Hospital-Acquired Infections Caused by Acinetobacter baumannii: A Comparative Analysis of Risk Factors with Other ESKAPE-E Pathogens in a Third-Level IMSS Hospital in Yucatan Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Type and Design
2.2. Sample and Sampling Methodology
2.3. Data Collection
2.4. Statistical Analysis
3. Results
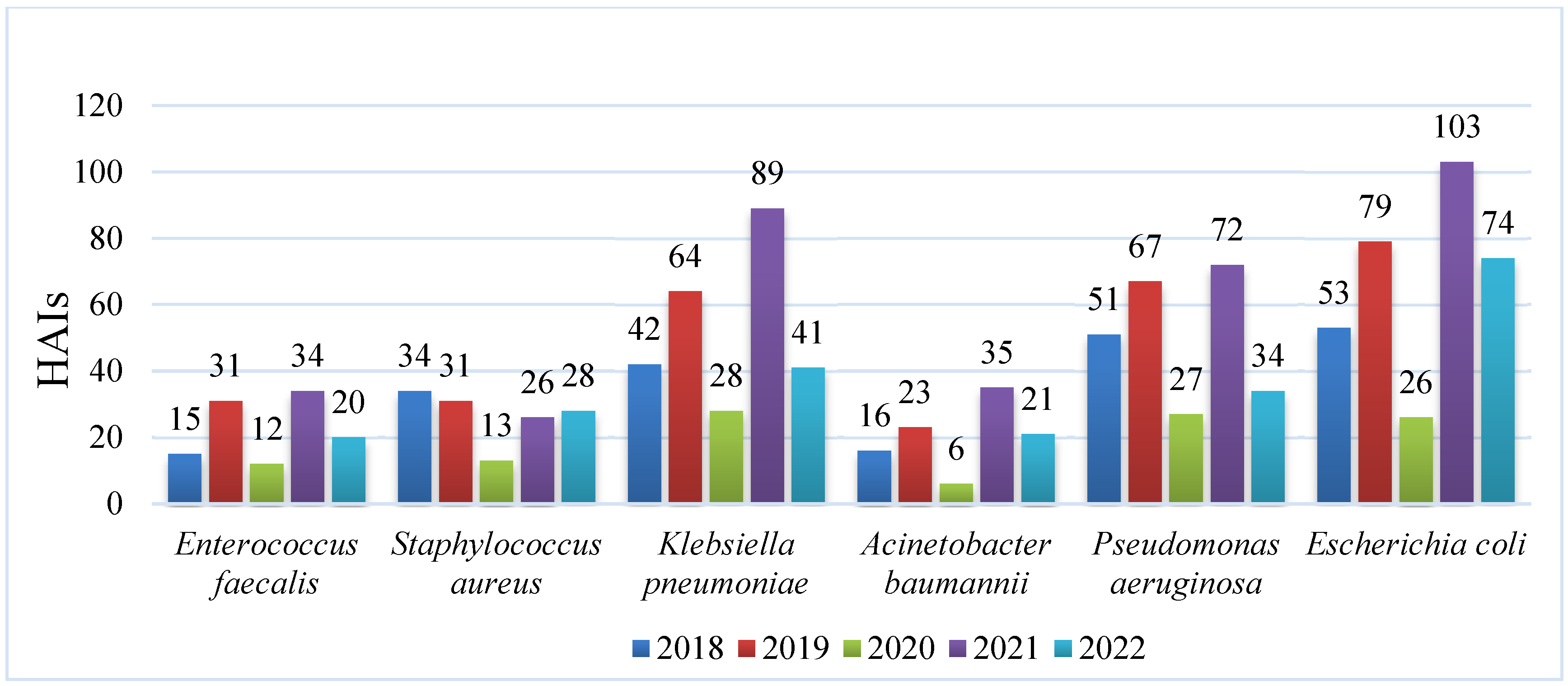
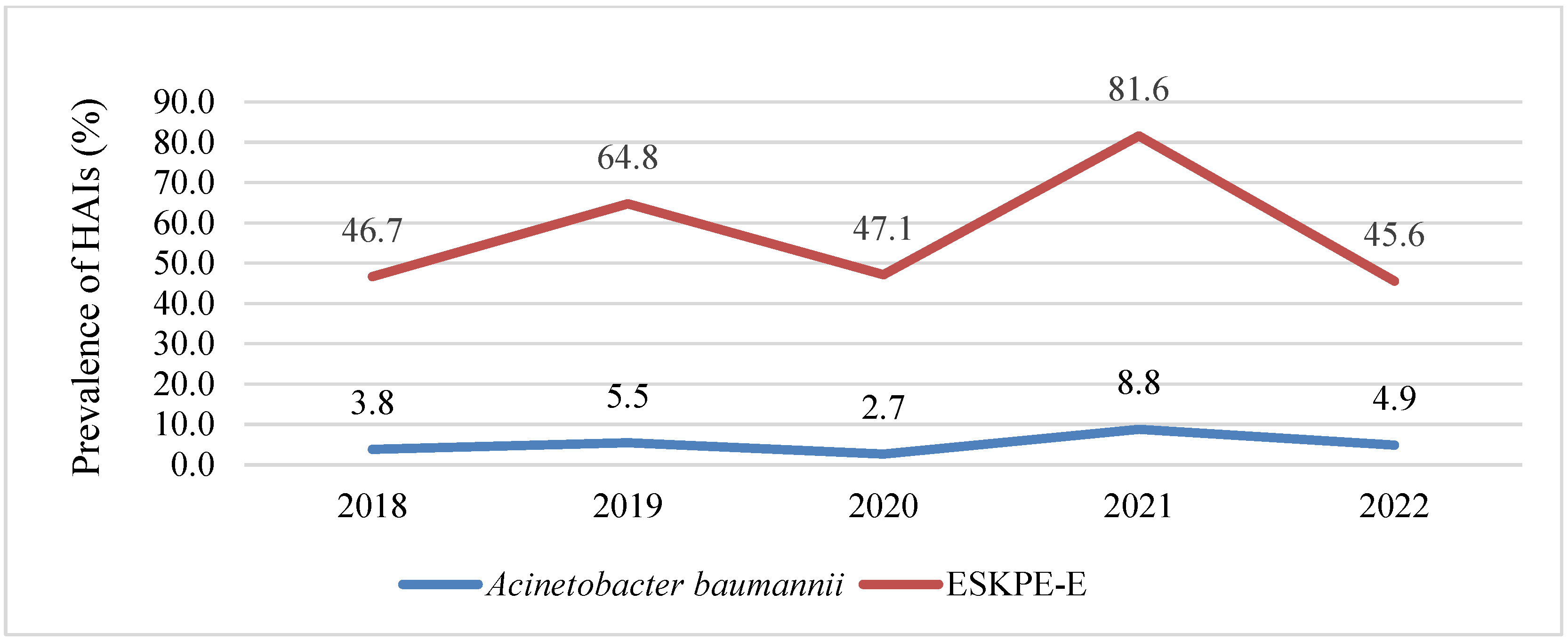
3.1. HAIs Caused by ESKAPE-E
3.2. Sociodemographic and Clinical Characteristics of Case and Control Groups and Univariate Analysis
3.3. Risk Factors Associated with HAIs Caused by A. baumannii vs. Other ESKAPE-E Pathogens
4. Discussion
4.1. Sociodemographic and Clinical Characteristics
4.2. Risk Factors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of Healthcare-Associated Infections Connected to Medical Devices-An Update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef] [PubMed]
- Raoofi, S.; Pashazadeh Kan, F.; Rafiei, S.; Hosseinipalangi, Z.; Noorani Mejareh, Z.; Khani, S.; Abdollahi, B.; Seyghalani Talab, F.; Sanaei, M.; Zarabi, F.; et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef]
- World Health Organization. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/80135/?sequence=1 (accessed on 8 July 2025).
- World Health Organization. Global Report on Infection Prevention and Control 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240103986 (accessed on 17 November 2025).
- Kollef, M.H.; Torres, A.; Shorr, A.F.; Martin-Loeches, I.; Micek, S.T. Nosocomial Infection. Crit. Care Med. 2021, 49, 169–187. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Abu Bakar, M. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Sati, H.; Carrara, E.; Savoldi, A.; Hansen, P.; Garlasco, J.; Campagnaro, E.; Boccia, S.; Castillo-Polo, J.A.; Magrini, E.; Garcia-Vello, P.; et al. The WHO Bacterial Priority Pathogens List 2024: A prioritisation study to guide research, development, and public health strategies against antimicrobial resistance. Lancet Infect Dis. 2025, 25, 1033–1043. [Google Scholar] [CrossRef]
- Bertagnolio, S.; Dobreva, Z.; Centner, C.M.; Olaru, I.D.; Donà, D.; Burzo, S.; Huttner, B.D.; Chaillon, A.; Gebreselassie, N.; Wi, T.; et al. WHO global research prioritiesfor antimicrobial resistance in human health. Lancet Microbe 2024, 5, 100902. [Google Scholar] [CrossRef]
- Ahuatzin-Flores, O.E.; Torres, E.; Chávez-Bravo, E. Acinetobacter baumannii, a Multidrug-Resistant Opportunistic Pathogen in New Habitats: A Systematic Review. Microorganisms 2024, 12, 644. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the critically ill: Complex infections get complicated. Front. Microbiol. 2023, 14, 1196774. [Google Scholar] [CrossRef]
- Castillo-Ramírez, S.; Aguilar-Vera, A.; Kumar, A.; Evans, B. Acinetobacter baumannii: Much more than a human pathogen. Antimicrob. Agents Chemother. 2025, 69, e0080125. [Google Scholar] [CrossRef]
- Mancilla-Rojano, J.; Ochoa, S.A.; Reyes-Grajeda, J.P.; Flores, V.; Medina-Contreras, O.; Espinosa-Mazariego, K.; Parra-Ortega, I.; Rosa-Zamboni, D.; Castellanos-Cruz, M.D.C.; Arellano-Galindo, J.; et al. Molecular Epidemiology of Acinetobacter calcoaceticus-Acinetobacter baumannii Complex Isolated from Children at the Hospital Infantil de México Federico Gómez. Front. Microbiol. 2020, 11, 576673. [Google Scholar] [CrossRef]
- Humberto, B.C.; Luis, L.A.; Josefina, D.B. Genomic analysis of the main epidemiological lineages of Acinetobacter baumannii in Mexico. Front. Cell Infect. Microbiol. 2025, 14, 1499839. [Google Scholar] [CrossRef] [PubMed]
- Alcántar-Curiel, M.D.; Rosales-Reyes, R.; Jarillo-Quijada, M.D.; Gayosso-Vázquez, C.; Fernández-Vázquez, J.L.; Toledano-Tableros, J.E.; Giono-Cerezo, S.; Garza-Villafuerte, P.; López-Huerta, A.; Vences-Vences, D.; et al. Carbapenem-Resistant Acinetobacter baumannii in Three Tertiary Care Hospitals in Mexico: Virulence Profiles, Innate Immune Response and Clonal Dissemination. Front. Microbiol. 2019, 10, 2116. [Google Scholar] [CrossRef] [PubMed]
- Garza-González, E.; Bocanegra-Ibarias, P.; Bobadilla-Del-Valle, M.; Ponce-de-León-Garduño, L.A.; Esteban-Kenel, V.; Silva-Sánchez, J.; Garza-Ramos, U.; Barrios-Camacho, H.; López-Jácome, L.E.; Colin-Castro, C.A.; et al. Drug resistance phenotypes and genotypes in Mexico in representative gram-negative species: Results from the infivar network. PLoS ONE 2021, 16, e0248614. [Google Scholar] [CrossRef] [PubMed]
- Universidad Autónoma de Mexico. Plan Universitario de Control de la Resistencia Antimicrobiana (PUCRA). Resistencia Antimicrobiana en México 2017 a 2023. Reporte de Los Hospitales de la Red PUCRA: Resistencia Antimicrobiana y Consumo de Antibióticos. Ciudad de México, Octubre 2024. Available online: https://puiree.cic.unam.mx/divulgacion/docs/pucra2024.pdf (accessed on 14 November 2025).
- Villarreal-Cruz, S.; Camacho-Ortiz, A.; Flores-Treviño, S.; Villarreal-Treviño, L.; Bocanegra-Ibarias, P. Intrahospital dissemination of multidrug-resistant Acinetobacter baumannii at a teaching hospital in Northeast of Mexico. Infect. Prev. Pract. 2025, 7, 100443. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Gracida-Osorno, C.; Luna-Chi, I.G.; Jiménez-Guillermo, J.G.; Molina-Salinas, G.M. High Prevalence of Antimicrobial Resistance Among Gram-Negative Isolated Bacilli in Intensive Care Units at a Tertiary-Care Hospital in Yucatán Mexico. Medicina 2019, 55, 588. [Google Scholar] [CrossRef]
- Uc-Cachón, A.H.; Molina-Salinas, G.M.; Dzul-Beh, A.J.; Rosado-Manzano, R.F.; Dzib-Baak, H.E. Bacterias Gram-negativas de prioridad crítica en pacientes de las UCI de un hospital de tercer nivel [Gram-negative bacteria of critical priority in ICU patients from a tertiary care hospital]. Rev. Med. Inst. Mex. Seguro Soc. 2023, 61, 552–558. [Google Scholar]
- Ardoino, I.; Zangirolami, F.; Iemmi, D.; Lanzoni, M.; Cargnelutti, M.; Biganzoli, E.; Castaldi, S. Risk factors and epidemiology of Acinetobacter baumannii infections in a university hospital in Northern Italy: A case-control study. Am. J. Infect. Control 2016, 44, 1600–1605. [Google Scholar] [CrossRef]
- Al-Gethamy, M.M.; Faidah, H.S.; Adetunji, H.A.; Haseeb, A.; Ashgar, S.S.; Mohanned, T.K.; Mohammed, A.-H.; Khurram, M.; Hassali, M.A. Risk factors associated with multi-drug-resistant Acinetobacter baumannii nosocomial infections at a tertiary care hospital in Makkah, Saudi Arabia-a matched case–control study. J. Int. Med. Res. 2017, 45, 1181–1189. [Google Scholar] [CrossRef]
- Schlosser, B.; Weikert, B.; Fucini, G.B.; Kohlmorgen, B.; Kola, A.; Weber, A.; Thoma, N.; Behnke, M.; Schwab, F.; Gastmeier, P.; et al. Risk factors for transmission of carbapenem-resistant Acinetobacter baumannii in outbreak situations: Results of a case-control study. BMC Infect. Dis. 2024, 24, 120. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Playford, E.G.; Craig, J.C.; Iredell, J.R. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: Risk factors for acquisition, infection and their consequences. J. Hosp. Infect. 2007, 65, 204–211. [Google Scholar] [CrossRef]
- Huertas Vaquero, M.; Asencio Egea, M.A.; Carranza González, R.; Padilla Serrano, A.; Conde García, M.C.; Tenias Burillo, J.M.; Redondo González, O. Association between antibiotic pressure and the risk of colonization/infection by multidrug-resistant Acinetobacter baumannii complex: A time series analysis. Rev. Esp. Quimioter. 2021, 34, 623–630. [Google Scholar] [CrossRef]
- Gu, Y.; Jiang, Y.; Zhang, W.; Yu, Y.; He, X.; Tao, J.; Hou, X.; Wang, H.; Deng, M.; Zhou, M.; et al. Risk factors and outcomes of bloodstream infections caused by Acinetobacter baumannii: A case-control study. Diagn. Microbiol. Infect. Dis. 2021, 99, 115229. [Google Scholar] [CrossRef]
- Combes, A.; Luyt, C.E.; Trouillet, J.L.; Nieszkowska, A.; Chastre, J. Gender impact on the outcomes of critically ill patients with nosocomial infections. Crit. Care Med. 2009, 37, 2506–2511. [Google Scholar] [CrossRef] [PubMed]
- Dossett, L.A.; Swenson, B.R.; Heffernan, D.; Bonatti, H.; Metzger, R.; Sawyer, R.G.; May, A.K. High levels of endogenous estrogens are associated with death in the critically injured adult. J. Trauma. 2008, 64, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, F.; Hydo, L.; Eachempati, S.; Pomp, A.; Shou, J.; Barie, P.S. Higher body mass index predicts need for insulin but not hyperglycemia, nosocomial infection, or death in critically ill surgical patients. Surg. Infect. 2008, 9, 121–130. [Google Scholar] [CrossRef]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, E.; Sánchez-Velázquez, L.D.; Rodríguez-Terán, G. Acinetobacter baumannii, un patógeno emergente: Estudio prospectivo en una unidad de terapia intensiva respiratoria. Col. Mex. Med. Crít. 2016, 30, 187–191. [Google Scholar]
- Real, C.; Peralta, L. Todos los caminos conducen a la pérdida de masa muscular: Desnutrición, fragilidad, sarcopenia y caquexia. Diaeta 2021, 39, 45–58. [Google Scholar]
- Schneider, S.M.; Veyres, P.; Pivot, X.; Soummer, A.M.; Jambou, P.; Filippi, J.; van Obberghen, E.; Hébuterne, X. Malnutrition is an independent factor associated with nosocomial infections. Br. J. Nutr. 2004, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sosa Hernández, Ó.; González Martínez, J.; Juárez Vargas, R.; Sánchez Rivas, M.P.; Cureño Díaz, M.A. Infecciones asociadas a la atención de la salud por bacterias del grupo ESKAPE en un hospital de la Ciudad de México. Enferm. Infecc. Microbiol. 2019, 39, 59–64. [Google Scholar]
- Rodriguez Buenahora, R.D.; Bustillo Zarate, D.E.; Caicedo Sanchez, D.C.; Cadena Sarmiento, D.C.; Castellanos Gomez, C. Acinetobacter baumannii: Patógeno multirresistente emergente. Med. UIS 2016, 29, 113–135. [Google Scholar] [CrossRef]
- Zuniga-Moya, J.C.; Caballero, C.A.; Loucel-Linares, M.; Benitez, M.J.; Zambrano-Garcia, E.; Fajardo, L.V.; Paz, J.S.; Bejarano, S.A.; Saavedra, E.B.; Romero, L.E. Antimicrobial profile of Acinetobacter baumannii at a tertiary hospital in Honduras: A cross-sectional analysis. Rev. Panam. Salud Publica 2020, 44, e46. [Google Scholar] [CrossRef]
- Swe-Han, K.S.; Mlisana, K.P.; Pillay, M. Analysis of clinical and microbiological data on Acinetobacter baumannii strains assist the preauthorization of antibiotics at the patient level for an effective antibiotic stewardship program. J. Infect. Public Health 2017, 10, 608–616. [Google Scholar] [CrossRef]
- Velázquez-Acosta, C.; Cornejo-Juárez, P.; Volkow-Fernández, P. Cepas E-ESKAPE multidrogorresistentes aisladas en hemocultivos de pacientes con cáncer [Multidrug resistance E-ESKAPE strains isolated from blood cultures in patients with cancer]. Salud Publica Mex. 2018, 60, 151–157. [Google Scholar] [CrossRef]
- René, C.A.J.; Arturo, R.R. Resistencia antimicrobiana de bacterias cultivadas en la Unidad de Cuidados Intensivos de Adultos. Enferm. Infecc. Microbiol. 2012, 32, 127–133. [Google Scholar]
- de Arriba Fernández, A.; Molina-Cabrillana, J.; Serra-Majem, L. Aplicación del cuestionario de autoevaluación de la estrategia multimodal de la OMS para mejorar la práctica de higiene de manos en un hospital de tercer nivel. Arch. Prev. Riesgos Labor. 2021, 24, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cuenca, F.; Tomás-Carmona, M.; Caballero-Moyano, F.; Bou, G.; Martínez-Martínez, L.; Vila, J.; Pachón, J.; Cisneros, J.M.; Rodríguez-Baño, J.; Pascual, Á. Actividad de 18 agentes antimicrobianos frente a aislados clínicos de Acinetobacter baumannii: Segundo estudio nacional multicéntrico (proyecto GEIH-REIPI-Ab 2010). Enferm Infecc Microbiol. 2013, 31, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidis, I.; Vasileiou, E.; Pana, Z.D.; Tragiannidis, A. Acinetobacter baumannii Antibiotic Resistance Mechanisms. Pathogens 2021, 10, 373. [Google Scholar] [CrossRef]
- Vanegas-Múnera, J.M.; Roncancio-Villamil, G.; Jiménez-Quiceno, J.N. Acinetobacter baumannii: Importancia clínica, mecanismos de resistencia y diagnóstico. CES Med. 2014, 28, 233–246. [Google Scholar]
- Torres, A.H.; Vázquez, E.G.; Yagüe, G.; Gómez, J.G. Acinetobacter baumannii multirresistente: Situación clínica actual y nuevas perspectivas. Rev. Esp. Quimioter. 2010, 23, 12–19. [Google Scholar] [PubMed]
- Ren, J.; Li, X.; Wang, L.; Liu, M.; Zheng, K.; Wang, Y. Risk Factors and Drug Resistance of the MDR Acinetobacter Baumannii in Pneumonia Patients in ICU. Open Med. 2019, 14, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Yehya, A.; Ezzeddine, Z.; Chakkour, M.; Dhaini, Z.; Bou Saba, M.S.; Bou Saba, A.S.; Nohra, L.; Nassar, N.B.; Yassine, M.; Bahmad, H.F.; et al. The intricacies of Acinetobacter baumannii: A multifaceted comprehensive review of a multidrug-resistant pathogen and its clinical significance and implications. Front. Microbiol. 2025, 16, 1565965. [Google Scholar] [CrossRef]
- Sharma, R.; Lakhanpal, D. Acinetobacter baumannii: A comprehensive review of global epidemiology, clinical implications, host interactions, mechanisms of antimicrobial resistance and mitigation strategies. Microbial Pathog. 2025, 204, 107605. [Google Scholar] [CrossRef]
| Selection Criteria | Cases | Controls |
|---|---|---|
| Inclusion |
|
|
| Exclusion |
|
|
| Sociodemographic and Clinical Characteristics | A. baumannii n (%) | ESKPE-E n (%) | p-Value | OR |
|---|---|---|---|---|
| Male | 45 (83.3%) | 90 (83.3%) | 1.000 | |
| Avg. Age (years) | 39.2 SD ± 26.6 | 39.8 SD ± 26.8 | ||
| Age (years) | 0.377 | |||
| 0–5 | 11 (20.4%) | 24 (22.2) | ||
| 6–11 | 2 (3.7%) | 0 (0.0%) | ||
| 12–18 | 2 (3.7%) | 8 (7.4%) | ||
| 19–26 | 1 (1.9%) | 4 (3.7%) | ||
| 27–59 | 21 (38.9%) | 40 (37.0%) | ||
| 60 and more | 17 (31.5%) | 32 (29.6%) | ||
| Type of HAIs | 0.161 | |||
| VAP | 27 (50.0%) | 54 (50.0%) | ||
| CLABSI | 16 (29.6%) | 32 (29.6%) | ||
| SSI | 5 (9.3%) | 10 (9.3%) | ||
| CAUTI | 5 (9.3%) | 10 (9.3%) | ||
| NV-HAP | 1 (1.9%) | 2 (1.9%) | ||
| Comorbidities | 0.102 | |||
| Hypertension | 24 (44.4%) | 36 (33.3%) | 0.173 | 1.600 |
| Diabetes mellitus | 13 (24.1%) | 26 (24.1%) | 1.000 | 1.000 |
| Oncohaematological diseases | 9 (16.7%) | 12 (11.1%) | 0.331 | 1.600 |
| Underweight | 9 (16.7%) | 9 (8.3%) | 0.120 | 2.200 |
| Cardiopathy | 3 (5.6%) | 11 (10.2%) | 0.389 | 0.519 |
| Chronic kidney disease | 3 (5.6%) | 5 (4.6%) | 1.000 | 1.212 |
| Rheumatological diseases | 2 (3.7%) | 1 (0.9%) | 0.258 | 4.115 |
| Obesity | 2 (3.7%) | 1 (0.9%) | 0.258 | 4.115 |
| Cancer | 1 (1.9%) | 5 (4.6%) | 0.665 | 0.389 |
| Asthma | 1 (1.9%) | 2 (1.9%) | 1.000 | 1.000 |
| HIV | 1 (1.9%) | 1 (0.9%) | 1.000 | 2.019 |
| Invasive Procedures | ||||
| Central venous catheter | 53 (98.1%) | 97 (89.8%) | 0.063 | 6.01 |
| Urinary catheter | 43 (79.6%) | 75 (69.4%) | 0.193 | 1.72 |
| Mechanical ventilation | 41 (75.9%) | 63 (58.3%) | 0.37 | 2.253 |
| Avg. invasive procedures (days) | ||||
| Central venous catheter | 14.2 | 10.2 | 0.005 | |
| Urinary catheter | 9.3 | 6.3 | 0.101 | |
| Mechanical ventilation | 9.0 | 6.6 | 0.150 | |
| Avg. of hospital stays (days) | 40.2 | 36.9 | 0.122 | |
| Avg. of ICU stays (days) | 21.4 | 22.4 | 0.196 | |
| Outcome | 0.360 | |||
| Hospital discharge | 31 (57.4%) | 65 (60.2%) | ||
| Death | 20 (37.0%) | 31 (28.7%) | ||
| Reference to 2nd. level hospital | 3 (5.6%) | 12 (11.1%) | ||
| Bacteria | ||||
| Acinetobacter baumannii | 54 (100%) | -- | ||
| Escherichia coli | -- | 30 (27.8%) | ||
| Pseudomonas aeruginosa | -- | 28 (25.9%) | ||
| Klebsiella pneumoniae | -- | 27 (25.0%) | ||
| Staphylococcus aureus | -- | 16 (14.8%) | ||
| Enterococcus faecalis | -- | 7 (6.5%) | ||
| Drug-resistant Profile | ||||
| Acinetobacter baumannii | ||||
| MDR | 5 (9.3%) | -- | ||
| XDR | 49 (90.7%) | -- | ||
| Escherichia coli | ||||
| MDR | -- | 5 (4.65%) | ||
| XDR | -- | 26 (24.1%) | ||
| Pseudomonas aeruginosa | ||||
| MDR | -- | 17 (15.8%) | ||
| XDR | -- | 11 (10.1%) | ||
| Klebsiella pneumoniae | ||||
| MDR | -- | 4 (3.7%) | ||
| XDR | -- | 22 (20.3%) | ||
| Staphylococcus aureus | ||||
| MDR | -- | 5 (4.6%) | ||
| XDR | -- | 11 (10.3%) | ||
| Enterococcus faecalis | ||||
| MDR | -- | 4 (3.7%) | ||
| XDR | -- | 3 (2.8%) | ||
| Previous Antibiotic Therapy | ||||
| Penicillins: AMP | 6 (11.1%) | 7 (6.5%) | ||
| 1st gen. CP: CET | 0 (0.0%) | 3 (2.8%) | ||
| 2nd gen. CP: CRX | 0 (0.0%) | 6 (5.6%) | ||
| 3rd gen. CP: CTX, CRO, CAZ | 18 (33.3%) | 45 (41.7%) | 0.143 | |
| 4th gen. CP: FEP | 6 (11.1%) | 9 (8.3%) | 0.565 | |
| Carbapenems: MEM, IMP | 12 (22.2%) | 28 (25.9%) | <0.000 | |
| Monobactams: PIP-TZ | 7 (13.0%) | 7 (6.5%) | 0.166 | |
| Aminoglycosides: AMK, GEN | 15 (27.8%) | 20 (18.5%) | 0.177 | |
| Glycopeptides: VAN | 14 (25.9%) | 18 (16.7%) | 0.163 | |
| Macrolides: CLI | 4 (7.4%) | 5 (4.6%) | 0.467 | |
| FQs: LEV, CIP, MXF | 29 (53.7%) | 42 (32.4%) | 0.009 | |
| Oxazolidinones: LZD | 13 (24.1%) | 5 (4.6%) | <0.000 | |
| No. of Antibiotics Prescribed | 0.002 | |||
| Cero | 0 | 9 (9.2%) | ||
| One | 12 (22.2%) | 47 (47.5%) | ||
| Two | 15 (27.8%) | 28 (28.3%) | ||
| Three | 17 (31.5%) | 15 (15.2%) | ||
| Four | 5 (9.3%) | 8 (8.1%) | ||
| Five to Seven | 5 (9.3%) | 1 (1.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eb-Rejón, J.d.R.; Paniagua-Sierra, J.R.; Gracida-Osorno, C.; Molina-Salinas, G.M. Hospital-Acquired Infections Caused by Acinetobacter baumannii: A Comparative Analysis of Risk Factors with Other ESKAPE-E Pathogens in a Third-Level IMSS Hospital in Yucatan Mexico. Diseases 2025, 13, 384. https://doi.org/10.3390/diseases13120384
Eb-Rejón JdR, Paniagua-Sierra JR, Gracida-Osorno C, Molina-Salinas GM. Hospital-Acquired Infections Caused by Acinetobacter baumannii: A Comparative Analysis of Risk Factors with Other ESKAPE-E Pathogens in a Third-Level IMSS Hospital in Yucatan Mexico. Diseases. 2025; 13(12):384. https://doi.org/10.3390/diseases13120384
Chicago/Turabian StyleEb-Rejón, Jael del Rosario, José Ramón Paniagua-Sierra, Carlos Gracida-Osorno, and Gloria María Molina-Salinas. 2025. "Hospital-Acquired Infections Caused by Acinetobacter baumannii: A Comparative Analysis of Risk Factors with Other ESKAPE-E Pathogens in a Third-Level IMSS Hospital in Yucatan Mexico" Diseases 13, no. 12: 384. https://doi.org/10.3390/diseases13120384
APA StyleEb-Rejón, J. d. R., Paniagua-Sierra, J. R., Gracida-Osorno, C., & Molina-Salinas, G. M. (2025). Hospital-Acquired Infections Caused by Acinetobacter baumannii: A Comparative Analysis of Risk Factors with Other ESKAPE-E Pathogens in a Third-Level IMSS Hospital in Yucatan Mexico. Diseases, 13(12), 384. https://doi.org/10.3390/diseases13120384






