.2 | 73.3 ± 7.4 | −8.6 | <0.001 | 0.84 | ||||
| (5) C-Pla | 79.0 ± 7.6 | 80.0 ± 6.3 | 1.3 | 0.005 | 0.13 | ||||
| (6) C-Cur | 77.3 ± 5.3 | 79.3 ± 4.7 | 2.7 | 0.061 | 0.42 | ||||
| Group | Mean ± SD (Pre) | Mean ± SD (Post) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) | |
|---|---|---|---|---|---|---|---|---|---|
| Glycemia (mg/dL) | (1) Aecc-Cur | 90.0 ± 9.9 | 79.9 ± 7.8 | −11.2 | <0.001 | 1.13 | 1–5 | <0.001 | 1.24 |
| 1–6 | <0.001 | 1.28 | |||||||
| (2) Aecc-Pla | 87.8 ± 4.7 | 79.3 ± 5.4 | −9.6 | <0.001 | 1.67 | 2–5 | <0.001 | 1.61 | |
| 2–6 | 0.001 | 1.45 | |||||||
| (3) Max-Cur | 91.6 ± 8.7 | 80.2 ± 10.2 | −12.4 | <0.001 | 1.20 | 3–5 | <0.001 | 1.05 | |
| 3–6 | <0.001 | 1.10 | |||||||
| (4) Max-Pla | 85.1 ± 8.8 | 79.1 ± 6.9 | −7.1 | 0.001 | 0.76 | 4–5 | 0.001 | 1.49 | |
| 4–6 | 0.014 | 1.36 | |||||||
| (5) C-Pla | 85.5 ± 5.5 | 88.4 ± 5.6 | 3.4 | 0.270 | 0.53 | ||||
| (6) C-Cur | 93.3 ± 11.8 | 91.8 ± 10.2 | −1.6 | 0.922 | 0.14 | ||||
| Creatinine (md/dL) | (1) Aecc-Cur | 0.91 ± 0.16 | 0.93 ± 0.17 | 2.2 | 0.015 | 0.12 | 1–3 | 0.002 | 0.97 |
| 1–5 | 0.023 | 0.39 | |||||||
| (2) Aecc-Pla | 0.92 ± 0.17 | 0.92 ± 0.16 | 0.0 | 0.645 | 0.00 | 2–3 | 0.001 | 0.93 | |
| 3–4 | 0.027 | 0.34 | |||||||
| (3) Max-Cur | 0.80 ± 0.16 | 0.94 ± 0.17 | 17.5 | 0.028 | 0.85 | 3–5 | <0.001 | 1.02 | |
| 3–6 | <0.001 | 0.44 | |||||||
| (4) Max-Pla | 0.83 ± 0.18 | 0.88 ± 0.17 | 6.0 | 0.066 | 0.29 | 4–5 | 0.006 | 0.68 | |
| (5) C-Pla | 0.81 ± 0.17 | 0.76 ± 0.18 | −6.2 | <0.001 | 0.28 | ||||
| (6) C-Cur | 0.85 ± 0.19 | 0.86 ± 0.19 | 1.2 | 0.207 | 0.06 | ||||
| Triglycerides (mg/dL) | (1) Aecc-Cur | 99.7 ± 36.5 | 82.62 ± 28.7 | −17.1 | <0.001 | 0.52 | 1–5 | <0.001 | 0.28 |
| 1–6 | 0.002 | 0.65 | |||||||
| (2) Aecc-Pla | 95.6 ± 24.1 | 79.50 ± 24.5 | −16.8 | 0.019 | 0.66 | 2–5 | <0.001 | 0.20 | |
| 2–6 | 0.008 | 0.94 | |||||||
| (3) Max-Cur | 127.8 ± 20.0 | 95.90 ± 15.5 | −24.9 | 0.005 | 1.78 | 3–5 | <0.001 | 1.47 | |
| 3–6 | 0.001 | 0.23 | |||||||
| (4) Max-Pla | 129.6 ± 18.5 | 107.63 ± 22.8 | −16.9 | 0.025 | 1.06 | 4–5 | <0.001 | 1.58 | |
| 4–6 | 0.007 | 0.31 | |||||||
| (5) C-Pla | 90.5 ± 29.1 | 101.25 ± 33.3 | 11.9 | 0.036 | 0.36 | ||||
| (6) C-Cur | 121.9 ± 30.6 | 119.20 ± 28.0 | −1.9 | 0.638 | 0.09 | ||||
| Total Cholesterol (mg/dL) | (1) Aecc-Cur | 188.4 ± 31.3 | 169.91 ± 35.1 | −9.8 | 0.037 | 0.56 | 1–5 | <0.001 | 0.34 |
| 1–6 | 0.003 | 0.77 | |||||||
| (2) Aecc-Pla | 195.3 ± 35.9 | 178.93 ± 34.5 | −8.4 | <0.001 | 0.46 | 2–5 | <0.001 | 0.29 | |
| 2–6 | 0.038 | 0.90 | |||||||
| (3) Max-Cur | 219.9 ± 29.7 | 191.70 ± 36.9 | −12.8 | 0.005 | 0.84 | 3–5 | <0.001 | 1.55 | |
| 3–6 | <0.001 | 0.26 | |||||||
| (4) Max-Pla | 199.8 ± 34.1 | 181.30 ± 33.2 | −9.3 | 0.005 | 0.55 | 4–5 | <0.001 | 1.64 | |
| 4–6 | 0.006 | 0.39 | |||||||
| (5) C-Pla | 208.8 ± 31.4 | 215.75 ± 29.7 | 3.3 | 0.003 | 0.24 | ||||
| (6) C-Cur | 177.2 ± 32.5 | 173.93 ± 30.7 | −1.8 | 0.604 | 0.11 | ||||
| High Density Lipoprotein (mg/dL) | (1) Aecc-Cur | 60.1 ± 13.9 | 67.2 ± 12.0 | 11.9 | <0.001 | 0.54 | 1–5 | <0.001 | 0.58 |
| 1–6 | 0.001 | 0.47 | |||||||
| (2) Aecc-Pla | 51.3 ± 11.6 | 57.1 ± 10.5 | 10.6 | 0.009 | 0.52 | 2–5 | 0.007 | 0.26 | |
| 2–6 | 0.014 | 0.16 | |||||||
| (3) Max-Cur | 57.3 ± 7.7 | 67.9 ± 12.1 | 18.5 | 0.011 | 1.04 | 3–5 | <0.001 | 0.44 | |
| 3–6 | <0.001 | 0.34 | |||||||
| (4) Max-Pla | 61.0 ± 14.6 | 70.7 ± 15.1 | 15.9 | 0.005 | 0.68 | 4–5 | <0.001 | 0.68 | |
| 4–6 | <0.001 | 0.57 | |||||||
| (5) C-Pla | 65.9 ± 11.2 | 62.5 ± 10.8 | −5.1 | 0.146 | 0.31 | ||||
| (6) C-Cur | 53.1 ± 11.0 | 54.5 ± 9.7 | 2.6 | 0.063 | 0.16 | ||||
| Low Density Lipoprotein (mg/dL) | (1) Aecc-Cur | 116.9 ± 27.1 | 105.0 ± 25.7 | −10.2 | <0.001 | 0.45 | 1–5 | <0.001 | 0.45 |
| 1–6 | 0.004 | 0.36 | |||||||
| (2) Aecc-Pla | 111.5 ± 16.9 | 102.3 ± 26.4 | −7.4 | 0.008 | 0.33 | 2–5 | <0.001 | 0.28 | |
| 2–6 | 0.015 | 0.19 | |||||||
| (3) Max-Cur | 142.9 ± 11.6 | 121.9 ± 16.5 | −13.6 | 0.012 | 0.63 | 3–5 | <0.001 | 0.63 | |
| 3–6 | 0.003 | 0.53 | |||||||
| (4) Max-Pla | 134.5 ± 26.7 | 116.1 ± 28.1 | −13.7 | 0.005 | 0.65 | 4–5 | <0.001 | 0.56 | |
| 4–6 | 0.004 | 0.46 | |||||||
| (5) C-Pla | 125.6 ± 25.0 | 130.6 ± 25.1 | 4.0 | 0.016 | 0.21 | 5–6 | 0.047 | 0.13 | |
| (6) C-Cur | 108.6 ± 17.5 | 106.8 ± 15.8 | −1.7 | 0.057 | 0.12 | ||||
| Variable | End-Point/Cut-Point | Reference | Benefits/Risks | Aecc-Cur (n = 16) | Aecc-Pla (n = 13) | Max-Cur (n = 10) | Max-Pla (n = 10) | C-Cur (n = 15) | C-Pla (n = 17) |
|---|---|---|---|---|---|---|---|---|---|
| Fat mass | 5% decrease | Poobalan et al., 2004 [68] | A 5% weight loss significantly improves obesity-related cardiovascular and metabolic health | 87% | 69% | 100% | 100% | 0% | 0% |
| Muscle mass | 1% increase | Oh et al., 2021 [69] | Decrease of 19% in the metabolic syndrome | 100% | 100% | 100% | 80% | 7% | 0% |
| Systolic blood pressure | 10 mm/Hg decrease | Law et al., 2009 [70] | Decrease of 21% in cardiovascular disease risk and 46% in stroke | 81% | 92% | 90% | 70% | 7% | 0% |
| Diastolic blood pressure | 5 mm/Hg decrease | Law et al., 2009 [70] | Decrease of 21% in cardiovascular disease risk and 46% in stroke | 75% | 54% | 100% | 90% | 7% | 0% |
| Glycemia | 7.92 mg/dL or 0.44 mmol/L decrease | Keutmann et al., 2020 [71], Qiao et al., 2021 [72] | Decrease of 14% in stroke | 63% | 69% | 70% | 50% | 7% | 0% |
| Creatinine | 0.97 mg/dL cut-point ♀ 1.15 mg/dL cut-point ♂ | Odden et al., 2009 [73] | ≤0.97 ♀ or ≤1.15 ♂ decrease odds of functional limitation | 75% | 77% | 80% | 60% | 79% | 87% |
| Triglycerides | 40 mg/dL | Marston et al., 2019 [74] | Decrease of 5% in cardiovascular disease risk | 6% | 0% | 30% | 20% | 0% | 0% |
| Total cholesterol | 23.20 mg/dL or 0.60 mmol/L decrease | Mann et al., 2014 [75] | Decrease of 19% in the cardiovascular disease | 31% | 30% | 60% | 20% | 0% | 0% |
| High-Density Lipoprotein | 1 mg/dL or 0.03 mmol/L increase | Gordon et al., 1989 [76] | Decrease of 3% in the cardiovascular disease risk and 4.7% in cardiovascular disease mortality | 100% | 77% | 90% | 100% | 60% | 18% |
| Low-Density Lipoprotein | 38.66 mg/dL or 1.00 mmol/L decrease | Ference et al., 2012 [77] | Decrease of 20–25% in cardiovascular disease risk | 17% | 0% | 0% | 0% | 0% | 0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juesas, A.; Saez-Berlanga, A.; Babiloni-Lopez, C.; Martin, E.G.; Garrigues-Pelufo, L.; Ferri-Caruana, A.; Gene-Morales, J.; Martin-Rivera, F.; Chulvi-Medrano, I.; Jiménez-Martínez, P.; et al. Effects of Accentuated Eccentric and Maximal Strength High-Resistance Training Programs with or Without a Curcumin-Based Formulation Supplement on Body Composition, Blood Pressure, and Metabolic Parameters in Older Adults. Diseases 2025, 13, 62. https://doi.org/10.3390/diseases13020062
Juesas A, Saez-Berlanga A, Babiloni-Lopez C, Martin EG, Garrigues-Pelufo L, Ferri-Caruana A, Gene-Morales J, Martin-Rivera F, Chulvi-Medrano I, Jiménez-Martínez P, et al. Effects of Accentuated Eccentric and Maximal Strength High-Resistance Training Programs with or Without a Curcumin-Based Formulation Supplement on Body Composition, Blood Pressure, and Metabolic Parameters in Older Adults. Diseases. 2025; 13(2):62. https://doi.org/10.3390/diseases13020062
Chicago/Turabian StyleJuesas, Alvaro, Angel Saez-Berlanga, Carlos Babiloni-Lopez, Ezequiel G. Martin, Luis Garrigues-Pelufo, Ana Ferri-Caruana, Javier Gene-Morales, Fernando Martin-Rivera, Iván Chulvi-Medrano, Pablo Jiménez-Martínez, and et al. 2025. "Effects of Accentuated Eccentric and Maximal Strength High-Resistance Training Programs with or Without a Curcumin-Based Formulation Supplement on Body Composition, Blood Pressure, and Metabolic Parameters in Older Adults" Diseases 13, no. 2: 62. https://doi.org/10.3390/diseases13020062
APA StyleJuesas, A., Saez-Berlanga, A., Babiloni-Lopez, C., Martin, E. G., Garrigues-Pelufo, L., Ferri-Caruana, A., Gene-Morales, J., Martin-Rivera, F., Chulvi-Medrano, I., Jiménez-Martínez, P., Alix-Fages, C., Cwiklinska, M., Gallo, V., Zarza, V., Gargallo, P., Fernandez-Garrido, J., Caballero, O., Casaña, J., Moretti, E., ... Colado, J. C. (2025). Effects of Accentuated Eccentric and Maximal Strength High-Resistance Training Programs with or Without a Curcumin-Based Formulation Supplement on Body Composition, Blood Pressure, and Metabolic Parameters in Older Adults. Diseases, 13(2), 62. https://doi.org/10.3390/diseases13020062














