Abstract
Background: Non-classical manifestations such as neuropsychiatric manifestations in primary hyperparathyroidism (PHPT) have long been documented as symptoms of PHPT and are commonly reported by these patients, despite this connection still being a matter of debate, and they (per se) do not represent an indication of parathyroidectomy. Objective: We aimed to overview the most recent findings regarding the link between depression and/or anxiety (D/A) in subjects confirmed with PHPT, including the impact of the surgery in improving their outcome. Methods: This was a comprehensive review of English-based original studies published between January 2020 and October 2024. Results: The studies (n = 16) included a total of 10,325 patients and an additional 152,525 patients with hypercalcemia (out of whom 13,136 had a PHPT diagnosis and 45,081 were at risk of PHPT diagnosis). Out of these subjects with PHPT, 10,068 underwent parathyroidectomy. Female prevalence was between 62.5 and 92%. Most individuals were over 50, with the youngest studied population having a mean age of 52.7 ± 13.8 years, and the oldest had a median of 71. Depression was documented based on ICD-10 codes (n = 3) and patients’ records (n = 2), Depression Anxiety Stress Scales (DASS) (n = 2), Beck Depression Inventory (BDI) (n = 3), BDI-II (n = 3), Symptom Check List 90-revised (SCL) (n = 1), Hamilton Depression Rating Scale (HAM-D) (n = 2), HADS (n = 2), Patient Health Questionnaire-9 (n = 1), and European Quality of Life 5 Dimensions 3-Level Version (EuroQOL-5D-3L) (n = 1). Patient records’ (n = 1) and ICD-10 codes (n = 2) were also used for anxiety. Most studies used questionnaires to identify anxiety in PHPT: DASS (n = 2), SCL90R (n = 1), Generalized Anxiety Disorder-7 (n = 1), HADS (n = 2), EuroQOL-5D-3L (n = 1), and State–Trait Anxiety Inventory (n = 1). Depression prevalence varied from 20–36.6% to 65.7% (scale-based assessment) and to 10.5% upon ICD-10. A rate of newly onset depression was reported of 10.7% and of 0.2% with concern to the prevalent suicidal ideation (an incidental rate of 0.4% after a median follow-up of 4.2 years). Most studies identified a moderate depression (when assessing its severity), affecting approximately one third of the surgery candidates. The prevalence of anxiety in PHPT varied between 10.4% and 38.6% (n = 8). Discordant results were generated when applying distinct questionnaires for the same population, and this might come as a potential bias. Other confounding factors are generated by the sub-population referred for surgery that typically displays a more severe parathyroid condition or non-endocrine overlapping conditions (e.g., related to the social or familial status). Conclusion: The modern approach of the patient with PHPT should be complex and go beyond the traditional frame. D/A had a high prevalence in the mentioned studies, associated with increased medication use. Yet, the underlying pathogenic mechanisms remain incompletely elucidated. No correlations between D/A and serum calcium levels were confirmed, while PTH had a slight positive correlation with depression. Parathyroid surgery appears to be beneficial for D/A as it improves the scores, prevalence, and severity. Cinacalcet might reduce depression scores, although more evidence is needed. Women are prone to both PHPT and D/A. The optimal method of D/A screening in PHPT remains to be determined, and the current scales need validation and perhaps adjustment for this specific population sub-group, while PHPT management should be refined upon D/A identification.
Keywords:
primary hyperparathyroidism; depression; anxiety; PTH; calcium; parathyroidectomy; parathyroid; hormone; scale; questionnaire; study 1. Introduction
Primary hyperparathyroidism (PHPT) is a complex disease with various clinical consequences beyond the classical bone and the kidney involvement [1,2]. Non-classical manifestations such as neuropsychiatric manifestations have long been documented as symptoms of PHPT and are commonly reported by these patients, despite this connection still being a matter of debate [3,4]. The underlying pathogenic pathways linking hypercalcemia and parathyroid hormone (PTH) excess to the co-presence of the neuropsychiatric symptoms are poorly understood [5]. The most common psychiatric manifestations in patients with PHPT are depression and anxiety, with a prevalence up to 30% and 49%, respectively, as reported by older studies [6,7]. Of note, fatigue, sleep disturbances, and even psychosis have been reported in PHPT, too [8,9].
Depression is accompanied by a wide spectrum of vegetative, emotional, and cognitive symptoms, including fatigue, appetite, weight fluctuations, insomnia, and anhedonia, all of which impair physical and emotional health, as well as social well-being [10]. Similarly, the psychological and physical symptoms occurring in patients with anxiety, including insomnia, fatigue, muscle tension, and attention difficulties, can be debilitating. Anxiety impairs one’s overall health, it increases the risk of cardiovascular events, and affects work productivity as well as the personal life [10,11]. Both anxiety and depression decrease the quality of life and increase the disease-related overall burden [12]. Moreover, they increase the associated healthcare costs due to medication and additional therapy, and they may impact the evolution of other comorbidities in PHPT such as osteoporosis/rehabilitation upon osteoporotic fractures, kidney ailments, and cardiovascular diseases [13,14,15,16,17]. Moreover, since the association between PHPT and depression/anxiety is not the norm, the acumen of differential diagnosis involves an overlapping diagnosis with the parathyroid tumour that might not be directly related to the calcium and/or PTH over-production, or it may actually be related to other non-endocrine ailments or even concurrent medications [10,18,19].
Identifying depression and anxiety in patients with PHPT may be challenging. Their screening is recommended, as seen in the general adult population, considering that early detection and associated treatment improve the outcome. There are various scales available and validated, such as Hospital Anxiety and Depression Scales (HADS), Patient Health Questionnaire-9 (PHQ-9), Generalized Anxiety Disorder (GAD), etc. [20,21]. While one of the most reliable tools seems to be the PHQ-9 [21,22,23], there is no standardized approach recommended and no distinct recommended instrument so far (at guideline level) [20]. Regarding PHPT patients, recent data suggested that in PHPT, the PHQ-9 score may be applied in surgery candidates since parathyroidectomy was shown to reduce the preoperatory scores and decrease the prevalence of clinically significant depression [24].
According to the American Association of Endocrine Surgeons, patients with psychological manifestations such as depression and anxiety might have indication for parathyroidectomy, as it could improve the outcome. However, the evidence in support of this claim is still scarce [25]. So far, there are no specific recommendations regarding the diagnosis and management of depression in patients with PHPT or to perform surgery if one patient presents this type of complication (unless the other traditional complications are co-presented, such as osteoporosis, kidney stones and/or failure, high levels of calcium, etc.) [26].
We aimed to overview the most recent findings regarding the link between depression and/or anxiety in subjects confirmed with PHPT, including the impact of the parathyroidectomy in improving the outcome of these conditions; finally, we provided a novel interdisciplinary framework.
2. Methods
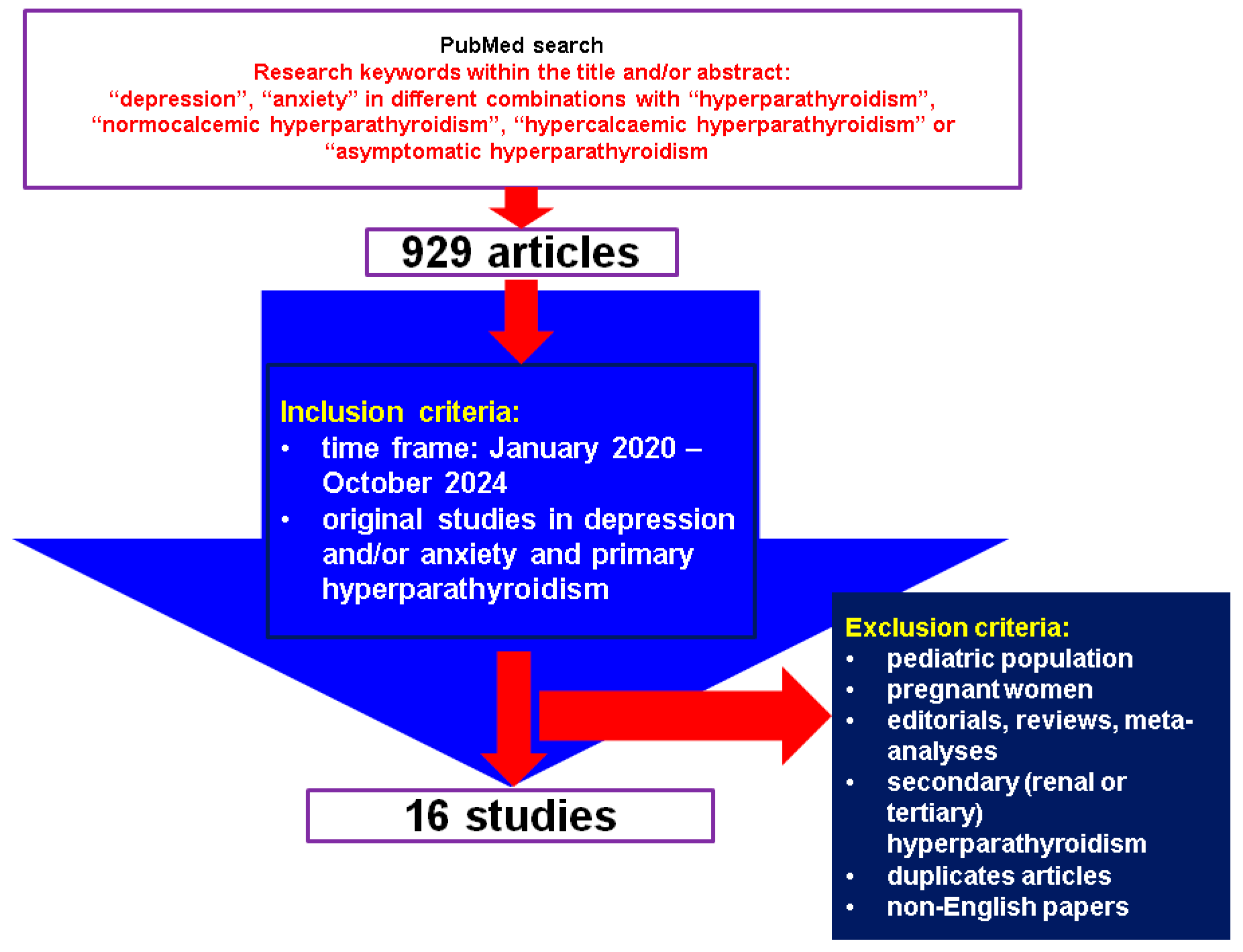
This was a comprehensive review of the English-based medical literature. We searched for papers published between January 2020 and October 2024, using the following keywords: “depression”, “anxiety” in different combinations with “hyperparathyroidism”, “normocalcemic hyperparathyroidism”, “hypercalcemic hyperparathyroidism” or “asymptomatic hyperparathyroidism”. The search based on the mentioned keywords identified 929 articles, the time frame restricted the search, and, finally, the articles were manually checked within title and abstract to provide original studies in the mentioned topics. We only included clinically relevant, original studies regarding depression and/or anxiety (including associated medication) in individuals confirmed with PHPT. We excluded papers on children, adolescents, pregnant women, editorials, reviews, meta-analyses, secondary (renal or tertiary) hyperparathyroidism, duplicates, non-English articles. Overall, we included 16 studies [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] (Figure 1).
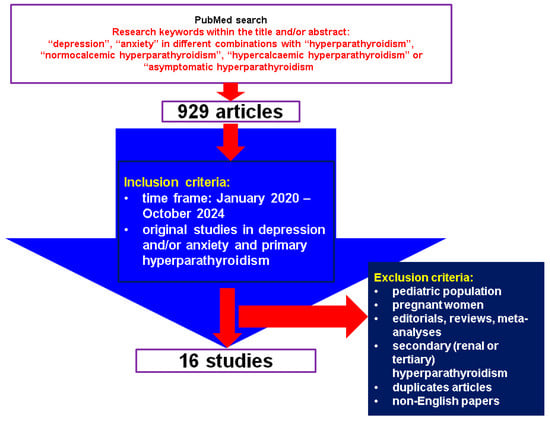
Figure 1.
Flow chart diagram of search.
3. Results
The studies (n = 16) [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] included a total of 10,325 patients diagnosed with PHPT and an additional 152,525 patients with hypercalcemia (out of whom 13,136 had a PHPT diagnosis and 45,081 were at risk of PHPT diagnosis). Out of the subjects with PHPT, 10,068 underwent parathyroidectomy, and the analysis was performed with respect to the pre- and postoperatory panel in surgery candidates [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] (Table 1).

Table 1.
Studies investigating depression and anxiety in patients with PHPT (the display starts with the most recent publication date) [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
The majority of the patients were women (between 62.5% [27] and 92% [28]). Most individuals were over 50 years, with the youngest studied population having a mean age of 52.7 ± 13.8 years [42] and the oldest with a median age of 71 (62–79) years [37]. Depression was documented based on ICD-10 (The International Classification of Diseases-Tenth Revision Clinical Modification) codes (n = 3) [27,30,34] and patients’ records (n = 2) [33,42], as well as upon applying the following questionnaires: Depression Anxiety Stress Scales (DASS) (n = 2) [28,32], Beck Depression Inventory (BDI) (n = 3) [32,36,40], BDI-II (n = 3) [29,31,41], Symptom Check List 90-revised (SCL90R) (n = 1) [32], Hamilton Depression Rating Scale (HAM-D) (n = 2) [35,41], HADS (n = 2) [37,41], Patient Health Questionnaire-9 (n = 1) [38], and European Quality of Life 5 Dimensions 3-Level Version (EuroQOL-5D-3L) (n = 1) [39]. Patient records (n = 1) [33] and ICD-10 codes (n = 2) [30,34] were also used for anxiety. Most studies, however, used questionnaires to identify anxiety in patients with PHPT: DASS (n = 2) [28,32], SCL90R (n = 1) [32], Generalized Anxiety Disorder-7 (n = 1) [35,38], HADS (n = 2) [37,41], EuroQOL-5D-3L (n = 1) [39], and State–Trait Anxiety Inventory (n = 1) [40].
3.1. Analysis of Depression in Patients Diagnosed with Primary Hyperparathyroidism
3.1.1. Depression: Prevalence, Severity, and Influencing Factors
The prevalence of depression in patients with PHPT varied with the studies, noting that distinct tools were used to assess it. The highest prevalence was 65.7% in the study conducted by Jovanovic et al. [32], BDI being used in this instance [32]. BDI-II was also applied in two other studies that identified depression rates of 57.1% and 51%, respectively [29,31]. The lowest prevalence (of 36.6%) based on BDI-II was reported by Kunert et al. [41]. The same study found a higher prevalence (of 60.4%) when HAM-D was applied and a lower prevalence of 20.79% upon HADS use, respectively [41]. Overall, the lowest rate was found at 10.5% based on ICD-10 codes. The same study also reported newly onset depression in 10.7% of the patients with parathyroid tumours, prevalent suicidal ideation in 0.2% of them, and newly onset suicidal ideation in 0.4% of these subjects after a median follow-up of 4.2 (3.7–4.6) years [30]. Another study based on the patients’ records identified that 27.2% of them had depression, women being more often affected (p = 0.016) [42].
Some studies provided depression scores in patients with PHPT. For instance, Wang et al. [40] found a median score of 5 (0.46) using BDI-II [40], while Chan et al. [28] reported a mean (SE) score of 6.89 (0.95) using DASS [28], both corresponding to the normal population. Similarly, Komman et al. [34] reported median scores of 4 (1–6) and 4 (1–8) in patients with PHPT ≥ 50 years and ≥70 years, respectively [34].
The variable results in the same study population due to using different methods/tools of depression assessment are worth mentioning. Two of the studies applied multiple methods within the same study population. For example, Kunert et al. [41] identified discordant rates via HAM-D, BDI-II, and HADS in 101 patients with PHPT who underwent parathyroidectomy and 50 controls with toxic goitre. At baseline, in patients with PHPT, the overall prevalence of depression was the highest according to HAM-D (60.4%) and the lowest according to HADS (20.79%). Moreover, when HAM-D and BDI-II were used, the prevalence of depression in PHPT patients was higher than controls (60.4% versus 12%, p < 0.001 and 36.36% versus 18%, p < 0.05, respectively), but the difference was not statistically significant when HADS was applied (20.79% versus 12%, p > 0.05) [41]. BDI and DASS were applied by Jovanovic et al. [32] in 101 individuals with PHPT who underwent surgery and found a higher rate of depression based on BDI (65.7%) versus DASS (19.8%) [32]. Compared to the control population, depression was more frequent and severe in patients with PHPT [31,38,41], while versus age- and sex-matched controls, the endocrine subjects with PHPT had a higher BDI-II score (16.8 ± 9.98 versus 6.10 ± 5.45, p < 0.001) [29]. Similar results were found between patients with PHPT and those diagnosed with a toxic goitre [41]. Another study compared subjects who underwent parathyroidectomy to those who were referred for thyroidectomy and revealed that subjects with parathyroid tumours had higher preoperative depression scores (6.7 ± 6.6 versus 4.4 ± 4.9, p < 0.01) [38].
Of note, the prevalence of depression might be influenced by the inclusion of surgical patients only, who typically display a more severe disease form [29,32,41]. Yet, Song et al. [30] analysed operative and non-operative subjects and found similar rates with respect to depression (p = 0.265), pre-existing suicidal ideation (p = 1), and newly onset suicidal ideation (p = 0.13) [30]. The study reported higher rates of newly onset depression in non-operative patients (12.2%) compared to the overall study population (10.7%) and operative patients (9.3%) (p = 0.046) [30].
Other factors influencing the depression rates were investigated by Febrero et al. [31], who identified higher depression scores in subjects aged between 40 and 60 years (p < 0.001), those with no primary education (p = 0.014), and individuals with children (p = 0.019). Regarding PHPT-related symptoms, depression rates were similar in patients experiencing bone pain (p = 0.54), nephrolithiasis (p = 0.215), gastrointestinal complains (p = 0.863), and hypertension (p = 0.323) [31].
Major depressive disorder was also evaluated in a very large study on 135,034 patients diagnosed with hypercalcemia (and 13,136 individuals had PHPT), the condition being more prevalent in subjects with PHPT as well as those at high risk of PHPT diagnosis with or without PTH data when compared to matched controls (p < 0.001). Moreover, the time to diagnosis might influence the development of major depression. The prevalence and risk of major depression were lower in subjects diagnosed with PHPT later (after more than one year from the moment of hypercalcemia confirmation) compared to those diagnosed earlier than one year [11.9% versus 16.1%, odds ratio (OR) = 0.7 (0.62–0.8), p < 0.001], but, overall, during the following one to three years, depression became more prevalent in patients diagnosed later with PHPT [17.3% versus 15.1%, OR = 1.18 (1.05–1.34), p = 0.008)] [33]. On the other hand, the identification of depression may influence the odds of PTH testing in patients with hypercalcemia, as suggested by Bunch et al. [27] in a large retrospective study (N = 17,491). The people suffering from depression and hypercalcemia had lower odds of having a PTH check-up [OR = 0.84 (0.74, 0.96), p = 0.0081], suggesting a potential testing gap on this specific matter [27] (Table A1).
3.1.2. The Use of Antidepressant Medication in Subjects with Primary Hyperparathyroidism
The prevalence of antidepressant use in PHPT was reported at 20% in surgery candidates [29]. The largest study investigating the matter of this drug administration was performed on 8279 subjects with PHPT [37]. The most common drugs were selective serotonin reuptake inhibitors (SSRIs) in 15.9% of the subjects, followed by selective serotonin and norepinephrine inhibitors (SNRIs) in 8.6%, while 4.5% of the people with PHPT were treated with tricyclic antidepressants. Compared to controls, the odds of using antidepressants were higher in subjects with PHPT, for SSRI [OR = 1.38 (1.3–1.47)], SNRI [OR = 1.36 (1.26–1.48)], and tricyclic antidepressants [OR = 1.4 (1.26–1.57)] [34] (Table A2).
3.1.3. The Relationship Between Depression and the Endocrine Assays Amid Primary Hyperparathyroidism
Six studies investigated potential associations between the presence of depression and the levels of calcium, parathyroid hormone, or other endocrine parameters such as serum cortisol and bone formation marker osteocalcin in PHPT [30,31,34,38,40,41]. Regarding the baseline serum calcium levels, no statistically significant relationship with depression was found. Song et al. [30] calculated a threshold of 11.5 mg/dL for the value of serum total calcium but found no statistical significance in terms of hazard ratio (HR) for depression [HR = 1.32 (0.97–1.81)]. However, patients with serum total calcium above 11.5 mg/dL were 6.69-times more likely to develop suicidal ideation [HR = 6.69 (1.2–37.35)] [30]. A lower serum total calcium threshold was applied by Febrero et al. [31] while comparing the depression scores in patients with preoperative serum calcium lower than 11 mg/dL versus those with a calcaemic level ≥11 mg/dL. Both groups had similar depression scores (15.5 ± 11.38 versus 14.5 ± 19, p = 0.399) [31]. Koman et al. [34] used different serum calcium ranges in order to assess the medication use in PHPT compared to controls. The study found the highest odds for SSRI use [OR = 1.61 (1.39–1.99)] and SNRI use [OR = 1.81 (1.53–2.14)] compared to controls at lower calcium levels (<1.38 mmol/L), while calcaemic values did not influence the odds of tricyclic antidepressive use (p = 0.1771) [34]. Notably, the correlations between depression and pre- and postoperative calcium levels [38], depression and calcaemic levels [40], or depression severity and calcium levels [41] did not confirm any association (Table A3).
3.1.4. The Impact of the Parathyroidectomy on Depression
The impact of parathyroid tumour removal on depression was studied in five original studies that analysed the presence of the pre- and postoperative depression scores in 448 surgery candidates [28,29,32,36,38]. Moreover, one study provided comparative data between parathyroidectomy and cholecystectomy [35], and another investigated the impact of parathyroidectomy in symptomatic and asymptomatic patients [39], while another analysed the antidepressant use before and after parathyroid surgery [34]. Most studies confirmed the depression prevalence decrease, as well as improvements in the depression scores and severity following parathyroidectomy. The largest study included 244 subjects with PHPT and 161 controls undergoing thyroidectomy. A higher rate of patients without depression following surgery (72.5% versus 47.9%) was confirmed, as well as an overall reduction in the mean depression scores after surgery (6.7 ± 6.6 versus 3.1 ± 3.9, p < 0.01). In addition, there was a decrease in depression severity based on a lower postoperatory proportion of moderate to severe depression (27.5% versus 8.2%, p < 0.01). Moreover, the study reported higher preoperative depression scores in PHPT compared with the subjects who underwent thyroidectomy (6.7 ± 6.6 versus 4.4 ± 4.9, p < 0.01), with similar postoperative scores following parathyroidectomy and thyroidectomy (3.1 ± 3.9 versus 3.3 ± 4.1, p = 0.52) [38].
An improvement in both depression scores and depression severity was also reported in a prospective study on 49 patients with PHPT who underwent parathyroidectomy compared with age- and sex-matched controls. At diagnosis, 42.9% of them did not have depression, but, one year after surgery, 72.9% had normal scores, suggesting the absence of depression. Not only was the prevalence of depression lower after parathyroidectomy but also the mean depression scores decreased, both at three months and one year after surgery compared to baseline (13.08 ± 10.76 versus 16.80 ± 9.98, p = 0.001, and 10.5 ± 10.79 versus 16.65 ± 10.03, p < 0.001, respectively). Despite score decreases at three months and one year after surgery, they remained higher than controls (13.08 ± 10.76 versus 6.10 ± 5.45, p < 0.001 and 10.5 ± 10.79 versus 6.10 ± 5.45, p < 0.001, respectively). Data provided by this study suggested that parathyroidectomy has a beneficial effect on depression in individuals confirmed with PHPT, while patients with PHPT may be more affected by depression than the healthy population [29].
The depression diagnosis was heterogeneous considering various scales. One study reduced this bias by using three different questionnaires, all showing an improvement in scores, suggesting an overall beneficial post-surgery effect. The study was conducted in 101 patients who underwent parathyroidectomy and showed a reduction in depression scores following surgery of up to 62.7%. The highest score reduction was observed when SCL90R was used, while the lowest reduction (of 56.2%) was confirmed based on DASS. According to BDI, there was a 60% reduction in the mean depression score following surgery. All scales showed lower scores after one month and six months after parathyroid surgery compared to preoperative scores, as well as lower scores six months post-surgery compared to one month after parathyroidectomy. Of note, the differences between questionnaires were supported by the fact that distinct scores provided distinct depression prevalence in the same study population. While BDI reported 65.7% of patients as having depression, DASS showed that only 19.8% had the condition. In addition, the mean preoperative BDI score (13.5) corresponded to depression, while the mean DASS depression score (6.3) corresponded to normal ranges [32].
While most studies found an improvement regarding depression symptoms following parathyroidectomy, Szalat et al. [36] did not confirm it at one month or six months following surgery (p = 0.697); of note, this was a small cohort (N = 18) [36]. When compared with other surgical procedures such as cholecystectomy, parathyroidectomy might be associated with more depressive symptoms in surgery candidates, as suggested by a retrospective study in 43 patients (parathyroidectomy candidates) and another 43 (cholecystectomy candidates). Subjects who underwent parathyroid surgery had higher median depression scores (via HAM-D) compared with those who underwent cholecystectomy at baseline (6 versus 2, p < 0.05), six months postoperatively (2 versus 1, p = 0.006) and one year post-surgery (2 versus 1, p = 0.007), respectively. However, all median scores corresponded to the normal ranges [35].
Medication against depression was analysed in a large study in 8279 parathyroidectomy candidates. The antidepressant use before and after surgery showed that SSRI use increased overall [relative risk (RR) at 0–6 months postoperatively = 1.25 (1.16–1.34) and RR at 7–12 months postoperatively = 1.28 (1.2–1.37)] but decreased in prevalent users [RR at 0–6 months postoperatively = 0.92 (0.87–0.97), RR at 7–12 months postoperatively = 0.94 (0.89–0.99)], with no statistically significant trend regarding the time frame [34]. The impact of parathyroidectomy in symptomatic and asymptomatic subjects was evaluated in a prospective study (N = 78 patients with PHPT who underwent parathyroidectomy, 28 of whom were asymptomatic, with the remaining having symptomatic PHPT). The study assessed depression and anxiety together. Overall, the 41% decrease in anxiety/depression was not statistically significant (p = 0.07), while asymptomatic patients had a similar anxiety/depression prevalence before and after surgery (p = 1.00), and symptomatic PHPT subjects had a reduction in the prevalence of moderate anxiety/depression postoperatively (25% versus 53%, p < 0.01) but no difference in extremely severe anxiety/depression prevalence (4% versus 4%) [39].
To summarize, these recent data suggested that parathyroidectomy improved the subjective depression symptoms and reduced depression prevalence (Table A5).
3.1.5. The Administration of Cinacalcet and Its Impact on Depression
The use of cinacalcet was reported in one prospective observational study on 35 adults with PHPT and mild cognitive impairment who underwent treatment with this drug before surgery. At 4 weeks of cinacalcet versus baseline, depression scores decreased [−1 (0, −2), p = 0.019], as well as depression and anxiety scores combined [−2 (0, −3), p = 0.020] via HDAS. The reduction was not statistically significant in the sub-group of adults aged 70 years and older (p = 0.053 and p = 0.095, respectively). Parathyroidectomy improved the depression scores further in the overall population [−3 (0, −6), p = 0.04]. Of note, the baseline median depression scores corresponded to no or few symptoms [37] (Table A5).
3.1.6. Depression in Relationship with the Conservative Versus Surgical Management of Parathyroid Tumours
Data regarding the impact of parathyroidectomy compared to the conservative approach were provided in a retrospective study, which included, after a propensity score match, 1918 patients with PHPT, out of who 959 underwent surgery and 959 were managed conservatively. The study reported a higher prevalence of newly onset depression in conservatively managed subjects versus those who underwent surgery (12.2% versus 10.7%, p = 0.046). However, there was no statistically significant difference in terms of pre-existing depression, pre-existing or newly onset suicidal ideation between the two groups, respectively. Moreover, the risk of depression and suicidal ideation was also similar in conservatively versus surgically managed subjects [30] (Table A6).
3.2. Analysis of Anxiety in Subjects Confirmed with Primary Hyperparathyroidism
3.2.1. The Prevalence and Severity of Anxiety
The prevalence of anxiety in patients with PHPT varied between 10.4% [30] and 38.6% [41], and this variability might be caused by different methods to assess anxiety across eight studies [28,30,32,33,34,38,40,41]. For instance, the lowest prevalence of 10.4% was found using ICD-10 codes, a method that may overlook the undiagnosed cases and, therefore, underestimate the exact prevalence [30]. The prevalence calculated based on DASS in surgery candidates was 22.8% [32], while the highest prevalence (of 38.6%) was identified via HADS [41]. Song et al. [30] found no differences in terms of pre-existing (p = 0.601) or newly onset anxiety (p = 0.089) between operative and non-operative subjects [30]. Compared to controls, some data suggested higher rates of anxiety in PHPT [33], while others did not [41]. In a large study on patients with hypercalcemia, patients at high risk of PHPT diagnosis had higher anxiety rates than matched controls (15.4% versus 10.8%, p < 0.001), but the study did not compare the subjects confirmed with PHPT diagnosis versus controls [33]. Liu et al. [38] found higher anxiety scores in individuals who underwent parathyroidectomy compared to those who were referred for thyroidectomy (4.5 ± 5.6 versus 3.2 ± 4.1, p < 0.01) [38]. On the other hand, Kunert et al. [41] found a similar anxiety prevalence in people with PHPT who had to undergo parathyroidectomy (38.6% versus 34%, p > 0.05) [41]. Of note, the controls had non-toxic goitre, an affliction that may have higher rates of anxiety than the general population according to some authors [43].
The severity of anxiety was analysed in 101 surgery candidates with PHPT. Most patients had mild (9.9%) or moderate (7.9%) anxiety, while 4% had severe anxiety. Extremely severe anxiety was identified in 1% of patients [32]. Other studies only provided anxiety scores, not the prevalence. For instance, Chan et al. [28] reported a mean score of 5.17 (1.01), corresponding for a lack of anxiety [28]. Similarly, Koman et al. [37] reported a median score of 6 (4–10) in patients with PHPT ≥ 50 years [37]. Wang et al. [40], however, found a higher score of proneness to anxiety (37.39 ± 10.34), which indicated a moderate tendency towards anxiety, according to the State–Trait Anxiety Inventory. The mean score (of 35.43 ± 11.56) was indicative for low anxiety [40] (Table A7).
3.2.2. Analysis of the Anxiolytic Medication
Data regarding medication against anxiety in patients confirmed with PHPT were provided in a single study that reported a prevalence of 13.3%, surgery candidates being at 1.4-times higher risk of anxiolytic drug use compared to a matched control population [OR = 1.4 (1.31–1.5)] [34].
3.2.3. Endocrine Assays and Anxiety
Limited data (n = 5 studies) suggested that patients with PHPT might be more affected by anxiety at lower calcium levels [30,34,38,40,41]. For instance, a negative weak correlation between ionized serum calcium and anxiety severity was found (r = −0.1863, p < 0.05) [41]. The highest rate of using anxiolytics in PHPT versus controls was confirmed at lower calcium levels (p = 0.004) by Koman et al. [34]. On the other hand, a retrospective study in 192 patients with PHPT found no correlation between the current anxiety (r = −0.060, p = 0.726) or anxiety proneness (r = 0.049, p = 0.782) and serum calcium levels [40]. A study on 244 subjects with PHPT also did not confirm this type of correlation [38]. While one study reported a small positive correlation between anxiety and serum PTH levels (r = 0.1797, p < 0.05) [41], others did not [38,40], nor to a potential correlation with serum cortisol [40]. Blood osteocalcin was negatively associated with current anxiety after multiple variable adjustments (r = −0.426, p = 0.027) in a single study [40] (Table A8).
3.2.4. The Potential Parathyroidectomy Impact on Anxiety
Most studies showed a reduction in anxiety scores following parathyroidectomy [28,32,34,38]. The largest of them (N = 244 patients who underwent parathyroidectomy for PHPT) reported lower anxiety scores postoperatively [mean difference of 2.5 (1.9–3.1), p < 0.01]. The same study compared parathyroidectomy with thyroidectomy candidates and found that, although preoperative scores were higher in subjects with PHPT (4.5 ± 5.6 versus 3.2 ± 4.1, p < 0.01), following surgery, anxiety scores were similar to those in persons who underwent thyroidectomy (2.0 ± 3.6 versus 2.1 ± 3.9, p = 0.75). Moreover, after parathyroid surgery, anxiety severity improved (moderate to severe: 18% versus 5.3%, p < 0.01) [38]. Another study (N = 101 patients with PHPT who were surgically treated) identified a reduction in anxiety scores, as assessed by DASS, of 62.4%. The lowering of anxiety scores was statistically significant, both at one-month post-surgery (p = 0.001) and at six months (p = 0.001). Similar results were reported via SCL90R [32]. Lower anxiety scores were also reported in a small study in 36 surgery patients, both at one week and at three months following surgery [5.17 (1.01) versus 3 (1.01) versus 2.46 (1.03), p = 0.01]. The trend, however, was not statistically significant (p = 0.2) [28]. Regarding anxiolytic medication, one large study (N = 8279 patients with PHPT) identified a higher risk following surgery compared to controls [0–6 months: RR (CI) = 1.31 (1.2–1.43)] but decreased over time (p for trend = 0.003). Prevalent users, however, had similar risk to controls [0–6 months: RR (CI) = 0.97 (0.9–1.05)] [34]. As mentioned, Scerrino et al. [35] compared cholecystectomy candidates and found that individuals who underwent parathyroidectomy had similar anxiety rates preoperatively (72% versus 93%, p = 0.7321), at six months (76.7% versus 97.6%, p = 0.3823) and at one year (83.72% versus 97.6%, p = 0.4329) after surgery, respectively [35] (Table A9).
3.2.5. Cinacalcet Use Before Parathyroidectomy and Anxiety Impact
One prospective observational study analysed the impact of preoperative exposure to cinacalcet in adults with PHPT; similar anxiety scores were found after four weeks into the drug compared to baseline (p = 0.140). Following parathyroidectomy, however, the median anxiety scores decreased [−2 (0, −3), p = 0.040] [37] (Table A9).
3.2.6. Conservative Management Versus Parathyroidectomy in Patients with PHPT and the Consequences on Anxiety
Song et al. [30] investigated the differences regarding the prevalence and incidence of anxiety in PHPT with concern to conservative management versus parathyroidectomy. The study reported no statistically significant difference in terms of pre-existing anxiety (10.8% versus 10%, p = 0.601) and newly onset anxiety (14.3% versus 11.6%, p = 0.089) [30] (Table A9).
4. Discussion
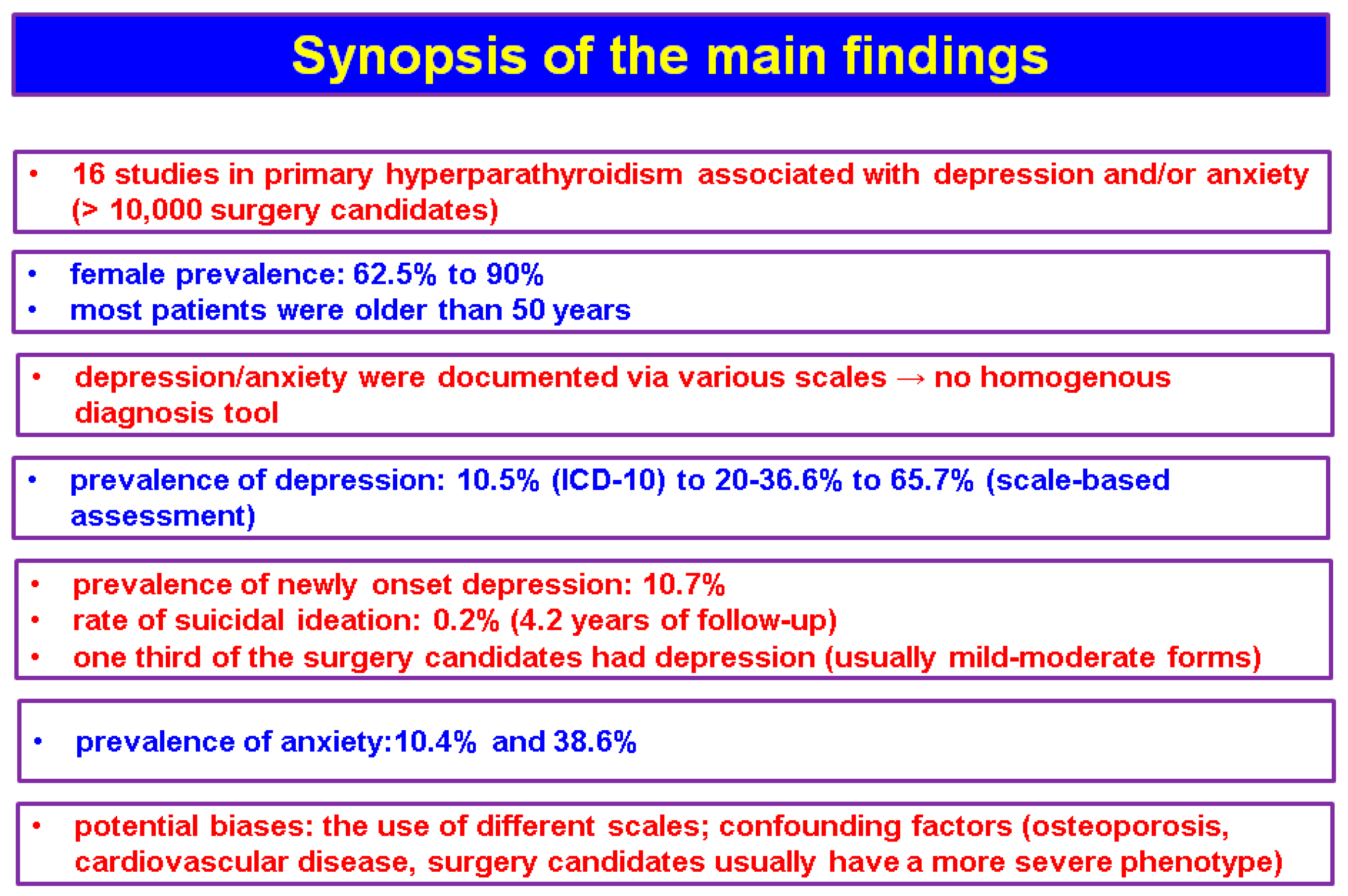
The studies (n = 16, N = 10,325) that we identified based on the mentioned methods showed an interesting association between depression and/or anxiety and the features of PHPT, either in terms of diagnosis or management [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42], but the data are not homogenous (Figure 2).
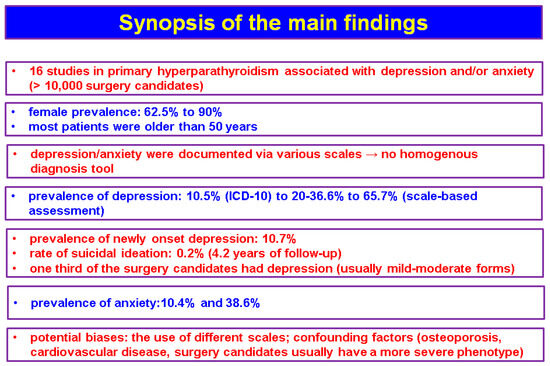
Figure 2.
Synopsis of the main findings according to our methods.
4.1. Tools and Methods Used for the Assessment of Depression and Anxiety
One of the most used depression scales in recent studies is BDI [44] and its revised version, BDI-II [29,31,32,36,40,41]. The first version underwent multiple revisions, and the latest version, BDI-II, was adjusted to align with DSM-IV criteria for depressive disorder [45]. Even though BDI-I and BDI-II have been validated and are extensively used in different medical areas [46,47,48,49,50], they might overestimate the prevalence of depression given that factors, such as fatigability, sleep disturbances, osteoporosis, fractures, weight loss and loss of appetite, commonly found in various chronic illnesses, are included [51,52,53]. Of note, fatigability, bone and muscle ailments, as well as sleep disturbances are highly prevalent in subjects with PHPT, and symptoms such as loss of appetite and weight loss may be related to nausea and vomiting due to hypercalcemia [54,55]. Liu et al. [38] applied PHQ-9, which evaluates both the presence of depression and its severity. However, it takes into consideration multiple symptoms, such as sleep troubles, tiredness, and appetite changes, that might not be a reflection of PTH and serum calcium excess [56,57]. Another scale that has been validated among different populations is DASS [52] [58,59,60]. Unlike BDI and PHQ-9, DASS does not include physical symptoms like fatigue and appetite changes, and it also differentiates between anxiety, stress, and depression [52]. HADS served as a tool in PHPT investigation amid two recent studies [37,41]. This widely validated scale might be more fitting as it avoids somatic symptoms and does not overlap with common symptoms of (non-PTH-related) chronic illness [61,62]. However, recently, it has been suggested that cut-off values should be adjusted in certain patients [63].
The anxiety scales that have been used in patients with PHPT in these recent studies included GAD-7 [35,38], HADS [37,41], DASS [28,32], and State–Trait Anxiety Inventory [40]. All are widely used and validated scales, and they mostly focus on subjective symptoms [64]. The main advantages of scales like HADS or DASS are the ability to evaluate patients in a time-efficient manner and with little prior training [65]. One of the major limitations of applying these scales in assessing depression and anxiety is the fact that they are non-diagnostic screening tools [66]. Another important point is the method of administration, considering that self-rating was shown to provide higher scores compared to interview administration, especially in the elderly [67]. There were also studies based on ICD-10 codes [30,34]. Although these may include an objective diagnosis, they may not always be accurate, as some authors suggested an underestimation of depression prevalence [68]. Overall, while data from questionnaires are practical, they might be biased due to relying on self-reporting symptoms; yet, they may be integrated in the evaluation of one patient with PHPT as a screening tool in daily endocrine practice. Further studies correlating standardized objective clinical assessments are needed to understand the extent of the PHPT effect on depression and anxiety.
4.2. Common Pathogenic Mechanisms Underlying PHPT and Depression/Anxiety
The mechanisms linking PHPT and psychiatric manifestations such as depression and anxiety are intricate and incompletely understood so far [69]. The influence of the parathyroid hormone on the brain is supported by the presence of PTH receptors, namely, PTH1R and PTH2R, in various brain regions. However, its ability to cross the blood–brain barrier is disputed, with no clear data [70]. The PTH1R receptor found in brain tissue is expressed in distinct regions of the limbic system, including the thalamus, amygdala, and entorhinal cortex, in vestibular nuclei, in the cerebellum, and astrocytes, being identical to the PTH1R from bones and kidneys according to animal studies [71]. Both PTH and PTHrP can bind to PTH1R. PTH1R activation was shown to reduce neuro-inflammation, to improve cell proliferation, and increase the blood flow in the brain [72,73]. Another PTHR that was found in the brain was PTH2R, which was described in 1995 by Usdin et al. [74]. Unlike PTH1R, PTH2R differentiates between PTH and PTHrP, and it is only activated by the former [75]. While PTH can bind to PTH2R, a much more potent ligand is TIP39. Not only does PTH2R have a higher affinity for TIP39 but the response is also more sustained [69]. The binding of TIP39 to PTH2R was linked in animal studies to fear, nociception, reproductive behaviour, and stress response by modulating the hypothalamic–pituitary–adrenal axis [76]. It was also shown to reduce depression and anxiety-like behaviours [76,77,78].
Apart from the possible effects of PTH on the central nervous system, other mechanisms involved in the development of depression and anxiety in patients with PHPT might be related to the influence exerted by excessive PTH and calcium on monoamine neurotransmitters; however, data are scarce and conflicting to date [79]. While it has been proposed that hypercalcemia might increase the neurotransmitters by reducing monoamine oxidases (MAO) activity and reducing the catabolism of serotonin, dopamine, and norepinephrine [79], other studies suggested quite the opposite, namely, that intracellular calcium stimulates MAO-A [80]. Overall, the mechanisms connecting PHPT to the development of depression/anxiety are complex and involve both the direct and indirect effects of PTH and calcium. Further studies are needed for a more in-depth understanding of these pathogenic pathways.
4.3. Confounding Factors in Assessing Anxiety/Depression in Patients with Parathyroid Tumours
4.3.1. Age and Gender
In order to accurately understand the relationship between PHPT and depression/anxiety, possible confounding elements must be taken into account. First of all, PHPT has a peak of incidence in menopausal women [81]. This is an important factor when assessing depression in patients with PHPT, as it has been shown that women are 2- to 2.5-times more likely to develop depression and major depressive disorder than men, with postmenopausal women having the highest risk [82,83]. Recent studies have shown that the depression affects about one-third of menopausal women worldwide, and it is influenced by various factors, including marital, social, and economic status, as well as the presence of menopausal symptoms [84,85]. Not only are women more likely to develop depression but anxiety might also be more frequent in this population due to various psychosocial and biological contributors [86,87]. Depression may have an age-related incidence, as recent studies have found peaks in middle adulthood and seniors [88,89]. In order to rule out this bias, some studies compared patients with PHPT with age- and sex-matched controls and found higher depression scores in patients with PHPT [29,31], suggesting that PHPT itself leads to a greater depression rate. While depression scores decreased following parathyroidectomy, they may remain higher than in controls [29]. Interestingly, the number of livebirths was shown to be a protective factor against depression in women [90], but Febrero et al. [31] found that in patients with PHPT, having children was associated with higher depression scores [31].
4.3.2. Comorbidities
The relationship between PHPT, comorbidities such as metabolic syndrome and heart disease, and depression and/or anxiety is intricate and often multidirectional. PHPT might be accompanied by multiple ailments, ranging from classical ones such as osteoporosis/osteoporotic fractures and chronic kidney disease to cardiovascular disease, type 2 diabetes mellitus, and metabolic syndrome. One of the more recently characterized complications of PHPT is diabetes, which not only has a higher prevalence in patients with PHPT but, also, displays a positive linear association with the value of serum calcium [91]. Depression and/or anxiety are also linked to diabetes in a bi-directional manner. While there is a causal relationship from diabetes to depression/anxiety, they also increase the risk of developing diabetes and are linked to poorer glycaemic control and, consequently, a higher disease burden and mortality [92,93,94].
Regarding metabolic syndrome, both depression and PHPT are contributing factors, and their independent and cumulative effect ought to be investigated [95,96,97]. Furthermore, metabolic syndrome might increase the risk of anxiety via chronic inflammation [98]. One recent study that we already mentioned [32] briefly covered the complex relationship between mental health, PHPT, and type 2 diabetes mellitus. The authors found that in subjects confirmed with PHPT, there were no statistically significant differences regarding depression and anxiety scores, as evaluated using multiple scales, between diabetic and non-diabetic individuals following parathyroid surgery [32]. Little is known of the cumulative effect of PHPT and depression and/or anxiety on diabetes. Further studies are needed to understand this complex relationship.
Apart from the link with PHPT, osteoporosis has been connected to depression both as a possible risk factor and as a possible effect. On the one hand, osteoporosis has been shown to independently increase the risk of depression [99]. Moreover, fragility fractures, orthopaedic surgery, and long-term immobilization may trigger depression [100,101]. On the other hand, depression has previously been associated with an increased risk of osteoporosis [102]. Multiple common mechanisms have been proposed, including the chronic inflammatory state, leading to apoptosis of osteoclasts, and the disruption of the endocrine system such as the decrease in oestrogens and testosterone and the increase in cortisol [103]. Another important aspect is the fact that drugs acting against osteoporosis might have depression as adverse reactions. For instance, when compared to teriparatide, alendronate was associated with a higher risk of depression [patients ≤65 years: ROR (reporting odds ratio) of 14.67 (11.55–18.63), individuals >65 years: 3.60 (2.82–4.59)] and anxiety [subjects ≤65 years: ROR of 7.10 (5.79–8.71), individuals >65 years: 2.28 (1.84–2.84)] in one study [104].
Depression risk in PHPT may also be mediated by the presence of cardiovascular involvement. In turn, depression and PHPT may exert a cumulative role on the development of cardiovascular conditions. Although not an indication for surgery, increased cardiovascular risks due to arterial stiffness, endothelial dysfunction, and high-risk hypertension are associated with PHPT [105,106]. Cardiovascular disease is also associated with an elevated risk of depression and/or anxiety [107]. On the other hand, depression and/or anxiety might contribute to the development of a cardiovascular disorder by speeding up the development of risk factors and promoting arterial stiffness [108,109,110].
4.3.3. Different PHPT Disease Forms: From Normocalcemic to Hypercalcemic Type
In recent years, the presentation of the PHPT patient changed, with disease being diagnosed in early stages, most frequently in asymptomatic stages, and even in patients with normocalcemia [111,112]. The various forms of PHPT raised the question whether depression and/or anxiety have different characteristics in asymptomatic and normocalcemic patients. Patients with PHPT are considered asymptomatic if they lack classical symptoms (e.g., osteoporosis, fragility fractures, kidney stones, and severe neurological consequences of the acute hypercalcemia) [113]. However, asymptomatic patients may present with various other minor complaints and, more often, have been shown to have different types of psychological symptoms when compared to the healthy population [114]. Moreover, the severity of the symptoms in PHPT is not always reflected by the PTH and calcium levels [115]. Regarding asymptomatic PHPT, Jovanovic et al. [32] showed that depression and anxiety had a high prevalence, and both comorbidities improved following parathyroidectomy [32]. Vadhwana et al. [39], however, did not find a reduction in anxiety/depression scores in subjects with asymptomatic PHPT, even though patients with symptomatic disease had better scores following surgery [39]. Normocalcemic PHPT has been recently recognized as a distinct disease form, characterized by elevated PTH levels and normal serum calcium in the absence of secondary causes for a PTH increase [116,117]. Older data, like those from a case report of an 80-year-old male with depression resistant to treatment, who experienced resolution of depression during a post-parathyroidectomy follow-up of three years, suggested that there might be a possible connection between normocalcemic PHPT and depression [118]. As mentioned, we did not identify distinct studies regarding normocalcemic patients and depression/anxiety. Notably, only a cohort from 2024 studied by Febrero et al. [29] included normocalcemic patients among hypercalcaemic subjects, but the study did not compare the two types of PHPT [29]. Given the limited data available, further studies are needed in order to determine the impact of the PHPT phenotype on depression and/or anxiety.
4.4. Current Limits and Further Perspectives
As limits of the current work, we mention that this was a non-systematic review since we did not intend to restrain the included studies, noting that their design and methods of depression/anxiety assessments varied. Also, we only included cohorts that specifically provided data with respect to depression and/or anxiety, which might remain undiagnosed in other studies that analysed other elements of the PHPT-related phenotype. Other related topics are still at a low level of statistical evidence, such as the impact of medical therapy against hypercalcemia on depression/anxiety versus surgery, the extended panel of psychiatric manifestations in PHPT (other than depression and anxiety), or the impact of the disease duration (PHPT or depression/anxiety) on the clinical picture. We found only limited data on cinacalcet, as mentioned. Generally, the normalization of serum calcium is crucial in hypercalcemia-induced neuro-psychiatric symptoms, as seen in the setting of PHPT and apart from the surgical approach, and, hence, cinacalcet may be used for lowering blood calcium [119]. Previous reports have shown that it may manage psychotic symptoms, too, but these remain at the case report level [120]. The question arises whether cinacalcet truly impacts depression and/or anxiety. As mentioned, a single study included patients treated with cinacalcet, and the drug was shown to reduce depression scores but did not impact anxiety scores [37]. Depression is not only a common comorbidity in patients with PHPT but it may sometimes be one of the first manifestations. For example, a recent paper reported the case of a 72-year-old male who was diagnosed with PHPT due to an ectopic mediastinal parathyroid adenoma after presenting with fatigue and depression, both of which improved and then remitted following the surgical cure [121]. Another case report suggested that newly onset depression and cognitive decline might be accompanying symptoms of PHPT, especially in the elderly. Vultaggio et al. [122] reported that an 80-year-old patient who presented with a soporous state, depression, and cognitive decline was diagnosed with a parathyroid carcinoma and had a neurological recovery after parathyroidectomy [122]. Other psychiatric symptoms may also raise the alarm for an underlying parathyroid pathology, such as PHPT (e.g., delirium and psychosis) [123,124]. While a surgical cure leads to the resolution of these symptoms in some cases [125,126], other patients with long-standing hypercalcemia are unresponsive to this treatment, in spite of the surgical cure for the parathyroid tumour [127,128]. Even though high levels of calcium are attributed to involvement in the onset of psychosis, in some cases, even mild hypercalcemia might induce psychosis [129]. The psychiatric pathology of patients with PHPT might be complex, with more manifestations overlapping [130]. These data support the idea that depression, anxiety, fatigue, and even psychosis are important symptoms of PHPT and may lead to an early diagnosis in patients otherwise asymptomatic, and further trials are necessary to pinpoint the prevalence and severity of the pathogenic connection with high PTH and/or calcium, in addition to finding more evidence for which sub-group of patients is at a higher risk for such ailments and if PHPT management should be adjusted under these circumstances.
5. Conclusions
The modern approach for patients with PHPT should be complex and go beyond classical symptoms. Recent studies revealed that mental health conditions such as depression and anxiety have a high prevalence in patients with PHPT, regardless of the traditional symptoms, and are associated with increased medication use. Moreover, the scores of depression were generally higher compared to control populations. However, the underlying mechanisms remain incompletely elucidated. No correlations between depression or anxiety and serum calcium levels were found, while PTH had a slight positive correlation with depression. Parathyroidectomy appears to be beneficial for these mental health aspects in subjects with PHPT as it improves the scores, prevalence, and severity of depression and reduces anxiety scores. Improvements in symptoms following surgery in otherwise asymptomatic patients raise the question whether depression might be taken into consideration when referring patients for surgery. Cinacalcet might reduce depression scores, although more evidence is needed. Women are at higher risk both for PHPT and depression and anxiety. The optimal method for depression and anxiety screening for PHPT patients remains to be determined, and the current scales need validation and perhaps adjustment for this specific population group.
Author Contributions
Conceptualization, A.-M.G., C.N., A.-E.R. and M.C.; methodology, A.-M.G., C.N., A.-E.R. and M.C.; software, A.-M.G., C.N., A.-E.R. and M.C.; validation, A.-M.G., C.N., A.-E.R. and M.C.; formal analysis, A.-M.G., C.N., A.-E.R. and M.C.; investigation, A.-M.G., C.N., A.-E.R. and M.C.; resources, A.-M.G., C.N., A.-E.R. and M.C.; data curation, A.-M.G., C.N., A.-E.R. and M.C.; writing—original draft preparation, A.-M.G.; writing—review and editing, M.C.; visualization, A.-M.G., C.N., A.-E.R. and M.C.; supervision, A.-M.G., C.N., A.-E.R. and M.C.; project administration, A.-M.G. and M.C.; funding acquisition, A.-M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Carol Davila” University of Medicine and Pharmacy, PhD research “Primary hyperparathyroidism: cardio-metabolic, osseous and surgical aspects”—2023.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This is part of the PhD research belonging to the PhD Doctoral School of “Carola Davila” University of Medicine and Pharmacy, entitled “Primary hyperparathyroidism: cardio-metabolic, osseous and surgical aspects”—28374/02.10.2023.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BDI | Beck Depression Inventory |
| DASS | Depression Anxiety Stress Scales |
| EuroQOL-5D-3L | European Quality of Life 5 Dimensions 3 Level Version |
| GAD | Generalized Anxiety Disorder |
| HADS | Hospital Anxiety and Depression Scales |
| HAM-D | Hamilton Depression Rating Scale |
| HR | hazard ratio |
| ICD-10 | The International Classification of Diseases-Tenth Revision Clinical Modification |
| MAO | monoamine oxidases |
| n | number of studies |
| N | number of patients |
| PHPT | primary hyperparathyroidism |
| PTH | parathyroid hormone |
| PHQ-9 | Patient Health Questionnaire-9 |
| PTHR | parathyroid hormone receptor |
| RR | relative risk |
| ROR | reporting odds ratio |
| OR | odds ratio |
| SSRI | selective serotonin reuptake inhibitors |
| SNRI | selective serotonin and norepinephrine inhibitors |
| SCL90R | Symptom Check List 90-revised |
| SE | standard error |
Appendix A

Table A1.
The analysis of the prevalence and severity with respect to the diagnosis of depression in patients confirmed with PHPT (the display starts with the most recent publication date; the studied population and sub-groups have been introduced in Table 1, as well as the method/tool that has been applied in order to confirm the depression and its severity) [27,28,29,30,31,32,33,34,38,40,41,42].
Table A1.
The analysis of the prevalence and severity with respect to the diagnosis of depression in patients confirmed with PHPT (the display starts with the most recent publication date; the studied population and sub-groups have been introduced in Table 1, as well as the method/tool that has been applied in order to confirm the depression and its severity) [27,28,29,30,31,32,33,34,38,40,41,42].
| Reference | Prevalence/Severity of Depression |
|---|---|
| [27] | N1 vs. N2—depression: 9% vs. 10.3%, p < 0.0001 odds of having PTH checked in those with depression: 0.84 (0.74, 0.96), p = 0.0081 |
| [28] | Mean (SE) depression score: preoperatively: 6.89 (0.95) |
| [29] | N1 at diagnosis: 42.9% with minimal affectation, 24.5% with mild depression, 20.4% with moderate depression, 12.2% with severe depression Depression score: N1 vs. N2 at diagnosis: 16.80 ± 9.98 vs. 6.10 ± 5.45, p < 0.001 |
| [30] | Pre-existing depression overall vs. N1 vs. N2: 10.5% vs. 11.4% vs. 9.7%, p = 0.265 Newly-onset depression overall vs. N1 vs. N2: 10.7% vs. 12.2% vs. 9.3%, p = 0.046 Pre-existing suicidal ideation overall vs. N1 vs. N2: 0.2% vs. 0.2% vs. 0.2%, p = 1 Newly-onset suicidal ideation overall vs. N1 vs. N2: 0.4% vs. 0.6% vs. 0.1%, p = 0.13 |
| [31] | N1: 49%, minimal affectation, 23% mild depression, 17% moderate depression, 11% severe depression median (IQR) depression score N1 vs. N2: 14 (15) vs. 5 (7), p < 0.001 Mean ± SD depression scores and socio-personal variables in N1: age (<40 y vs. 40–60 y vs. >60 y): 4.18 ± 7.36 vs. 18.24 ± 10.28 vs. 15.38 ± 9.57, p < 0.001 gender: p = 0.218 education (no education vs. primary education vs. secondary education vs. higher education): 19.75 ± 6.88 vs. 17.43 ± 9.93 vs. 14.92 ± 14 vs. 9.29 ± 8.18, p = 0.014 work: p = 0.359 marital status (single vs. married vs. living with a partner vs. separated vs. widowed): 7.15 ± 9.15 vs. 14.71 ± 8.92 vs. 19.2 ± 15.15 vs. 21.5 ± 15.28 vs. 18.75 ± 10.02, p = 0.034 children (yes vs. no): 16.59 ± 9.78 vs. 11.23 ± 11.39, p = 0.019 Mean ± SD depression scores and clinical variables in N1: clinical symptoms (p = 0.438); hypertension (p = 0.323); fatigue (p = 0.067); gastrointestinal symptoms (p = 0.863); psychological symptoms (22.85 ± 9.73 vs. 12.52 ± 9.84, p = 0.001); renal crisis (p = 0.713); nephrolithiasis (p = 0.215); BMD (p = 0.644); bone pain (p = 0.54) |
| [32] | Prevalence of depression (preoperatively): BDI: normal = 34.3%, mild depression = 34.3%, mild to moderate = 8.1%, moderate to severe = 21.2%, severe = 2% DASS depression: normal = 80.2%, mild = 7.9%, moderate = 3%, severe = 7.9%, extreme severe = 1% |
| [33] | N1: time-to-diagnosis >1 y vs. ≤1 y after hypercalcemia: during 1 y from hypercalcemia: Prevalence of major depressive disorder: 11.9% vs. 16.1% OR (95% CI) = 0.7 (0.62–0.8); p < 0.001 during 1–3 y: Prevalence of major depressive disorder:17.3% vs. 15.1% OR (95% CI) = 1.18 (1.05–1.34); p = 0.008 time-to-surgery >1 y vs. ≤1 y after diagnosis: during 1 y from hypercalcemia: Prevalence of major depressive disorder:13.8% vs. 20.6% OR (95% CI) = 0.62 (0.47–0.81); p < 0.001 during 1–3 y: Prevalence of major depressive disorder: 19.9% vs. 18.2% OR (95% CI) = 1.12 (0.87–1.45); p = 0.39 N2 vs. matched controls: Prevalence of major depressive disorder: 16% vs. 11.2% OR (95% CI) = 1.51 (1.43–1.6); p < 0.001 N3 vs. matched controls: Prevalence of major depressive disorder:15.4% vs. 10% OR (95% CI) = 1.63 (1.55–1.72); p < 0.001 |
| [34] | Median (IQR) depression score in N (baseline): 4 (1–6) Median (IQR) depression score in N1 (baseline): 4 (1–8) Median (IQR) depression and anxiety score in N (baseline): 8 (6–16) Median (IQR) depression and anxiety score in N1 (baseline): 8 (5–15) |
| [38] | N1 vs. N2 preoperative: 6.7 ± 6.6 vs. 4.4 ± 4.9, p < 0.01 |
| [40] | Depression score (median): 5 (0.46) |
| [41] | N1 vs. N2 HAM-D: Overall (median): 9 vs. 2, p < 0.001; (prevalence): 60.4% vs. 12%, p < 0.001 Women (median): 9 vs. 2, p < 0.001; (prevalence): 62.35% vs. 9.5%, p < 0.001 Men (median): 2.5 vs. 4, p > 0.05; (prevalence): 31.25% vs. 25%, p > 0.05 BDI-II: Overall (median): 10 vs. 5.5, p < 0.01; (prevalence): 36.36% vs. 18%, p < 0.05 Women (median): 10 vs. 5, p < 0.01; (prevalence): 38.8% vs. 16.6%, p < 0.05 Men (median): 2.5 vs. 4, p > 0.05; (prevalence): 18.75% vs. 25%, p > 0.05 HADS depression: Overall (median): 5 vs. 2, p < 0.01; (prevalence): 20.79% vs. 12%, p > 0.05 Women (median): 5 vs. 2.5, p < 0.01; (prevalence): 22.35% vs. 11.9%, p > 0.05 Men (median): 4 vs. 2, p > 0.05; (prevalence): 12.5% vs. 12.5%, p > 0.05 |
| [42] | Prevalence of depression: overall = 27.2%, females = 32.6%, males = 3%, p = 0.016 |
Abbreviations: BDI = Beck Depression Inventory; BMD = bone mineral density; CI = confidence interval; DASS = Depression Anxiety Stress Scales; IQR = interquartile range; HAM-D = Hamilton Depression Rating Scale; HADS = Hospital Anxiety and Depression Scale; N = number of patients; PTH = parathormone; SD = standard deviation; SE = standard error; vs. = versus.

Table A2.
Antidepressant use in patients with PHPT (the studied population and sub-groups have been introduced in Table 1, as well as the method/tool that has been applied in order to confirm the depression and its severity) [29,34].
Table A2.
Antidepressant use in patients with PHPT (the studied population and sub-groups have been introduced in Table 1, as well as the method/tool that has been applied in order to confirm the depression and its severity) [29,34].
| Reference | Main Findings |
|---|---|
| [29] | 20%: antidepressant use |
| [34] | N1 vs. N2 OR (95% CI) for SSRI use: 15.9% vs. 12.1%, 1.38 (1.3–1.47) SNRI use: 8.6% vs. 6.5% 1.36 (1.26–1.48) Tricyclic antidepressants use: 4.5% vs. 3.3%, 1.4 (1.26–1.57) |
Abbreviations: CI = confidence interval; N = number of patients, OR = odds ratio; SNRI = selective serotonin and norepinephrine inhibitors; SSRI = selective serotonin reuptake inhibitors.

Table A3.
Correlations between depression and endocrine parameters in PHPT (the studied population and sub-groups have been introduced in Table 1, as well as the method/tool that has been applied in order to confirm the depression and its severity) [30,31,34,38,40,41].
Table A3.
Correlations between depression and endocrine parameters in PHPT (the studied population and sub-groups have been introduced in Table 1, as well as the method/tool that has been applied in order to confirm the depression and its severity) [30,31,34,38,40,41].
| Reference | Main Findings |
|---|---|
| [30] | Hazard ratio of depression: at Ca > 11.5 mg/dL: 1.32 (0.97–1.81) Hazard ratio of suicidal ideation: at Ca > 11.5 mg/dL: 6.69 (1.2–37.35) |
| [31] | mean ± SD depression scores and clinical variables in N1: pre-surgery blood calcium level <11 mg/dL vs. ≥11 mg/dL: 15.5 ± 11.38 vs. 14.5 ± 19, p = 0.399 |
| [34] | N1 vs. N2, OR (95% CI) SSRI: Ionized calcium (mmol/L): <1.38: 18.3% vs. 12.2%, 1.61 (1.39–1.99) 1.38–1.43: 17.2% vs. 12.2%, 1.52 (1.33–1.87) 1.44–1.49: 16.1% vs. 12.5%, 1.36 (1.19–1.69) ≥1.5: 12.6% vs. 11.4%, 1.11 (1.02–1.51) p = 0.002 SNRI: Ionized calcium (mmol/L): <1.38: 11.1% vs. 6.6%, 1.81 (1.53–2.14) 1.38–1.43: 9.1% vs. 1.45%, (1.22–1.72) 1.44–1.49: 7.9% vs. 6.8%, 1.18 (0.98–1.41) ≥1.5: 7.1 vs. 6.1% (0.98–1.43) p = 0.0013 Tricyclic antidepressants: Ionized calcium (mmol/L): <1.38: 5.4% vs. 3.2%, 1.71 (1.35–2.16) 1.38–1.43: 4.7% vs. 3.4%, 1.46 (1.15–1.84) 1.44–1.49: 4.1% vs. 3.5%, 1.19 (0.93–1.52) ≥1.5: 3.7% vs. 3%, 1.29 (0.99–1.67) p = 0.1771 |
| [38] | No statistically significant correlation between calcium and PTH preoperative and postoperative levels and depression |
| [40] | Correlations: Unadjusted: PTH: r = −0.166, p = 0.327 Ca: r = 0.027, p = 0.874 Cortisol at 8:00: r = 0.123, p = 0.467 Osteocalcin: r = −0.345, p = 0.037 Adjusted for multiple variables: Cortisol at 8:00: r = 0.084, p = 0.636 Osteocalcin: r = −0.337, p = 0.086 |
| [41] | No statistically significant correlation between severity of depression and Ca, PTH |
Abbreviations: Abbreviations: Ca = serum calcium; CI = confidence interval; N = number of patients; OR = odds ratio; PHPT = primary hyperparathyroidism; PTH = parathyroid hormone; SNRI = serotonin and norepinephrine reuptake inhibitors; SSRI = selective serotonin reuptake inhibitors; SD = standard deviation; vs. = versus.

Table A4.
Main findings with respect to the impact of parathyroidectomy on depression (the display starts with the most recent publication date; the studied cohorts and sub-groups have been introduced in Table 1) [28,29,31,34,35,36,38,39].
Table A4.
Main findings with respect to the impact of parathyroidectomy on depression (the display starts with the most recent publication date; the studied cohorts and sub-groups have been introduced in Table 1) [28,29,31,34,35,36,38,39].
| Reference | Main Findings Regard Depression Outcome Following Parathyroid Surgery |
|---|---|
| [28] | Mean (SE) depression score: preoperatively: 6.89 (0.95) 1 week postoperatively: 2.46 (0.94) 3 mo postoperatively: 2.72 (0.96) p < 0.001 p for trend = 0.13 |
| [29] | N1 at diagnosis: 24.5% with mild depression, 20.4% with moderate depression, 12.2% with severe depression N1 at 3 mo after surgery: 22.4% with mild depression, 16.3% with moderate depression, 8.2% with severe depression N1 at 1 y after surgery: 12.5% with mild depression, 8.3% with moderate depression, 6.3% with severe depression Depression score: N1 vs. N2: at diagnosis: 16.80 ± 9.98 vs. 6.10 ± 5.45, p < 0.001 3 months after surgery: 13.08 ± 10.76 vs. 6.10 ± 5.45, p < 0.001 1 y after surgery: 10.50 ± 10.79 vs. 6.10 ± 5.45, p < 0.001 N1 at diagnosis vs. 3 mo after surgery: 16.80 ± 9.98 vs. 13.08 ± 10.76, p = 0.001 N1 at diagnosis vs. 1 y after surgery: 16.65 ± 10.03 vs. 10.50 ± 10.79, p < 0.001 |
| [31] | 1 mo postoperatively: BDI: normal = 69.1%, mild depression = 18.8%, mild to moderate = 9.3%, moderate to severe = 2.1%, severe = 0% DASS depression: normal = 89.7%, mild = 5.2%, moderate = 4.1%, severe = 1%, extreme severe = 0% 6 mo postoperatively: BDI: normal = 87.2%, mild depression = 11.7%, mild to moderate = 1.1%, moderate to severe = 0%, severe = 0% DASS depression: normal = 95.7%, mild = 3.2%, moderate = 1.1%, severe = 0%, extreme severe = 0% Changes in depression scores: BDI: preoperatively: 13.5 1 mo postoperatively: 7.8 6 mo postoperatively: 5.4 difference: −60% preoperatively vs. 1 mo postoperatively p = 0.001 preoperatively vs. 6 mo postoperatively p = 0.001 1 mo postoperatively vs. 6 mo postoperatively p = 0.001 DASS depression: preoperatively: 6.3 1 mo postoperatively: 3.7 6 mo postoperatively: 2.7 difference: −56.2% preoperatively vs. 1 mo postoperatively p = 0.001 preoperatively vs. 6 mo postoperatively p = 0.001 1 mo postoperatively vs. 6 mo postoperatively p = 0.002 SCL90R: preoperatively: 17 1 mo postoperatively: 8.9 6 mo postoperatively: 6.3 difference: −62.7% preoperatively vs. 1 mo postoperatively p = 0.001 preoperatively vs. 6 mo postoperatively p = 0.001 1 mo postoperatively vs. 6 mo postoperatively p = 0.001 |
| [34] | RR (CI) of SSRI use following PTX: Overall: 0–6 mo: 1.25 (1.16–1.34) 7–12 mo: 1.28 (1.2–1.37) 13–18 mo: 1.27 (1.19–1.36) 19–24 mo: 1.25 (1.17–1.34) 25–30 mo: 1.28 (1.19–1.37) 31–36 mo:1.27 (1.18–1.36) p trend = 0.78 In prevalent users: 0–6 mo: 0.92 (0.87–0.97) 7–12 mo: 0.94 (0.89–0.99) 13–18 mo: 0.94 (0.89–0.99) 19–24 mo: 0.9 (0.85–0.96) 25–30 mo: 0.91 (0.86–0.97) 31–36 mo: 0.88 (0.83–0.94) p trend = 0.11 Incident users: 0–6 mo: 1.8 (1.48–2.2) 7–12 mo: 1.75 (1.49–2.06) 13–18 mo: 1.62 (1.4–1.88) 19–24 mo: 1.62 1.42–1.85) 25–30 mo: 1.68 (1.47–1.91) 31–36 mo: 1.69 (1.5–1.91) p trend = 0.69 |
| [35] | HAM-D score N1 vs. N3 baseline: 6 vs. 2, p < 0.05 6 mo postoperatively: 2 vs. 1, p = 0.006 1 y postoperatively: 2 vs. 1, p = 0.0007 |
| [36] | Depression scores before surgery vs. 1 mo after surgery vs. 6 mo after surgery: 5.9 ± 4 vs. 5.5 ± 5.3 vs. 5.2 ± 4.7, p = 0.697 |
| [38] | Depression scores: N1 preoperative vs. postoperative: 6.7 ± 6.6 vs. 3.1 ± 3.9, mean difference = 3.7 (2.9–4.4), p < 0.01 N1 vs. N2 preoperative: 6.7 ± 6.6 vs. 4.4 ± 4.9, p < 0.01 N1 vs. N2 following PTX: 3.1 ± 3.9 vs. 3.3 ± 4.1, p = 0.52 Depression severity in N1 preoperative vs. following PTX: moderate to severe: 27.5% vs. 8.2%, p < 0.01 no symptoms: 47.9% vs. 72.5%, p < 0.01 |
| [39] | N: 41% decrease in anxiety/depression in N, p = 0.07 Anxiety/depression levels before surgery vs. 6 weeks after surgery: N1: no anxiety/depression: 75% vs. 75% moderate anxiety/depression: 25% vs. 25% extreme anxiety/depression: 0% vs. 0% p = 1.00 N2: no anxiety/depression: 43% vs. 71% moderate anxiety/depression: 53% vs. 25% extreme anxiety/depression: 4% vs. 4% p < 0.01 |
Abbreviations: BDI = Beck Depression Inventory; CI = confidence interval; DASS = Depression Anxiety Stress Scales; HAM-D = Hamilton Depression Rating Scale; mo = month; N = number of patients; PTX = parathyroidectomy; RR = relative risk; SCL90R = Symptom Check List 90-revised; SE = standard error; SSRI = selective serotonin reuptake inhibitors; y = years; vs. = versus.

Table A5.
The impact of cinacalcet use before parathyroidectomy on depression in patients confirmed with PHPT [37].
Table A5.
The impact of cinacalcet use before parathyroidectomy on depression in patients confirmed with PHPT [37].
| Reference | Study Design/Studied Population | Main Findings |
|---|---|---|
| [37] | Prospective observational N = 35, with age ≥ 50 y (out of which N1 = 19 with age ≥ 70 y) with PHPT and mild cognitive impairment who underwent cinacalcet treatment 4 weeks before PTX F:M = 31:4 (88.6% females) Median (IQR) age = 71 (62–79) y N1: F:M = 17:2 (89.5% females) Median (IQR) age = 77 (72–82) y | Median (IQR) depression score in N: baseline: 4 (1–6) change between baseline and 4 weeks of cinacalcet: –1 (0 to –2), p = 0.019 change between baseline and 6 mo following PTX: –3 (0 to –6), p = 0.040 Median (IQR) depression score in N1: baseline: 4 (1–8) change between baseline and 4 weeks of cinacalcet: –1 (0 to –3), p = 0.053 change between baseline and 6 mo following PTX: –1 (0 to –3), p = 0.189 Median (IQR) depression and anxiety score in N: baseline: 8 (6–16) change between baseline and 4 weeks of cinacalcet: –2 (0 to –3), p = 0.020 change between baseline and 6 mo following PTX: –3 (0 to –6), p = 0.005 Median (IQR) depression and anxiety score in N1: baseline: 8 (5–15) change between baseline and 4 weeks of cinacalcet: –2 (0 to –3), p = 0.095 change between baseline and 6 mo following PTX: –3 (0 to –6), p = 0.073 |
Abbreviations: F = female; IQR = interquartile range; M = male; mo = month; N = number of patients; PHPT = primary hyperparathyroidism; PTX = parathyroidectomy; y = years.

Table A6.
Conservative management versus parathyroidectomy in relationship with the assessment of depression [30].
Table A6.
Conservative management versus parathyroidectomy in relationship with the assessment of depression [30].
| Reference | Study Design/Studied Population | Main Findings |
|---|---|---|
| [30] | Retrospective cohort study N = 3728 with PHPT After propensity score match: N1 = 959 non-operative patients F:M = 766:193 (79.9% females) Mean age = 62 ± 14 y N2 = 959 operative patients F:M = 763:196 (79.6% females) Mean age = 62 ± 13 y | Pre-existing depression overall vs. N1 vs. N2: 10.5% vs. 11.4% vs. 9.7%, p = 0.265 Newly-onset depression overall vs. N1 vs. N2: 10.7% vs. 12.2% vs. 9.3%, p = 0.046 Pre-existing suicidal ideation overall vs. N1 vs. N2: 0.2% vs. 0.2% vs. 0.2%, p = 1 New-onset suicidal ideation overall vs. N1 vs. N2: 0.4% vs. 0.6% vs. 0.1%, p = 0.13 Hazard ratio of depression: in N2 compared to N1: 1.02 (0.77–1.36) in normohormonal PHPT compared to classic PHPT: 0.82 (0.61–1.11) Hazard ratio of suicidal ideation: in N2 compared to N1: 0.31 (0.04–2.71) in normohormonal PHPT compared to classic PHPT: 3.42 (0.47–24.8) likelihood of newly-onset depression in patients without pre-existing neuropsychiatric disorders: within 5 y: N1: 6.2%, N2: 6.8% within 10 y: N1:23.7%, N2:22.9% likelihood of newly-onset suicidal ideation in patients without pre-existing neuropsychiatric disorders: within 5 y: N1:0.6%, N2:0.2% within 10 y: N1:1.6%, N2:0.2% |
Abbreviations: F = female; M = male; N = number of patients; PHPT = primary hyperparathyroidism; y = years.

Table A7.
The anxiety prevalence and severity in patients with PHPT (the display starts with the most recent publication date) [28,30,32,33,34,38,40,41].
Table A7.
The anxiety prevalence and severity in patients with PHPT (the display starts with the most recent publication date) [28,30,32,33,34,38,40,41].
| Reference | Study Design/Studied Population | Prevalence/Severity of Anxiety | Anxiety Evaluation Method |
|---|---|---|---|
| [28] | Longitudinal prospective study N = 36 with PHPT who underwent PTX F:M = 33:3 (92% females) Mean age = 59 ± 12.8 y | Mean (SE) anxiety score: preoperatively: 5.17 (1.01) | Depression Anxiety Stress Scales |
| [30] | Retrospective cohort study N = 3728 with PHPT After propensity score match: N1 = 959 non-operative patients F:M = 766:193 (79.9% females) Mean age = 62 ± 14 y N2 = 959 operative patients F:M = 763:196 (79.6% females) Mean age = 62 ± 13 y | Pre-existing anxiety overall vs. N1 vs. N2: 10.4% vs. 10.8% vs. 10%, p = 0.601 New onset anxiety overall vs. N1 vs. N2: 12.9% vs. 14.3% vs. 11.6%, p = 0.089 | ICD-10 codes |
| [32] | Prospective study N = 101 with asymptomatic PHPT who underwent PTX F:M = 88:13 (87.1% females) Average age = 60.7 (range 27–80) y | Prevalence of anxiety: preoperatively: DASS anxiety: normal = 77.2%, mild = 9.9%, moderate = 7.9%, severe = 4%, extreme severe = 1% 1 mo postoperatively: DASS anxiety: normal = 97.9%, mild = 0%, moderate = 2.1%, severe = 0%, extreme severe = 0% 6 mo postoperatively: DASS anxiety: normal = 97.8%, mild = 1.1%, moderate = 1.1%, severe = 0%, extreme severe = 0% | Depression Anxiety Stress Scales |
| [33] | Retrospective cohort study N = 135,034 with hypercalcemia F:M = 96,554:38,466 (72% females) Mean age = 63 ± 10 y N1 = 13,136 with PHPT diagnosis N2 = 20,176 with high risk of PHPT diagnosis with PTH data N3 = 24,905 with high risk of PHPT diagnosis without PTH data | N1: time-to-diagnosis >1 y vs. ≤1 y after hypercalcemia: during 1 y from hypercalcemia: Prevalence of anxiety: 10.4% vs. 13.4% OR (95% CI) = 0.75 (0.65–0.86) p < 0.001 during 1–3 y: Prevalence of anxiety: 15.7% vs. 13.4% OR (95% CI) = 1.2 (1.05–1.36) p = 0.007 during 1 y from hypercalcemia: Prevalence of anxiety: 10.7% vs. 15.1% OR (95% CI) = 0.67 (0.5–0.91) p < 0.01 during 1–3 y: Prevalence of anxiety: 17.4% vs. 15.1% OR (95% CI) = 1.18 (0.9–1.56) p = 0.23 N2 vs. matched controls: Prevalence of anxiety: 15.4% vs. 10.8% OR (95% CI) = 1.5 (1.41–1.59) p < 0.001 N3 vs. matched controls: Prevalence of anxiety:15.6% vs. 11.1% OR (95% CI) = 1.48 (1.40–1.56) p < 0.001 | Patient records |
| [34] | Prospective observational N = 35, with age ≥50 y (out of which N1 = 19 with age ≥70 y) with PHPT and mild cognitive impairment who underwent cinacalcet treatment 4 weeks before PTX F:M = 31:4 (88.6% females) Median (IQR) age = 71 (62–79) y N1: F:M = 17:2 (89.5% females) Median (IQR) age = 77 (72–82) y | Median (IQR) anxiety score in N: baseline: 6 (4–10) Median (IQR) anxiety score in N1: baseline: 4 (2–10) | Hospital Depression and Anxiety Scale |
| [38] | Prospective multicentre observational study N = 405 F:M= (76.6% females) Mean age = 59 ± 13.9 y N1 = 244 who underwent PTX F:M = 192:52 (78.7% females) Mean age = 63 ± 12.2 y N2 = 161 who underwent thyroidectomy F:M = 120:41 (74.5% females) Mean age = 52.4 ± 14.2 y | N1 vs. N2 preoperative: 4.5 ± 5.6 vs. 3.2 ± 4.1, p < 0.01 N1 vs. N2 following PTX: 2.0 ± 3.6 vs. 2.1 ± 3.9, p = 0.75 | Generalized Anxiety Disorder-7 |
| [40] | Retrospective study N = 192 with PHPT F:M = 147:45 (76.6% females) Mean age = 52.7 ± 13.8 y | Anxiety proneness (trait anxiety): Mean score = 37.39 ± 10.34 Current anxiety (state anxiety): Mean score = 35.43 ± 11.56 | State-Trait Anxiety Inventory |
| [41] | Observational retrospective cohort study N1 = 101 with PHPT who underwent PTX, out of whom: F:M = 85:16 (84.15% females) Median (range) age: 60 (20–86) N2 = 50 controls (with non-toxic goitre) F:M = 42:8 (84% females) Median (range) age: 58 (25–75) | HADS anxiety: Overall: Median: 6 vs. 6, p > 0.05 Prevalence: 38.6% vs. 34%, p > 0.05 Women: Median: 7 vs. 6, p > 0.05 Prevalence: 42.35% vs. 35.71%, p > 0.05 Men: Median: 5 vs. 6, p > 0.05 Prevalence: 18.75% vs. 25%, p > 0.05 | Hospital Anxiety and Depression Scale |
Abbreviations: CI = confidence interval; DASS = Depression Anxiety Stress Scales; F = female; IQR = interquartile range; ICD = International Classification of Diseases; M = male; mo = month; N = number of patients; OR = odds ratio; SE = standard error; PHPT = primary hyperparathyroidism; PTX = parathyroidectomy; y = years; vs. = versus.

Table A8.
Correlations between anxiety and endocrine parameters in patients with PHPT (the display starts with the most recent publication date; the cohorts are characterized in Table 1) [30,34,38,40,41].
Table A8.
Correlations between anxiety and endocrine parameters in patients with PHPT (the display starts with the most recent publication date; the cohorts are characterized in Table 1) [30,34,38,40,41].
| Main Findings | |
|---|---|
| [30] | Hazard ratio of anxiety: at Ca > 11.5 mg/dL: 0.98 (0.72–1.33) |
| [34] | Anxiolytic, benzodiazepine use: N1 vs. N2, OR (95% CI) Ionized calcium (mmol/L): <1.38: 15.5% vs. 9.9%, 1.72 (1.48–1.99) 1.38–1.43: 12.6% vs. 10%, 1.34 (1.15–1.55) 1.44–1.49: 13.1% vs. 9.8, 1.42 (1.22–1.64) ≥1.5: 11.5% vs. 10%, 1.17 (1.00–1.36) p = 0.004 |
| [38] | No statistically significant correlation between calcium and PTH preoperative and postoperative levels and anxiety |
| [40] | Anxiety proneness (trait anxiety): Correlations: Unadjusted PTH: r = −0.168, p = 0.328 Ca: r = 0.049, p = 0.782 Cortisol at 8:00: r = 0.172, p = 0.316 Osteocalcin: r = −0.250, p = 0.141 Adjusted for multiple variables: Cortisol at 8:00: r = 0.127, p = 0.538 Osteocalcin: r = −0.363, p = 0.069 Current anxiety (state anxiety): Correlations: Unadjusted PTH: r = −0.148, p = 0.383 Ca: r = −0.060, p = 0.726 Cortisol at 8:00: r = 0.092, p = 0.587 Osteocalcin: r = −0.323, p = 0.051 Adjusted for multiple variables: Cortisol at 8:00: r = 0.044, p = 0.827 Osteocalcin: r = −0.426, p = 0.027 |
| [41] | HADS anxiety and iCa: overall: r = −0.1863, p < 0.05, in women: r = −0.2404, p < 0.05 HADS anxiety and PTH: in women: r = 0.1797, p < 0.05 |
Abbreviations: Ca = serum calcium; CI = confidence interval; iCa = ionized serum calcium; HADS = Hospital Anxiety and Depression Scales; N = number of patients; OR = odds ratio; PTH = parathyroid hormone; r = correlation coefficient.

Table A9.
Studies in PHPT with the following outcomes: the impact of parathyroidectomy on anxiety [28,32,34,35,38]; the preoperatory use of cinacalcet and anxiety impact [37]; comparative analysis between conservative and surgical approach and anxiety dynamics [30] (the display starts with the most recent publication date; the detailed studied sub-groups are provided in Table 1).
Table A9.
Studies in PHPT with the following outcomes: the impact of parathyroidectomy on anxiety [28,32,34,35,38]; the preoperatory use of cinacalcet and anxiety impact [37]; comparative analysis between conservative and surgical approach and anxiety dynamics [30] (the display starts with the most recent publication date; the detailed studied sub-groups are provided in Table 1).
| Reference | Main Findings |
|---|---|
| [28] | Mean (SE) anxiety score: preoperatively: 5.17 (1.01) 1 week postoperatively: 3 (1.01) 3 mo postoperatively: 2.46 (1.03) p = 0.01 p for trend = 0.20 |
| [30] | Preexisting anxiety overall vs. N1 vs. N2: 10.4% vs. 10.8% vs. 10%, p = 0.601 Newly-onset anxiety overall vs. N1 vs. N2: 12.9% vs. 14.3% vs. 11.6%, p = 0.089 Hazard ratio of anxiety: in N2 compared to N1: 1.07 (0.83–1.37) in normohormonal PHPT compared to classic PHPT: 1.01 (0.77–1.32) likelihood of newly-onset anxiety in patients without pre-existing neuropsychiatric disorders: within 5 y: N1:6.7%, N2:9.1% within 10 y: N1:27%, N2:28.2% |
| [32] | Changes in anxiety scores: DASS anxiety: preoperatively: 4.9 1 mo postoperatively: 2.3 6 mo postoperatively: 1.8 difference: −62.4% preoperatively vs. 1 mo postoperatively p = 0.001 preoperatively vs. 6 mo postoperatively p = 0.001 1 mo postoperatively vs. 6 mo postoperatively p = 0.033 SCL90R: preoperatively: 7.6 1 mo postoperatively: 3.8 6 mo postoperatively: 2.9 difference: −62.4% preoperatively vs. 1 mo postoperatively p = 0.001 preoperatively vs. 6 mo postoperatively p = 0.001 1 mo postoperatively vs. 6 mo postoperatively p = 0.001 |
| [34] | RR (CI) of anxiolytic use following PTX: Overall: 0–6 mo: 1.31 (1.2–1.43) 7–12 mo: 1.28 (1.17–1.39) 13–18 mo: 1.17 (1.07–1.28) 19–24 mo: 1.18 (1.08–1.28) 25–30 mo: 1.21 (1.11–1.32) 31–36 mo: 1.14 (1.04–1.24) p trend = 0.003 In prevalent users: 0–6 mo: 0.97 (0.9–1.05) 7–12 mo: 0.99 (0.91–1.07) 13–18 mo: 0.95 (0.88–1.03) 19–24 mo: 0.95 (0.88–1.03) 25–30 mo: 0.95 (0.87–1.03) 31–36 mo: 0.93 (0.85–1.01) p trend = 0.23 Incident users: 0–6 mo: 1.72 (1.41–2.09) 7–12 mo: 1.37 (1.13–1.65) 13–18 mo: 1.05 (0.86–1.27) 19–24 mo: 1.1 (0.92–1.32) 25–30 mo: 1.25 (1.06–1.47) 31–36 mo: 1.07 (0.9–1.27) p trend = 0.004 |
| [35] | Anxiety prevalence N1 vs. N3: baseline: 72% vs. 93%, p = 0.7321 6 mo postoperatively: 76.7% vs. 97.6%, p = 0.3823 1 y postoperatively: 83.72% vs. 97.6%, p = 0.4329 |
| [38] | Anxiety scores: N1 preoperative vs. postoperative: 4.5 ± 5.6 vs. 2.0 ± 3.6, mean difference = 2.5 (1.9–3.1), p < 0.01 N1 vs. N2 preoperative: 4.5 ± 5.6 vs. 3.2 ± 4.1, p < 0.01 N1 vs. N2 following PTX: 2.0 ± 3.6 vs. 2.1 ± 3.9, p = 0.75 Anxiety severity in N1 preoperative vs. following PTX: moderate to severe: 18% vs. 5.3%, p < 0.01 no symptoms: 63.1% vs. 83.2%, p < 0.01 |
| [37] | Median (IQR) anxiety score in N: baseline: 6 (4–10) change between baseline and 4 weeks of cinacalcet: –1 (0 to –2), p = 0.140 change between baseline and 6 mo following PTX: –2 (0 to –3), p = 0.040 Median (IQR) anxiety score in N1: baseline: 4 (2–10) change between baseline and 4 weeks of cinacalcet: –1 (0 to –3), p = 0.505 change between baseline and 6 mo following PTX: –1 (0 to –3), p = 0.189 |
Abbreviations: DASS = Depression Anxiety Stress Scales, IQR = interquartile range, mo = month, N = number of patients, PHPT = primary hyperparathyroidism, PTX = parathyroidectomy, RR = relative risk; SCL90R= Symptom Check List 90-revised; SE = standard error, y = years; vs. = versus.
References
- Silverberg, S.J.; Clarke, B.L.; Peacock, M.; Bandeira, F.; Boutroy, S.; Cusano, N.E.; Dempster, D.; Lewiecki, E.M.; Liu, J.M.; Minisola, S.; et al. Current issues in the presentation of asymptomatic primary hyperparathyroidism: Proceedings of the Fourth International Workshop. J. Clin. Endocrinol. Metab. 2014, 99, 3580–3594. [Google Scholar] [CrossRef]
- Walker, M.D.; Rubin, M.; Silverberg, S.J. Nontraditional manifestations of primary hyperparathyroidism. J. Clin. Densitom. 2013, 16, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Borer, M.S.; Bhanot, V.K. Hyperparathyroidism: Neuropsychiatric manifestations. Psychosomatics 1985, 26, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Dandurand, K.; Ali, D.S.; Khan, A.A. Primary Hyperparathyroidism: A Narrative Review of Diagnosis and Medical Management. J. Clin. Med. 2021, 10, 1604. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Bajgain, A.; Ali, A.; Dutta, C.; Pasha, K.; Paul, S.; Abbas, M.S.; Nassar, S.T.; Tasha, T.; Khan, S. Association of Major Depressive Disorder in Hyperparathyroidism: A Systematic Review. Cureus 2023, 15, e40150. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, R.P.; Kearns, A.E.; Vickers, K.S.; Grant, C.; Ryu, E.; Wermers, R.A. Depression in primary hyperparathyroidism: Prevalence and benefit of surgery. J. Clin. Endocrinol. Metab. 2011, 96, E1737–E1745. [Google Scholar] [CrossRef]
- Weber, T.; Eberle, J.; Messelhäuser, U.; Schiffmann, L.; Nies, C.; Schabram, J.; Zielke, A.; Holzer, K.; Rottler, E.; Henne-Bruns, D.; et al. Parathyroidectomy, elevated depression scores, and suicidal ideation in patients with primary hyperparathyroidism: Results of a prospective multicenter study. JAMA Surg. 2013, 148, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, G.J.; Buła, G.; Żądło, D.; Gawrychowska, A.; Gawrychowski, J. Primary hyperparathyroidism. Endokrynol. Pol. 2020, 71, 260–270. [Google Scholar] [CrossRef]
- Alarcón, R.D.; Franceschini, J.A. Hyperparathyroidism and paranoid psychosis. Case report and review of the literature. Br. J. Psychiatry 1984, 145, 477–486. [Google Scholar] [CrossRef] [PubMed]
- DeMartini, J.; Patel, G.; Fancher, T.L. Generalized Anxiety Disorder. Ann. Intern. Med. 2019, 170, ITC49–ITC64. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, C.; Cianferotti, L. Quality of Life in Primary Hyperparathyroidism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.; Traunfellner, M.; Gleitsmann, M.; Zalesak, M.; Helmenstein, C. The cost-of-illness and burden-of-disease of treatment-resistant depression in Austria. J. Med. Econ. 2023, 26, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Richards, D.A.; Ekers, D.; McMillan, D.; Taylor, R.S.; Byford, S.; Warren, F.C.; Barrett, B.; Farrand, P.A.; Gilbody, S.; Kuyken, W.; et al. Cost and Outcome of Behavioural Activation versus Cognitive Behavioural Therapy for Depression (COBRA): A randomised, controlled, non-inferiority trial. Lancet 2016, 388, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, N.; Carsote MCocolos, A.; Petrova, E.; Olaru, M.; Dumitrache, C.; Ghemigian, A. The Link Between Bone Osteocalcin and Energy Metabolism in a Group of Postmenopausal Women. Curr. Health Sci. J. 2019, 45, 47–51. [Google Scholar] [CrossRef]
- Touloumis, C. The burden and the challenge of treatment-resistant depression. Psychiatriki 2021, 32 (Suppl. S1), 11–14. [Google Scholar] [CrossRef] [PubMed]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union 2016, 51, 476–480. [Google Scholar]
- Nica, S.; Sionel, R.; Maciuca, R.; Csutak, O.; Ciobica, M.L.; Nica, M.I.; Chelu, I.; Radu, I.; Toma, M. Gender-Dependent Associations Between Digit Ratio and Genetic Polymorphisms, BMI, and Reproductive Factors. Rom. J. Mil. Med. 2025, 128, 78–86. [Google Scholar] [CrossRef]
- Vasiliu, O. Impact of SGLT2 inhibitors on metabolic status in patients with psychiatric disorders undergoing treatment with second-generation antipsychotics (Review). Exp. Ther. Med. 2023, 25, 125. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Barry, M.J.; Nicholson, W.K.; Silverstein, M.; Chelmow, D.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; Jaén, C.R.; et al. Screening for Depression and Suicide Risk in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2023, 329, 2057–2067. [Google Scholar] [CrossRef]
- Siu, A.L.; US Preventive Services Task Force (USPSTF); Bibbins-Domingo, K.; Grossman, D.C.; Baumann, L.C.; Davidson, K.W.; Ebell, M.; García, F.A.; Gillman, M.; Herzstein, J.; et al. Screening for Depression in Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2016, 315, 380–387. [Google Scholar] [CrossRef] [PubMed]
- El-Den, S.; Chen, T.F.; Gan, Y.L.; Wong, E.; O’Reilly, C.L. The psychometric properties of depression screening tools in primary healthcare settings: A systematic review. J. Affect. Disord. 2018, 225, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Pasquarella, C.; Odone, A.; Colucci, M.E.; Costanza, A.; Serafini, G.; Aguglia, A.; Belvederi Murri, M.; Brakoulias, V.; Amore, M.; et al. Screening for depression in primary care with Patient Health Questionnaire-9 (PHQ-9): A systematic review. J. Affect. Disord. 2021, 279, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Caraway, J.; Ryan, M.; Yang, A.; Watson, N.; Allard, R.; Orestes, M. PHQ-9 and GAD-7 Score Response After Parathyroidectomy for Primary Hyperparathyroidism: A Systematic Review and Meta-analysis. Otolaryngol. Neck Surg. 2024, 171, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Wang, T.S.; Ruan, D.T.; Lee, J.A.; Asa, S.L.; Duh, Q.Y.; Doherty, G.M.; Herrera, M.F.; Pasieka, J.L.; Perrier, N.D.; et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surg. 2016, 151, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J.; Fuleihan, G.E.; Marcocci, C.; Minisola, S.; Perrier, N.; Sitges-Serra, A.; Thakker, R.V.; Guyatt, G.; et al. Evaluation and Management of Primary Hyperparathyroidism: Summary Statement and Guidelines from the Fifth International Workshop. J. Bone Miner. Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Bunch, P.M.; Rigdon, J.; Lenchik, L.; Gorris, M.A.; Randle, R.W. Testing for Primary Hyperparathyroidism in 17,491 Patients With Hypercalcemia. J. Surg. Res. 2024, 296, 456–464. [Google Scholar] [CrossRef]
- Chan, K.; Tseng, C.C.; Milarachi, E.; Goldrich, D.Y.; King, T.S.; Fernandez-Mendoza, J.; Saadi, R.A.; Saunders, B.; Boltz, M.; Goldenberg, D. Actigraphy measures show sleep improvement after parathyroidectomy for primary hyperparathyroidism. Am. J. Otolaryngol. 2024, 45, 104297. [Google Scholar] [CrossRef]
- Febrero, B.; Ruiz-Manzanera, J.J.; Ros-Madrid, I.; Vergara, A.; Rodríguez, J.M. Improvement of mood and sleep quality in patients with primary hyperparathyroidism after parathyroidectomy: A prospective case-control study. Surgery 2024, 175, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Balachandra, S.; Wu, C.; Wang, R.; Zmijewski, P.; Gillis, A.; Fazendin, J.; Lindeman, B.; Chen, H. Risk of neuropsychiatric disorders in primary hyperparathyroidism: Parathyroidectomy versus nonoperative management. World J. Surg. 2024, 49, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Febrero, B.; Ruiz-Manzanera, J.J.; Ros-Madrid, I.; Teruel, E.; Rodríguez, J.M. Quality of life, mood and sleep quality in patients with primary hyperparathyroidism. Impact of socio-personal and clinical profile. Ann. d’Endocrinol. 2023, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Zivaljevic, V.; Sipetic Grujicic, S.; Tausanovic, K.; Slijepcevic, N.; Rovcanin, B.; Jovanovic, K.; Odalovic, B.; Buzejic, M.; Bukumiric, Z.; et al. Effects of successful parathyroidectomy on neuropsychological and cognitive status in patients with asymptomatic primary hyperparathyroidism. Endocrine 2023, 81, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, F.J.; Beauchamp-Perez, F.; Manni, A.; Chung, T.; Goldenberg, D.; Goyal, N. Analysis of Time to Diagnosis and Outcomes Among Adults With Primary Hyperparathyroidism. JAMA Netw. Open 2022, 5, e2248332. [Google Scholar] [CrossRef]
- Koman, A.; Bränström, R.; Pernow, Y.; Bränström, R.; Nilsson, I.L.; Granath, F. Neuropsychiatric Comorbidity in Primary Hyperparathyroidism Before and After Parathyroidectomy: A Population Study. World J. Surg. 2022, 46, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Scerrino, G.; Melfa, G.; Lo Brutto, D.; Mazzola, S.; Corigliano, A.; Vitale, I.; Tutino, R.; Rotolo, G.; Orlando, G.; Cocorullo, G. Chronic asthenia in patients who have undergone endocrine neck surgery. Endocrine 2022, 75, 159–168. [Google Scholar] [CrossRef]
- Szalat, A.; Tamir, N.; Mazeh, H.; Newman, J.P. Successful parathyroidectomy improves cognition in patients with primary hyperparathyroidism: A prospective study in a tertiary medical center and comprehensive review of the literature. Front. Endocrinol. 2022, 13, 1095189. [Google Scholar] [CrossRef] [PubMed]
- Koman, A.; Bränström, R.; Pernow, Y.; Bränström, R.; Nilsson, I.L. Prediction of cognitive response to surgery in elderly patients with primary hyperparathyroidism. BJS Open 2021, 5, zraa029. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Peine, B.S.; Mlaver, E.; Patel, S.G.; Weber, C.J.; Saunders, N.D.; Pofahl, W.E.; Sharma, J. Neuropsychologic changes in primary hyperparathyroidism after parathyroidectomy from a dual-institution prospective study. Surgery 2021, 169, 114–119. [Google Scholar] [CrossRef]
- Vadhwana, B.; Currow, C.; Bowers, D.; Groot-Wassink, T. Impact on Quality of Life After Parathyroidectomy for Asymptomatic Primary Hyperparathyroidism. J. Surg. Res. 2021, 261, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; He, Y.; Zhu, M.T.; Tao, B.; Zhao, H.Y.; Sun, L.H.; Liu, J.M. The Associations of Serum Osteocalcin and Cortisol Levels With the Psychological Performance in Primary Hyperparathyroidism Patients. Front. Endocrinol. 2021, 12, 692722. [Google Scholar] [CrossRef]
- Kunert, Ł.; Gawrychowski, J.; Sobiś, J.; Buła, G.; Pudlo, R. Depressive and anxiety disorders in patients with primary hyperparathyroidism. Psychiatr. Pol. 2020, 54, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Weber, T.; Hillenbrand, A.; Peth, S.; Hummel, R. Symptoms of Primary Hyperparathyroidism in Men and Women: The Same but Different? Visc. Med. 2020, 36, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.R.; Watt, T.; Cramon, P.; Døssing, H.; Hegedüs, L.; Bonnema, S.J.; Godballe, C. Quality of life after thyroidectomy in patients with nontoxic nodular goiter: A prospective cohort study. Head Neck 2017, 39, 2232–2240. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Smarr, K.L.; Keefer, A.L. Measures of depression and depressive symptoms: Beck Depression Inventory-II (BDI-II), Center for Epidemiologic Studies Depression Scale (CES-D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire-9 (PHQ-9). Arthritis Rheum. 2011, 63 (Suppl. S11), S454–S466. [Google Scholar] [CrossRef]
- Vasiliu, O. Therapeutic management of atypical antipsychotic-related metabolic dysfunctions using GLP-1 receptor agonists: A systematic review. Exp. Ther. Med. 2023, 26, 355. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Gorenstein, C. Psychometric properties of the Beck Depression Inventory-II: A comprehensive review. Braz. J. Psychiatry 2013, 35, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Bucurica, S.; Lupanciuc, M.; Ionita-Radu, F.; Stefan, I.; Munteanu, A.E.; Anghel, D.; Jinga, M.; Gaman, E.L. Estrobolome and Hepatocellular Adenomas-Connecting the Dots of the Gut Microbial β-Glucuronidase Pathway as a Metabolic Link. Int. J. Mol. Sci. 2023, 24, 16034. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.; Munteanu, A.; Mititelu, R.; Onciul, S.; Deleanu, D.; Iliescu, V.A.; Popescu, B.A.; Jurcut, R. Severe Aortic Stenosis and ATTRwt Amyloidosis—Beware in the Aging: A Case Report and Review of the Literature. Clin. Interv. Aging 2020, 15, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- García-Batista, Z.E.; Guerra-Peña, K.; Cano-Vindel, A.; Herrera-Martínez, S.X.; Medrano, L.A. Validity and reliability of the Beck Depression Inventory (BDI-II) in general and hospital population of Dominican Republic. PLoS ONE 2018, 13, e0199750. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, S.; Enciu, C.; Raduta, I.; Stoicescu, D.; Anghel, A.; Anghel, D.; Olan, B.; Ciobica, L. The role of contrast-enhanced ultrasound in risk assessment of carotid atheroma. Rom. J. Mil. Med. 2016, 119, 9–11. [Google Scholar] [CrossRef]
- Lovibond, P.F.; Lovibond, S.H. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Anghel, D.; Ciobica, L.M.; Negru, M.M.; Jurcut, C.; Otlocan, L.; Coca, A. Bone mineral density and vitamin D levels in patients with rheumatoid arthritis. Osteoporos. Int. 2017, 28, S435–S436. [Google Scholar]
- Kochman, M. Primary hyperparathyroidism: Clinical manifestations, diagnosis and evaluation according to the Fifth International Workshop guidelines. Reumatologia 2023, 61, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Zelano, L.; Locantore, P.; Rota, C.A.; Policola, C.; Corsello, A.; Rossi, E.D.; Rufini, V.; Zagaria, L.; Raffaelli, M.; Pontecorvi, A. Parathyroid Carcinoma All-In-One, a Rare Life-Threatening Case With Multiple Systemic Manifestations: Case Report and Review of the Literature. Front. Endocrinol. 2022, 13, 881225. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Martin, A.; Rief, W.; Klaiberg, A.; Braehler, E. Validity of the Brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen. Hosp. Psychiatry 2006, 28, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Al-Dassean, K.A.; Murad, O.S. Factor structure and psychometric properties of the Jordanian version of the depression anxiety stress scale (DASS-21). Neuropsychopharmacol. Rep. 2024, 44, 447–456. [Google Scholar] [CrossRef]
- Hekimoglu, L.; Altun, Z.O.; Kaya, E.Z.; Bayram, N.; Bilgel, N. Psychometric properties of the Turkish version of the 42 item Depression Anxiety Stress Scale (DASS-42) in a clinical sample. Int. J. Psychiatry Med. 2012, 44, 183–198. [Google Scholar] [CrossRef]
- Vignola, R.C.; Tucci, A.M. Adaptation and validation of the depression, anxiety and stress scale (DASS) to Brazilian Portuguese. J. Affect. Disord. 2014, 155, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Snaith, R.P. The Hospital Anxiety And Depression Scale. Health Qual. Life Outcomes 2003, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Levis, B.; Sun, Y.; He, C.; Krishnan, A.; Neupane, D.; Bhandari, P.M.; Negeri, Z.; Benedetti, A.; Thombs, B.D.; et al. Accuracy of the Hospital Anxiety and Depression Scale Depression subscale (HADS-D) to screen for major depression: Systematic review and individual participant data meta-analysis. BMJ 2021, 373, n972. [Google Scholar] [CrossRef] [PubMed]
- Julian, L.J. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. 2011, 63 (Suppl. S11), S467–S672. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.B.; Shin, I.H.; Lim, K.H.; Lee, S.H.; Cho, G.A.; Sung, H.M.; Jung, S.W.; Zmimmerman, M.; Lee, Y. Current use of depression rating scales in mental health setting. Psychiatry Investig. 2010, 7, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Bunevicius, A.; Peceliuniene, J.; Mickuviene, N.; Valius, L.; Bunevicius, R. Screening for depression and anxiety disorders in primary care patients. Depress. Anxiety 2007, 24, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Stepankova Georgi, H.; Horakova Vlckova, K.; Lukavsky, J.; Kopecek, M.; Bares, M. Beck Depression Inventory-II: Self-report or interview-based administrations show different results in older persons. Int. Psychogeriatr. 2019, 31, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, L.; Karlsson, B.; Atti, A.R.; Skoog, I.; Fratiglioni, L.; Wang, H.X. Prevalence of depression: Comparisons of different depression definitions in population-based samples of older adults. J. Affect. Disord. 2017, 221, 123–131. [Google Scholar] [CrossRef]
- Dettori, C.; Ronca, F.; Scalese, M.; Saponaro, F. Parathyroid Hormone (PTH)-Related Peptides Family: An Intriguing Role in the Central Nervous System. J. Pers. Med. 2023, 13, 714. [Google Scholar] [CrossRef] [PubMed]
- Joborn, C.; Hetta, J.; Niklasson, F.; Rastad, J.; Wide, L.; Agren, H.; Akerström, G.; Ljunghall, S. Cerebrospinal fluid calcium, parathyroid hormone, and monoamine and purine metabolites and the blood-brain barrier function in primary hyperparathyroidism. Psychoneuroendocrinology 1991, 16, 311–322. [Google Scholar] [CrossRef]
- Hironaka, T.; Morimoto, S.; Fukuo, K.; Koh, E.; Imanaka, S.; Sumida, T.; Onishi, T.; Kumahara, Y. Immunoreactive parathyroid hormones in the circulation and cerebrospinal fluid from patients with renal failure: Possible restriction of parathyroid hormone by the blood-brain barrier. Bone Miner. 1987, 2, 487–494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weaver, D.R.; Deeds, J.D.; Lee, K.; Segre, G.V. Localization of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor mRNAs in rat brain. Mol. Brain Res. 1995, 28, 296–310. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, L.; Yao, L.; Pan, J.; Arzola, E.; Zhu, X.; Mei, L.; Xiong, W.C. Attenuation of Alzheimer’s brain pathology in 5XFAD mice by PTH1-34, a peptide of parathyroid hormone. Alzheimer’s Res. Ther. 2023, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Usdin, T.B.; Gruber, C.; Bonner, T.I. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J. Biol. Chem. 1995, 270, 15455–15458. [Google Scholar] [CrossRef]
- Hoare, S.R.; Bonner, T.I.; Usdin, T.B. Comparison of rat and human parathyroid hormone 2 (PTH2) receptor activation: PTH is a low potency partial agonist at the rat PTH2 receptor. Endocrinology 1999, 140, 4419–4425. [Google Scholar] [CrossRef]
- Keller, D.; Tsuda, M.C.; Usdin, T.B.; Dobolyi, A. Behavioural actions of tuberoinfundibular peptide 39 (parathyroid hormone 2). J. Neuroendocr. 2022, 34, e13130. [Google Scholar] [CrossRef] [PubMed]
- Dobolyi, A.; Dimitrov, E.; Palkovits, M.; Usdin, T.B. The neuroendocrine functions of the parathyroid hormone 2 receptor. Front. Endocrinol. 2012, 3, 121. [Google Scholar] [CrossRef]
- Gellén, B.; Zelena, D.; Usdin, T.B.; Dobolyi, Á. The parathyroid hormone 2 receptor participates in physiological and behavioral alterations of mother mice. Physiol. Behav. 2017, 181, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Serdenes, R.; Lewis, M.; Chandrasekhara, S. A Clinical Review of the Psychiatric Sequelae of Primary Hyperparathyroidism. Cureus 2021, 13, e19078. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, X.M.; Mousseau, D.D. Calcium alters monoamine oxidase-A parameters in human cerebellar and rat glial C6 cell extracts: Possible influence by distinct signalling pathways. Life Sci. 2009, 85, 262–268. [Google Scholar] [CrossRef]
- Minisola, S.; Arnold, A.; Belaya, Z.; Brandi, M.L.; Clarke, B.L.; Hannan, F.M.; Hofbauer, L.C.; Insogna, K.L.; Lacroix, A.; Liberman, U.; et al. Epidemiology, Pathophysiology, and Genetics of Primary Hyperparathyroidism. J. Bone Miner. Res. 2022, 37, 2315–2329. [Google Scholar] [CrossRef]
- Kuehner, C. Why is depression more common among women than among men? Lancet Psychiatry 2017, 4, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Sassarini, D.J. Depression in midlife women. Maturitas 2016, 94, 149–154. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Liu, Z.; Li, M.; Wang, Y.; Shang, Y.; Li, Y. Prevalence and associated factors of depression in postmenopausal women: A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 431. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, Z.; Xiang, F.; Hu, W.; Cao, X. Global prevalence of depression in menopausal women: A systematic review and meta-analysis. J. Affect. Disord. 2024, 358, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Farhane-Medina, N.Z.; Luque, B.; Tabernero, C.; Castillo-Mayén, R. Factors associated with gender and sex differences in anxiety prevalence and comorbidity: A systematic review. Sci. Prog. 2022, 105, 368504221135469. [Google Scholar] [CrossRef] [PubMed]
- Santoro, N. Perimenopause: From Research to Practice. J. Women’s Health 2016, 25, 332–339. [Google Scholar] [CrossRef]
- Arias de la Torre, J.; Vilagut, G.; Ronaldson, A.; Dregan, A.; Ricci-Cabello, I.; Hatch, S.L.; Serrano-Blanco, A.; Valderas, J.M.; Hotopf, M.; Alonso, J. Prevalence and age patterns of depression in the United Kingdom. A population-based study. J. Affect. Disord. 2021, 279, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Abdoli, N.; Salari, N.; Darvishi, N.; Jafarpour, S.; Solaymani, M.; Mohammadi, M.; Shohaimi, S. The global prevalence of major depressive disorder (MDD) among the elderly: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2022, 132, 1067–1073. [Google Scholar] [CrossRef]
- Wang, H.; Chen, M.; Xin, T.; Tang, K. Number of children and the prevalence of later-life major depression and insomnia in women and men: Findings from a cross-sectional study of 0.5 million Chinese adults. BMC Psychiatry 2020, 20, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, H.; Yang, A.; YH Ng, N.; Zhang, X.; Lau, E.S.H.; Chow, E.W.K.; Kong, A.P.S.; Chow, E.Y.K.; Chan, J.C.N.; et al. Higher risk of incident diabetes among patients with primary hyperparathyroidism. Clin. Endocrinol. 2024, 101, 605–613. [Google Scholar] [CrossRef]
- Basiri, R.; Seidu, B.; Rudich, M. Exploring the Interrelationships between Diabetes, Nutrition, Anxiety, and Depression: Implications for Treatment and Prevention Strategies. Nutrients 2023, 15, 4226. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, R.S.; Rapp, A.; Kelly, K.M.; Weiss, D.; Mezuk, B. Understanding the relationship between type 2 diabetes and depression: Lessons from genetically informative study designs. Diabet. Med. 2021, 38, e14399. [Google Scholar] [CrossRef] [PubMed]
- Mitrică, M.; Lorusso, L.; Badea, A.A.; Sîrbu, C.A.; Pleșa, A.; Stănescu, A.A.; Pleșa, F.C.; Sîrbu, O.M.; Munteanu, A.E. The Hidden Heart: Exploring Cardiac Damage Post-Stroke: A Narrative Review. Medicina 2024, 60, 1699. [Google Scholar] [CrossRef]
- Ferriani, L.O.; Silva, D.A.; Molina, M.D.C.B.; Mill, J.G.; Brunoni, A.R.; da Fonseca, M.J.M.; Moreno, A.B.; Benseñor, I.M.; de Aguiar, O.B.; Barreto, S.M.; et al. Depression is a risk factor for metabolic syndrome: Results from the ELSA-Brasil cohort study. J. Psychiatr. Res. 2023, 158, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, J.; Yin, Z.; Wang, L.; Peng, L. The association between depression and metabolic syndrome and its components: A bidirectional two-sample Mendelian randomization study. Transl. Psychiatry 2021, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, S.; Mantovani, G.; Spada, A. Metabolic Syndrome in Parathyroid Diseases. Front. Horm. Res. 2018, 49, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Cen, M.; Song, L.; Fu, X.; Gao, X.; Zuo, Q.; Wu, J. Associations between metabolic syndrome and anxiety, and the mediating role of inflammation: Findings from the UK Biobank. Brain Behav. Immun. 2024, 116, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wang, T.; Tong, X.; Song, Y.; Hong, J.; Sun, Y.; Zhuang, Y.; Shen, H.; Yao, X.I. Osteoporosis is associated with depression among older adults: A nationwide population-based study in the USA from 2005 to 2020. Public Health 2024, 226, 27–31. [Google Scholar] [CrossRef]
- Heidari, M.E.; Naghibi Irvani, S.S.; Dalvand, P.; Khadem, M.; Eskandari, F.; Torabi, F.; Shahsavari, H. Prevalence of depression in older people with hip fracture: A systematic review and meta-analysis. Int. J. Orthop. Trauma Nurs. 2021, 40, 100813. [Google Scholar] [CrossRef] [PubMed]
- Davison, R.; Daniel, J.A.; Idarraga, A.J.; Perticone, K.M.; Lin, J.; Holmes, G.B.; Lee, S.; Hamid, K.S.; Bohl, D.D. Depression Following Operative Treatments for Achilles Ruptures and Ankle Fractures. Foot Ankle Int. 2021, 42, 1579–1583. [Google Scholar] [CrossRef] [PubMed]
- Bistrović, I.L.; Roncević-Grzeta, I.; Crncević-Orlić, Z.; Francisković, T.; Ljubicić, R.; Orlić, A.; Kapović, M. Connection of depression and bone loss in perimenopausal and postmenopausal women. Coll. Antropol. 2012, 36, 1219–1223. [Google Scholar] [PubMed]
- Kashfi, S.S.; Abdollahi, G.; Hassanzadeh, J.; Mokarami, H.; Khani Jeihooni, A. The relationship between osteoporosis and depression. Sci. Rep. 2022, 12, 11177. [Google Scholar] [CrossRef] [PubMed]
- Keshishi, D.; Makunts, T.; Abagyan, R. Common osteoporosis drug associated with increased rates of depression and anxiety. Sci. Rep. 2021, 11, 23956. [Google Scholar] [CrossRef] [PubMed]
- Tournis, S.; Makris, K.; Cavalier, E.; Trovas, G. Cardiovascular Risk in Patients with Primary Hyperparathyroidism. Curr. Pharm. Des. 2020, 26, 5628–5636. [Google Scholar] [CrossRef] [PubMed]
- Pepe, J.; Cipriani, C.; Sonato, C.; Raimo, O.; Biamonte, F.; Minisola, S. Cardiovascular manifestations of primary hyperparathyroidism: A narrative review. Eur. J. Endocrinol. 2017, 177, R297–R308. [Google Scholar] [CrossRef] [PubMed]
- Hare, D.L.; Toukhsati, S.R.; Johansson, P.; Jaarsma, T. Depression and cardiovascular disease: A clinical review. Eur. Heart J. 2014, 35, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Maitra, N.S.; Qadeer, Y.K.; Wang, Z.; Fogg, S.; Storch, E.A.; Celano, C.M.; Huffman, J.C.; Jha, M.; Charney, D.S.; et al. Association of Depression and Cardiovascular Disease. Am. J. Med. 2023, 136, 881–895. [Google Scholar] [CrossRef]
- Civieri, G.; Abohashem, S.; Grewal, S.S.; Aldosoky, W.; Qamar, I.; Hanlon, E.; Choi, K.W.; Shin, L.M.; Rosovsky, R.P.; Bollepalli, S.C.; et al. Anxiety and Depression Associated With Increased Cardiovascular Disease Risk Through Accelerated Development of Risk Factors. JACC Adv. 2024, 3, 101208. [Google Scholar] [CrossRef] [PubMed]
- Dregan, A.; Rayner, L.; Davis, K.A.S.; Bakolis, I.; Arias de la Torre, J.; Das-Munshi, J.; Hatch, S.L.; Stewart, R.; Hotopf, M. Associations Between Depression, Arterial Stiffness, and Metabolic Syndrome Among Adults in the UK Biobank Population Study: A Mediation Analysis. JAMA Psychiatry 2020, 77, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Tabacco, G.; Makras, P.; Zavatta, G.; Trimboli, P.; Castellano, E.; Yavropoulou, M.P.; Naciu, A.M.; Anastasilakis, A.D. Primary hyperparathyroidism: From guidelines to outpatient clinic. Rev. Endocr. Metab. Disord. 2024, 25, 875–896. [Google Scholar] [CrossRef]
- Eberly, H.W.; Sciscent, B.Y.; Lorenz, F.J.; Goyal, N.; Goldenberg, D. Asymptomatic Primary Hyperparathyroidism: A Misnomer. OTO Open 2024, 8, e70039. [Google Scholar] [CrossRef]
- McDow, A.D.; Sippel, R.S. Should Symptoms Be Considered an Indication for Parathyroidectomy in Primary Hyperparathyroidism? Clin. Med. Insights: Endocrinol. Diabetes 2018, 11, 1179551418785135. [Google Scholar] [CrossRef]
- Bollerslev, J.; Jansson, S.; Mollerup, C.L.; Nordenström, J.; Lundgren, E.; Tørring, O.; Varhaug, J.E.; Baranowski, M.; Aanderud, S.; Franco, C.; et al. Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: A prospective, randomized trial. J. Clin. Endocrinol. Metab. 2007, 92, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Bargren, A.E.; Repplinger, D.; Chen, H.; Sippel, R.S. Can biochemical abnormalities predict symptomatology in patients with primary hyperparathyroidism? J. Am. Coll. Surg. 2011, 213, 410–414. [Google Scholar] [CrossRef]
- Liu, Y.; Sinha Gregory, N.; Andreopoulou, P.; Kashyap, S.; Cusano, N. Approach to the Patient: Normocalcemic Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2024, 00, 1–10. [Google Scholar] [CrossRef]
- Díaz-Soto, G.; Julián, M.T.; Puig-Domingo, M. Normocalcemic primary hyperparathyroidism: A newly emerging disease needing therapeutic intervention. Hormones 2012, 11, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Grønli, O.; Wynn, R. Normocalcemic hyperparathyroidism and treatment resistant depression. Psychosomatics 2013, 54, 493–497. [Google Scholar] [CrossRef]
- Parks, K.A.; Parks, C.G.; Onwuameze, O.E.; Shrestha, S. Psychiatric Complications of Primary Hyperparathyroidism and Mild Hypercalcemia. Am. J. Psychiatry 2017, 174, 620–622. [Google Scholar] [CrossRef]
- El-Husari, A.; Phrathep, D.D.; Ibrahim, M.; Chism, D.; Narvel, R. Acute Psychosis in the Setting of Undiagnosed Normocalcemic Hyperparathyroidism: A Case Report. Cureus 2023, 15, e35840. [Google Scholar] [CrossRef]
- Strother, R.K.; Meunier, M. Hypercalcemia in the Presence of an Ectopic Mediastinal Mass. J. Prim. Care Community Health 2020, 11, 2150132720932411. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, F.; Martino, B.; Nitro, L.; Fuccillo, E.; Felisati, G.; De Pasquale, L. A parathyroid cancer with soporous state, depression, and severe cognitive decline in acute renal failure. Clin. Case Rep. 2023, 11, e7627. [Google Scholar] [CrossRef]
- Meng, A.Z.; Tan, Y.; Ong, S.J.; Wee, B.B.; Teo, L. Primary Hyperparathyroidism Causing Psychosis: A Case Report. Cureus 2022, 14, e31935. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Mlawa, G.; Mahdi, H.; Abumedian, M. Acute Psychosis Related to Primary Hyperparathyroidism in a Patient With Bipolar Disorder. Cureus 2023, 15, e42567. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.J.; Paul, S.; Primelo, R. Parathyroid Paranoia: Unveiling Psychosis in Hyperparathyroidism. Case Rep. Psychiatry 2024, 2024, 8126125. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bauernfreund, Y.; Arya, P.; Singh, E.; Shute, J. Primary hyperparathyroidism presenting as acute psychosis secondary to hypercalcaemia requiring curative parathyroidectomy. J. Surg. Case Rep. 2018, 2018, rjy023. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Mangini, P.; Schroen, C.; Aziz, R.; Tobia, A. Prolonged Hypercalcemia-Induced Psychosis. Case Rep. Psychiatry 2020, 2020, 6954036. [Google Scholar] [CrossRef]
- Babar, G.; Alemzadeh, R. A case of acute psychosis in an adolescent male. Case Rep. Endocrinol. 2014, 2014, 937631. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, K.; Izuhara, M.; Miura, S.; Yamashita, S.; Nagahama, M.; Hayashida, M.; Hashioka, S.; Miyaoka, T.; Hotta, Y.; Shimizu, Y.; et al. Psychosis in a primary hyperparathyroidism patient with mild hypercalcemia: A case report. Medicine 2021, 100, e25248. [Google Scholar] [CrossRef] [PubMed]
- Corredor-Orlandelli, D.; Valenzuela-Vallejo, L.; Aguirre-Ruiz, J.F.; Valenzuela Rincon, A. Ectopic parathyroid adenoma causing hyperparathyroidism-induced psychosis: A case report. SAGE Open Med Case Rep. 2023, 11, 2050313X231180752. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).