Simple Summary
Probiotics are live microorganisms that support health by balancing gut bacteria, boosting immunity and metabolism, and potentially preventing or treating various conditions. Their effectiveness varies by strain and proper use. Advances in microbiome research are leading to personalized probiotic treatments with significant benefits. Different strains play unique roles in aging, cancer, autoimmune diseases, and neurodegenerative disorders. Understanding these strain-specific effects is crucial for personalized healthcare. Studies show probiotics can modulate immunity, gut health, and overall well-being. Tailoring probiotics to individual needs may help address complex health challenges. This review provides an overview of probiotic strains Lactobacillus and Bifidobacterium mediated unique benefits, suggesting that targeted use can maximize their therapeutic potential.
Abstract
Probiotics are known for their health benefits, and new studies suggest they could help with various conditions. However, the specific formulations and mechanisms of probiotics in addressing these issues are still being explored. This review focuses on four key areas: cancer, aging, autoimmune diseases, and neurodegenerative disorders, highlighting the potential benefits of Lactobacillus and Bifidobacterium probiotics. Their interaction with the immune system plays a crucial role in offering protection and therapeutic effects, particularly in enhancing immunity in older adults. The review sheds light on how these probiotics affect the immune system, gut microbiome, and related processes to manage or combat these health problems. It emphasizes the importance of customizing probiotic formulations for specific conditions, as different combinations of Lactobacillus and Bifidobacterium uniquely activate immune cells. Some combinations work as effective treatments for diseases, while others boost immunity in aging. While the potential of these probiotics is significant, challenges remain in using them for cancer, age-related diseases, autoimmune diseases neurodegenerative disorder treatments. Limited evidence calls for further research to define their role and establish guidelines. Future approaches like strain engineering, nanoencapsulation, synbiotics, and personalized microbiome analysis aim to overcome these challenges, making probiotics a more viable option for disease prevention and care. Additionally, there is an urgent need for clinical trials to ensure patients can benefit from these probiotics.
Keywords:
NK cells; T cells; CD8+ T cells; immunotherapy; probiotics; cancer; aging; autoimmune; neurodegenerative; cytotoxicity; IFN-γ 1. Introduction and Background
In the early 20th century, Elie Metchnikoff discovered certain strains of gut bacteria essential for maintaining balance in the gut, which he named probiotics [1]. These live, beneficial bacteria, often derived from fermented foods or the gut itself, offer numerous health benefits supported by studies and clinical trials [2,3]. Probiotics help maintain the intestinal barrier and microbial balance by enhancing proteins and mucus that prevent pathogens and excessive immune responses [4,5]. They interact with immune cells through direct contact and signaling molecules, boosting immunity while maintaining gut tolerance [6]. Probiotics also strengthen defenses against diseases and infections by activating immune cells and increasing cytokines and antibodies [7,8]. By competing with pathogens and producing antimicrobial agents, probiotics contribute to immune stability [9]. Components like exopolysaccharides, surface proteins, and secreted peptides interact with immune receptors, influencing signaling pathways [10,11,12]. They also adhere to the gut lining and engage with immune cells via Toll-like receptors (TLRs) like TLR2, TLR4, and TLR9 [8,13].
Probiotics play a crucial role in maintaining immune balance by activating regulatory T (Tregs) cells, which promote tolerance to commensals and food antigens, reducing inflammation and allergic responses [14]. They also impact the differentiation and function of T helper (Th) cell subsets and Tregs, driving immune responses like cell-mediated immunity or tolerance through cytokines like IL-10 and TGF-β [8,13]. Probiotics aid in B-cell maturation into plasma cells that produce secretory IgA (sIgA), essential for defending mucosal surfaces against pathogens [15]. The immune-modulating effects of probiotics depend heavily on the specific strains used, as different species and strains can uniquely affect cytokine responses and immune cell activity. For instance, Lactobacillus strains often enhance Th1-type immune responses, while Bifidobacterium strains typically promote anti-inflammatory effects [16]. Most probiotics are lactic acid-producing bacteria, such as lactobacilli, streptococci, and bifidobacteria, emphasizing their potential for human health [17]. Certain strains, like Lactococcus lactis subsp. Cremoris C60, have even been found to boost antigen presentation by dendritic cells (DCs), enhancing cytotoxic T cell responses critical for combating tumors and viruses [18,19].
Lactobacillus and Bifidobacterium offer promising benefits for cancer, aging, autoimmune diseases, and neurodegenerative disorders through their role in the gut–brain axis. However, their practical application faces hurdles like strain-specific variability, challenges with oral delivery and formulation stability, individual differences in gut microbiota, inconsistent clinical trial results, unclear mechanisms, disease-specific effectiveness, safety concerns for vulnerable groups, and environmental and lifestyle influences. Selecting the right strain and dosage is crucial for boosting immunity and maximizing health benefits. This review explores their mechanisms of action, applications, and challenges, emphasizing the importance of careful Lactobacillus and Bifidobacterium strain selection to achieve therapeutic outcomes. It highlights how specific strains enhance immune cell activity, maintain immune balance, and improve treatments for these conditions. Additionally, it stresses the need for faster clinical trials to bridge gaps between preclinical and clinical studies, ensuring patients can benefit from probiotics.
2. Mechanism of Action, Application, and Challenges of Lactobacillus and Bifidobacterium in Cancer Prevention and Their Role as Adjuvant Cancer Therapies
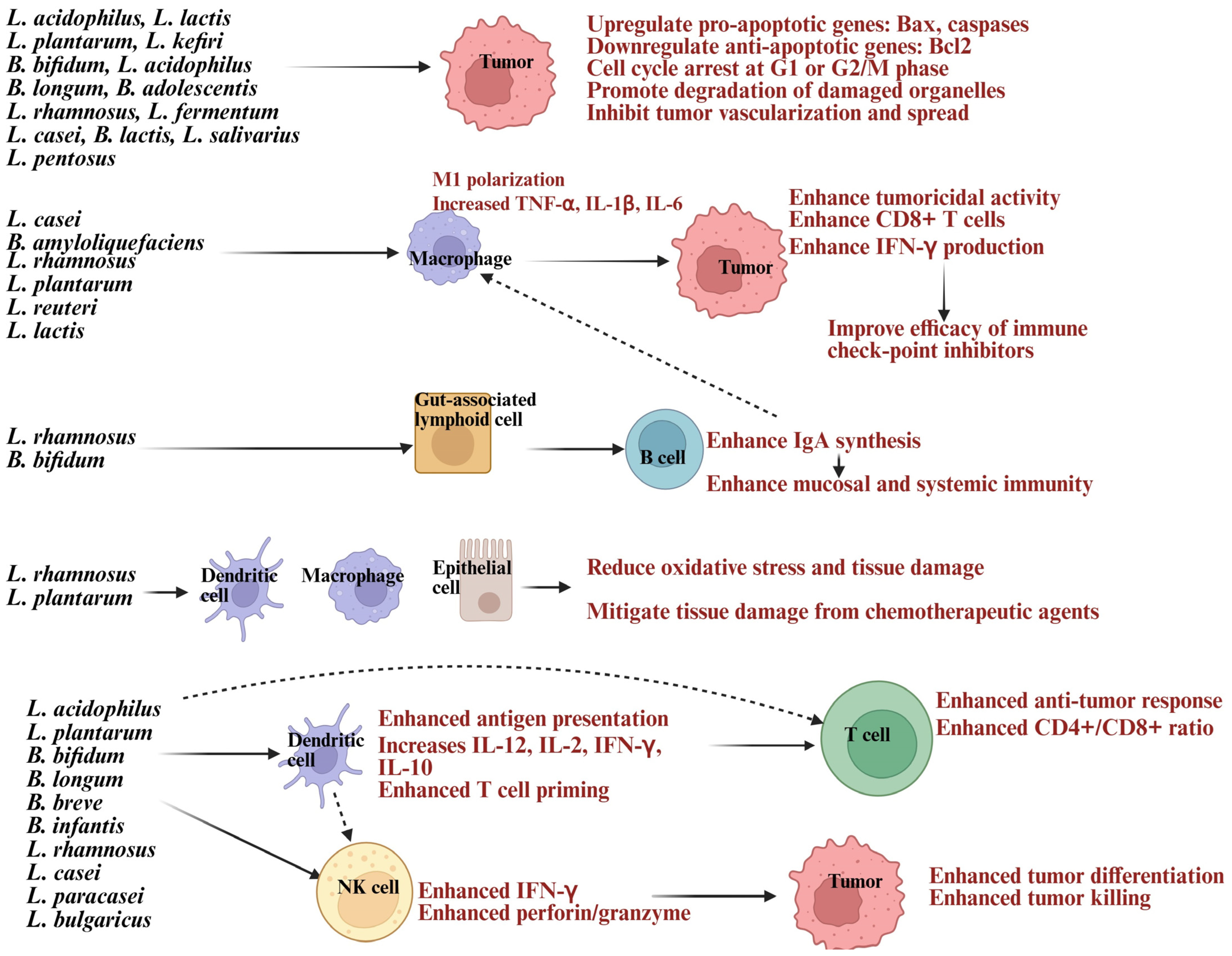
Cancer remains the leading cause of death worldwide, with cancer-related fatalities expected to increase [20,21,22]. There is a pressing demand for treatments that are both effective and have minimal side effects [23]. Current therapies often come with challenges like side effects, drug resistance, and affordability issues, which can affect quality of life [24]. Interestingly, studies show that probiotics may play a role in cancer prevention and work as complementary treatments [25]. Probiotics can prevent cancer by boosting immune function and enhancing the effectiveness of therapies. Even inactive probiotics and their byproducts provide similar benefits, making them a promising option for cancer prevention and treatment [26]. Among the probiotics being studied, this review focuses on Lactobacillus and Bifidobacterium strains as illustrated in Figure 1. Lactobacillus and Bifidobacterium species are recognized for their anti-cancer properties, achieved through direct toxicity to tumor cells and indirect effects on host physiology [27,28].
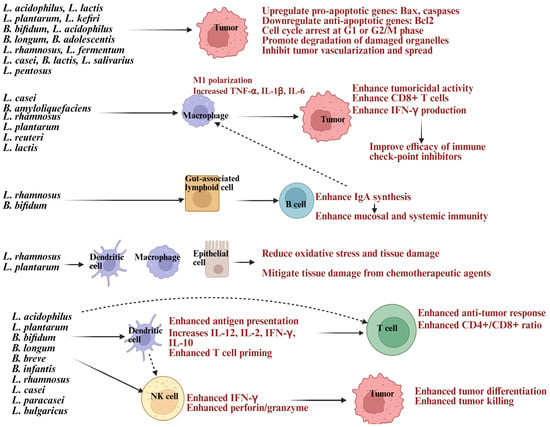
Figure 1.
Illustration showing Lactobacillus and Bifidobacterium strains inducing direct apoptosis in tumor or modulating immune cells to enhance anti-cancer activity. Created in BioRender. Kaur, K. (2025) https://BioRender.com/a2qdrnd (accessed on 3 October 2025).
Lactobacillus, a prominent group of probiotic lactic acid bacteria (LAB), promotes gut health by inhibiting harmful pathogens like Fusobacterium nucleatum, Escherichia coli, and Clostridium difficile, which are linked to colorectal and other cancers [29,30]. Bifidobacterium combats cancer by inducing apoptosis, modulating oncogenic signaling pathways, activating the immune system, altering metabolism, and colonizing tumor hypoxic regions [31,32]. It also serves as a therapeutic agent and delivery vector for drugs, genes, and nanomaterials, offering a comprehensive approach to cancer prevention and treatment [33]. These probiotics induce apoptosis, growth cycle arrest, inhibit tumor vascularization, and metastasis [34]. Lactobacillus and Bifidobacterium produce antimicrobial substances, organic acids, bacteriocins, hydrogen peroxide, and short-chain fatty acids (SCFAs) like butyrate, acetate, and propionate, which reduce carcinogen-producing bacteria or trigger cancer cell apoptosis [35]. SCFAs regulate tumor suppressor genes and silence oncogenes through histone acetylation and methylation [36]. Exopolysaccharides (EPS) from Lactobacillus plantarum and Lactobacillus acidophilus activate TLR2/MyD88 pathways, promoting Fas-mediated apoptosis. They suppress tumor growth by inhibiting cyclin D1, causing G0/G1 phase arrest, and reducing cell proliferation [37,38]. EPS also activates pattern recognition receptors like TLR2, encouraging apoptosis, autophagy, and immune responses [11,37]. Bacteriocins and peptides damage tumor cell membranes, inducing apoptosis [39]. Probiotics induce cancer cell apoptosis by increasing pro-apoptotic proteins like Bax and BAD, decreasing anti-apoptotic proteins like Bcl-2, and activating caspase cascades, leading to deoxyribonucleic acid (DNA) fragmentation [40,41]. Lactobacillus and Bifidobacterium induced reduction in pro-angiogenic factors, further suppressing tumor growth, adding to on therapeutic value [42,43].
Lactobacillus and Bifidobacterium have anti-cancer properties by modulating the immune system. They enhance immune responses and tumor immunity by interacting with dendritic cells, macrophages, natural killer (NK) cells, and neutrophils, boosting their function and cytokine production [32]. They enhance the cytotoxic activity of innate immune cells like NK cells and CD8+ T cells, activate antigen-presenting cells such as DCs, and regulate cytokines by increasing interferon-gamma (IFN-γ) and IL-2 while reducing immunosuppressive IL-10 and TGF-β [44,45]. DCs are activated through CpG-rich bacterial DNA to promote Th1 responses via IL-12 signaling and IFN-γ production [44,45]. This leads to the upregulation of co-stimulatory molecules on antigen-presenting cells and the release of cytokines to manage inflammation and recruit immune cells, balancing pro- and anti-inflammatory pathways [19,46,47,48,49,50,51] (Figure 1). These probiotics influence macrophages, DCs, and intestinal epithelial cells, lowering the activity of inducible nitric oxide synthase (iNOS), which produces nitric oxide (NO) during inflammation [13,52]. By reducing iNOS levels, probiotics help prevent excessive NO production, minimizing oxidative stress and tissue damage [13]. They lower pro-inflammatory cytokines like TNF-α, IL-6, and IL-1β while increasing anti-inflammatory cytokines such as IL-10, maintaining balance to control chronic inflammation, strengthening the gut barrier, and regulating immune responses throughout the body [13,52]. In individuals consuming Bifidobacterium, DCs showed increased expression of anti-tumor immunity genes, boosting T cell activation [53]. These bacteria activate macrophages and aid in cancer prevention and treatment by influencing IgA production, stimulating macrophage activity, and reducing the toxicity of anti-cancer therapies [44,45,54]. These probiotics also promote macrophage polarization to an M1 anti-tumor phenotype, enhancing phagocytosis and cytokine-driven tumor suppression [44,45,54] (Figure 1).
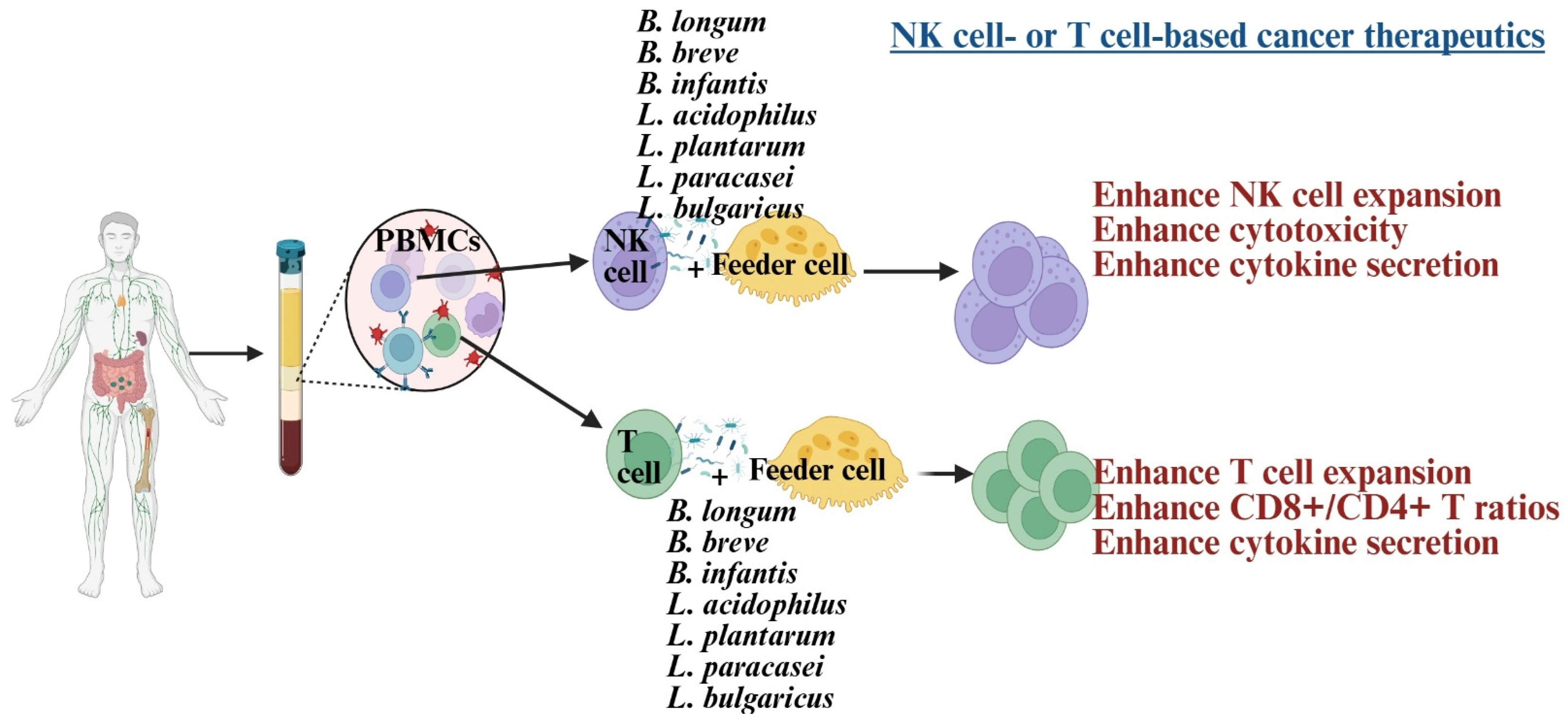
NK cells and CD8+ T cells play a crucial role in cancer therapy, serving as the basis of many current treatments [32,55,56]. Dysfunction of these cells is linked to poorer outcomes in cancer patients [57]. In such patients, NK cells exhibit reduced cytotoxicity, IFN-γ secretion, survival, and expansion, associated with lower expression of CD16, NKG2D, and the CD3 zeta chain (CD3z) [58,59,60,61]. Similarly, CD8+ T cells show decreased IFN-γ secretion and reduced expression of CD62L, CD28, CCR7, and CD127 [62]. Probiotic bacteria have been explored to develop NK or CD8+ T cell-based immunotherapies, significantly enhancing these cells’ functions [58,60,62,63] (Figure 2). Combining probiotics with feeder cells has proven more effective for NK cell therapy than using feeder cells alone [58,62,64]. These expanded NK cells were more effective at inducing tumor killing and differentiation in both in vivo and in vitro studies [59,65]. The efficacy test of expanded T cells is still under investigation in tumor-bearing humanized mice.
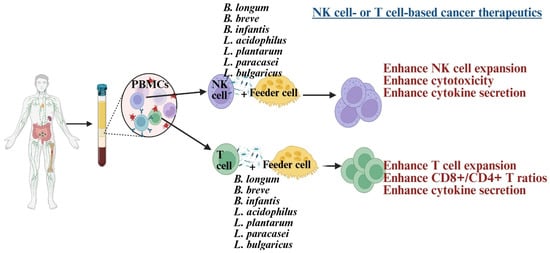
Figure 2.
Illustration showing Lactobacillus and Bifidobacterium strains, when combined with feeder cells, induce cell expansion and enhance anti-cancer activity in NK cells and T cells. Created in BioRender. Kaur, K. (2025) https://BioRender.com/1smaneg (accessed on 3 October 2025).
Consuming Lactobacillus acidophilus is linked to increased serum levels of IFN-γ, IL-10, and higher counts of CD4+ and CD8+ T cells [66,67,68]. Oral administration of B. longum and B. breve to melanoma-bearing mice significantly reduced tumor volume, showing effects comparable to programmed death protein-1 (PD-1) therapy alone. When combined with PD-1 therapy, these probiotics further reduced tumor size [53]. Lactobacillus and Bifidobacterium probiotic-fed humanized mice showed improved NK-mediated cytotoxicity, increased IFN-γ secretion across tissues, reduced tumor load, and restored cancer-induced bone defects [59,60,65]. In mice with breast tumors, oral Lactobacillus acidophilus administration lowered tumor burden and influenced cytokine production [69]. Bifidobacterium longum, Lactobacillus acidophilus, and Lactobacillus plantarum have shown promise in preventing and inhibiting cancers such as colon, breast, liver, intestines, lungs, oral cavity, and pancreas [35,51,66,67,68,70,71,72,73,74,75,76,77,78].
These findings emphasize the potential of probiotics in cancer treatment, demonstrating their ability to modulate immune responses for therapeutic benefits [75,76,79]. When combined with chemotherapy and radiotherapy, probiotics enhance drug accumulation in tumors and reduce systemic side effects like diarrhea, fever, and disruptions to intestinal microbiota in cancer patients. These benefits improve patients’ quality of life and help prevent interruptions or halts in treatment [80]. Additionally, engineered Bifidobacterium can deliver drugs, cytokines, anti-angiogenic genes (such as endostatin or tumstatin), or prodrug-converting enzymes (like cytosine deaminase for converting 5-FC to 5-FU) directly to tumors, increasing local impact while minimizing systemic toxicity. They may also enhance focused ultrasound and imaging-based cancer therapies for precise targeting.
Lactobacillus and Bifidobacterium show potential in cancer care by improving gut microbiota, triggering cancer cell death, and boosting immune responses. However, their benefits face hurdles like strain-specific effects, individual variability, low survival rates, risks for immunocompromised individuals, formulation challenges, limited clinical evidence, and regulatory concerns, such as antibiotic resistance [32,81,82]. These probiotics may interact with other treatments, with effectiveness varying by individual and cancer type. Standardizing doses and formulations is crucial for safety and consistency [32,81,82]. Their anti-cancer effects are strain-dependent, influencing apoptosis, immune responses, and tumor environments, though some strains, like Bifidobacterium dentium, could be harmful [83]. Mechanisms include inducing tumor cell death and modulating the immune system, but the molecular pathways and tumor-specific impacts remain unclear [31]. Factors like dysbiosis, age, diet, genetics, gut microbiota, and health affect colonization and immune response [32,81,82]. Long-term colonization relies on adhesion mechanisms like exopolysaccharides or biofilms, supported by prebiotics. Probiotics face challenges from stomach acid, bile, and digestion, but encapsulation methods like alginate or chitosan enhance survival with strain-specific adjustments [32,81,82]. Effective doses depend on the strain and therapeutic goals, ranging from immune support to anti-proliferative effects [32,81,82]. Future advancements like strain engineering, nanoencapsulation, synbiotics, and personalized microbiome analysis aim to address these challenges, making probiotics a more viable option in cancer care and prevention [84].
3. Mechanism of Action, Application, and Challenges of Utilizing Lactobacillus and Bifidobacterium to Restore Immune Function in Older Individuals
Aging brings about immunosenescence, characterized by a decline in both innate and adaptive immune functions, increased systemic inflammation, and alterations in the composition and diversity of gut microbiota [85,86]. It significantly affects innate immunity, causing changes in the number, characteristics, and functionality of immune cells, which are closely linked to various diseases and infections [87,88,89,90]. Additionally, cytokine levels, especially IFN-γ, are notably lower in the immune cells of older individuals [91]. These changes increase susceptibility to infections, chronic diseases, and reduced vaccine effectiveness. In older adults, diminished immune function leads to higher risks of infections, cancer, autoimmune disorders, Alzheimer’s disease, atherosclerosis, vision problems like age-related macular degeneration, cardiovascular issues, coronary heart disease, liver fibrosis, neurodegenerative diseases, and exposure to pathogens [90,92,93]. The gut microbiota is essential for immune regulation, with aging-related dysbiosis playing a major role in immune dysfunction [94,95].
NK cells are vital for fighting age-related illnesses and activating adaptive immune cells, ensuring a robust immune system in old age [87,91]. NK cells are thought to significantly influence longevity, as their proper function protects the elderly from infections, cancer, autoimmune disorders, and neurodegenerative diseases [87,90,91]. Aging leads to decreased NK cell function, contributing to age-related health issues [90]. Additionally, NK cell proliferation declines in the elderly. Research by Guo et al. found that the pro-inflammatory CD52+ NK cell subset increases in older adults, facilitating infection spread [96]. Feeder cells, which support NK cell activation and growth, also lose effectiveness in older individuals due to reduced ligand expression and factor secretion [91]. Probiotics can restore gut microbial balance, enhance innate immunity, such as NK cells, and regulate inflammation [4,13,97]. Probiotic supplements have been found to moderately improve immune markers and boost NK cell function, an essential part of innate immunity, in healthy older adults [13]. Studies show that probiotics significantly enhance NK cell cytotoxic activity in individuals aged 60 and above, helping with early infection defense and tumor surveillance [98]. They also lower chronic low-grade inflammation, prevent infections, and promote healthy aging [98].
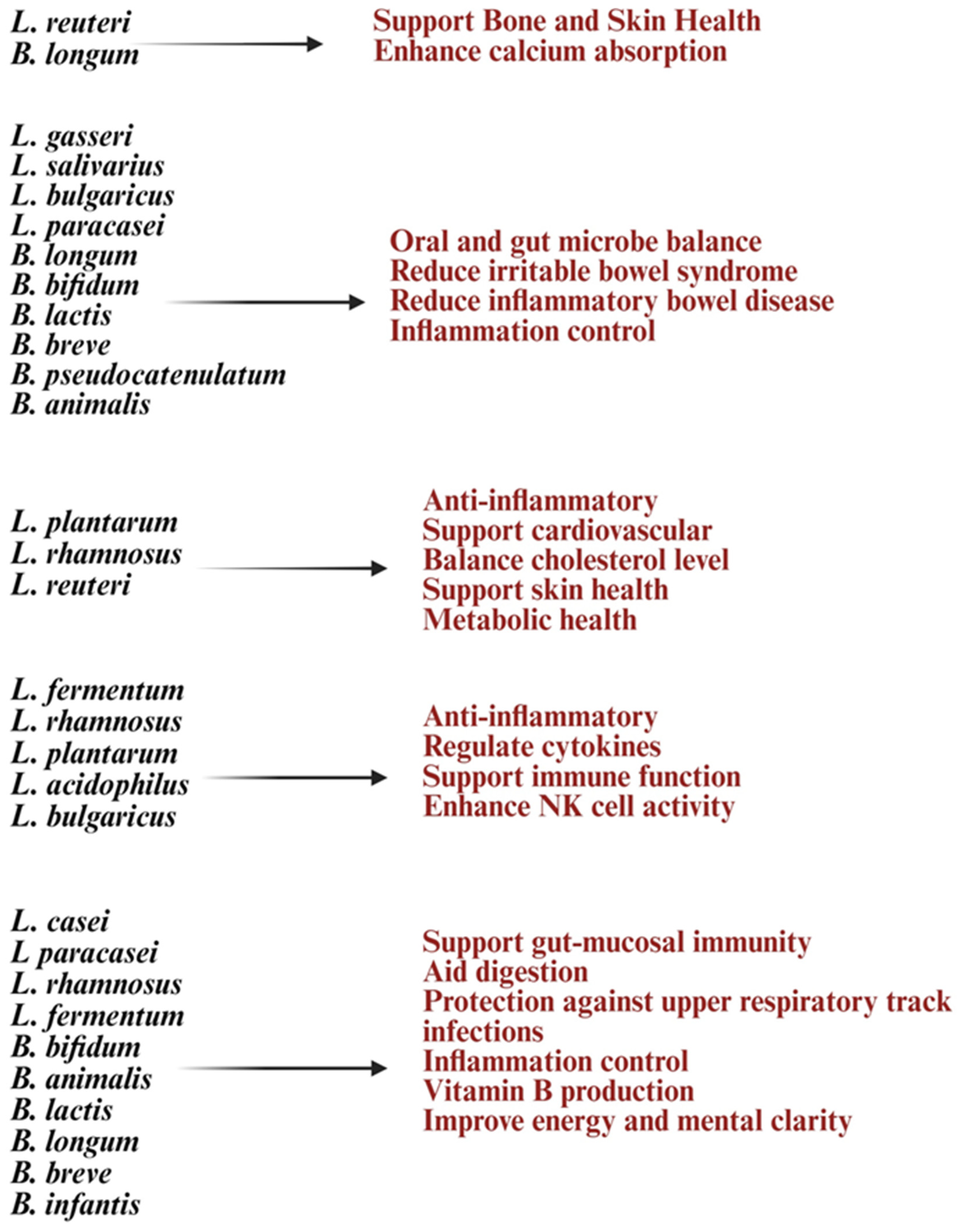
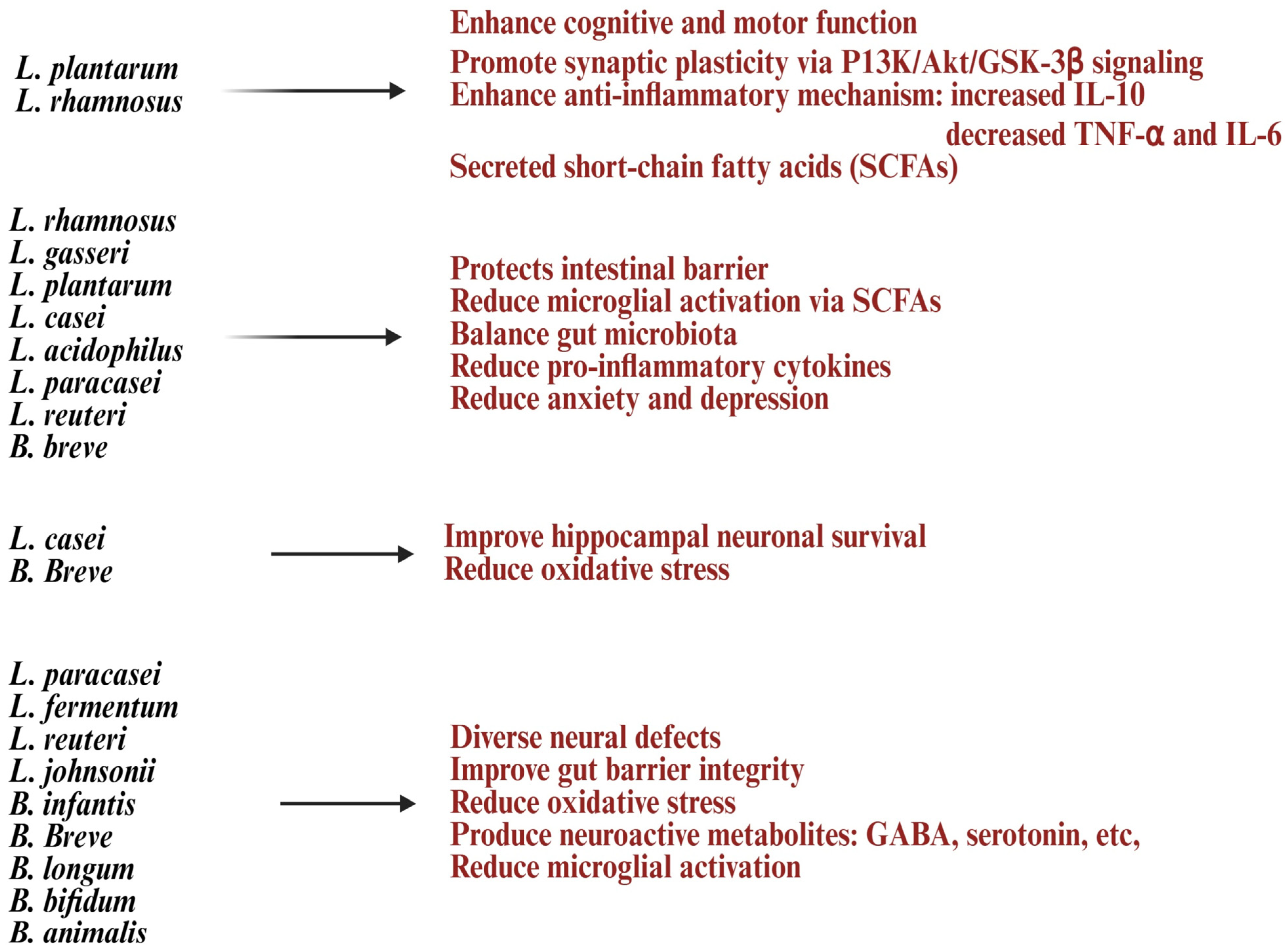
Early studies suggest that strains like Lactobacillus plantarum, Lactobacillus rhamnosus, Bifidobacterium longum, and Lactobacillus helveticus may help improve aging processes and strengthen immunity [27]. Lactobacillus and Bifidobacterium increase anti-inflammatory cytokines like IL-10 and TGF-β while reducing pro-inflammatory markers such as IL-6 and CRP, combating chronic inflammation [8,99]. These treatments can activate T and B cells, improve immune monitoring, reduce the occurrence and severity of colds and gut infections, and strengthen vaccine responses [8,99]. Also, in vitro treatment with Lactobacillus and Bifidobacterium probiotics, alone or with feeder cells, has restored NK cell numbers and function in the elderly [91] (Figure 2). Lactobacillus acidophilus helps balance gut bacteria and boost immunity during illness or antibiotic use [9]. Lactobacillus fermentum strengthens the immune system and helps prevent gastrointestinal and respiratory infections [30,100]. Lactobacillus casei and Lactobacillus paracasei have anti-inflammatory properties, support the gut barrier, and aid in immune modulation [101]. Lactobacillus plantarum promotes immune response and reduces gut inflammation [102]. Lactobacillus rhamnosus enhances gut health, strengthens immune defenses, and eases digestive discomfort. Bifidobacterium longum reduces inflammation, protects against intestinal infections, and supports immune balance [103]. Bifidobacterium bifidum improves digestive health and boosts the immune system. Bifidobacterium lactis helps prevent infections and supports vitamins such as vitamin B and vitamin K production [83]. These vitamins are vital for metabolism and immune health. Probiotic formulations can be single-strain or multi-strain combinations, often including both Lactobacillus and Bifidobacterium species for synergistic benefits (Figure 3). Probiotics are generally safe for healthy elderly individuals [104].
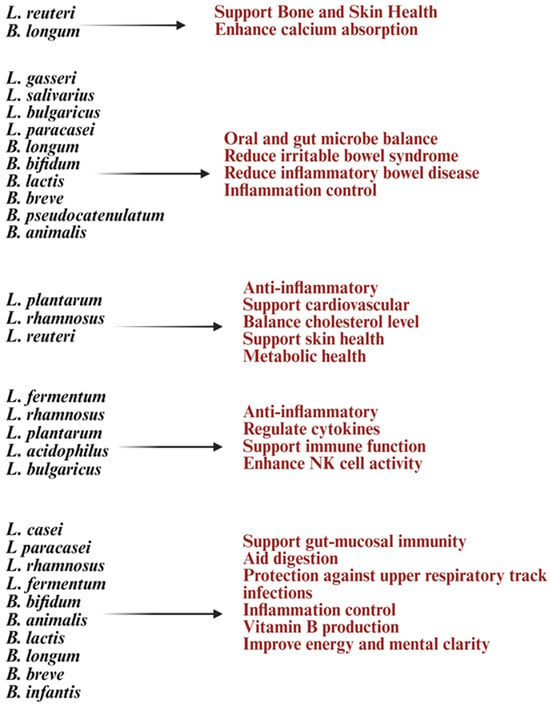
Figure 3.
Illustration list of Lactobacillus and Bifidobacterium strains and their health benefits at old age. Created in BioRender. Kaur, K. (2025) https://BioRender.com/08dx43n (accessed on 3 October 2025).
Lactobacillus and Bifidobacterium improve antioxidant capacity in the gut and circulation, regulate microbial balance to maintain homeostasis, and may counteract age-related dysbiosis and illness [105,106]. Strains like Lactobacillus plantarum and Lactobacillus reuteri are linked to reduced oxidative stress and neuroinflammation [107]. They also support cognitive health and neuroprotection by modulating the gut–brain axis, benefiting memory, mood, and learning [108,109]. These probiotics influence neurotransmitters such as gamma-aminobutyric acid (GABA), serotonin, and dopamine, which help delay age-related cognitive decline and affect mood, stress, and cognition [110,111,112]. SCFAs and microbial metabolites reduce neuroinflammation, potentially slowing cognitive decline, while also improving lipid metabolism, lowering cholesterol absorption, and preventing age-related metabolic issues [113,114,115]. Lactobacillus and Bifidobacterium’s anti-inflammatory effects indirectly promote bone density and muscle function by enhancing nutrient absorption and reducing tissue breakdown [65,116,117]. Supplementing Lactobacillus and Bifidobacterium in older adults can ease conditions like constipation, irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and antibiotic-associated diarrhea while maintaining microbial diversity, which often decreases with age [118,119].
Lactobacillus and Bifidobacterium are well-known probiotics that may support healthy aging by improving gut microbiota, boosting immune responses, producing beneficial metabolites like SCFAs, and potentially managing inflammation and oxidative stress linked to aging [27]. However, using these probiotics in older adults poses challenges. Benefits depend on the strain, as their effects on immunity, gut health, and metabolism vary, making it hard to target aging-specific issues [120]. Their precise impact on aging markers like telomere shortening, mitochondrial dysfunction, or epigenetic changes remains unclear, as most studies are preclinical [121,122,123,124]. Colonization is often hindered by stomach acid, bile salts, and oxygen exposure, especially for anaerobic strains like Bifidobacterium [125]. Factors such as pH and temperature affect traits like surface hydrophobicity and mucosal adhesion, and many strains require continuous supplementation to stay in the gut [126]. Aging-related immune decline, gut microbiota shifts, chronic conditions, medications, and antibiotics further reduce their effectiveness [127]. Delivering enough viable bacteria is challenging, as stability varies in products like fermented foods, powders, and capsules, with some methods reducing bacterial viability during storage or digestion [128]. Multi-strain formulations require careful balancing due to interactions that may affect efficacy or metabolism [128]. Clinical evidence is limited; trials using Lactobacillus rhamnosus and Bifidobacterium animalis did not significantly reduce antibiotic use or improve systemic immunity in older adults [129]. Many studies are short-term, underpowered, or involve diverse elderly populations, making conclusions difficult. The obligate anaerobic nature of Bifidobacterium adds to its culturing challenges [130]. Despite this, Lactobacillus and Bifidobacterium show great potential for promoting healthy aging.
4. Mechanism of Action, Application, and Challenges of Utilizing Lactobacillus and Bifidobacterium in Autoimmune Disease Therapy
Autoimmune diseases occur when the immune system becomes overactive and mistakenly attacks the body’s own tissues [131]. Studies show that gut microbiota dysbiosis, an imbalance in the intestinal microbial community, is connected to the onset and progression of autoimmune conditions such as Amyotrophic lateral sclerosis (ALS), type 1 diabetes, rheumatoid arthritis, lupus, and multiple sclerosis [131]. Probiotics offer a promising way to manage gut microbiota, restoring balance by promoting beneficial microbes and outcompeting harmful ones [132]. Research in animals and humans indicates probiotics can delay or prevent autoimmune diabetes in non-obese diabetic (NOD) mice, lower inflammatory cytokines, and reduce joint damage in rheumatoid arthritis [104,133]. They also improve gut microbiota composition in conditions like systemic lupus and multiple sclerosis. However, results vary based on strain, disease, and study design [134,135]. Various probiotic strains hold promise for supporting patients with autoimmune diseases through their differentiation effects [136]. Probiotic formulations may involve single-strain or multispecies blends tailored to specific autoimmune conditions [137]. Creating probiotic formulations for autoimmune disease treatment involves selecting strains with known immunomodulatory benefits, ensuring they survive and thrive in the right locations, and tailoring them to the host and microbiome’s specific needs [138,139]. Next-generation probiotics (NGPs), developed through sequencing and bioinformatics, leverage synthetic biology and gene editing for targeted therapeutic applications [140]. These formulations often blend strains like Lactobacillus and Bifidobacterium, sometimes enhanced with technologies like nano-encapsulation or genetic engineering to improve their impact. While promising, current research indicates that probiotics are most effective as supplementary therapies rather than standalone treatments until more clinical studies are completed [138].
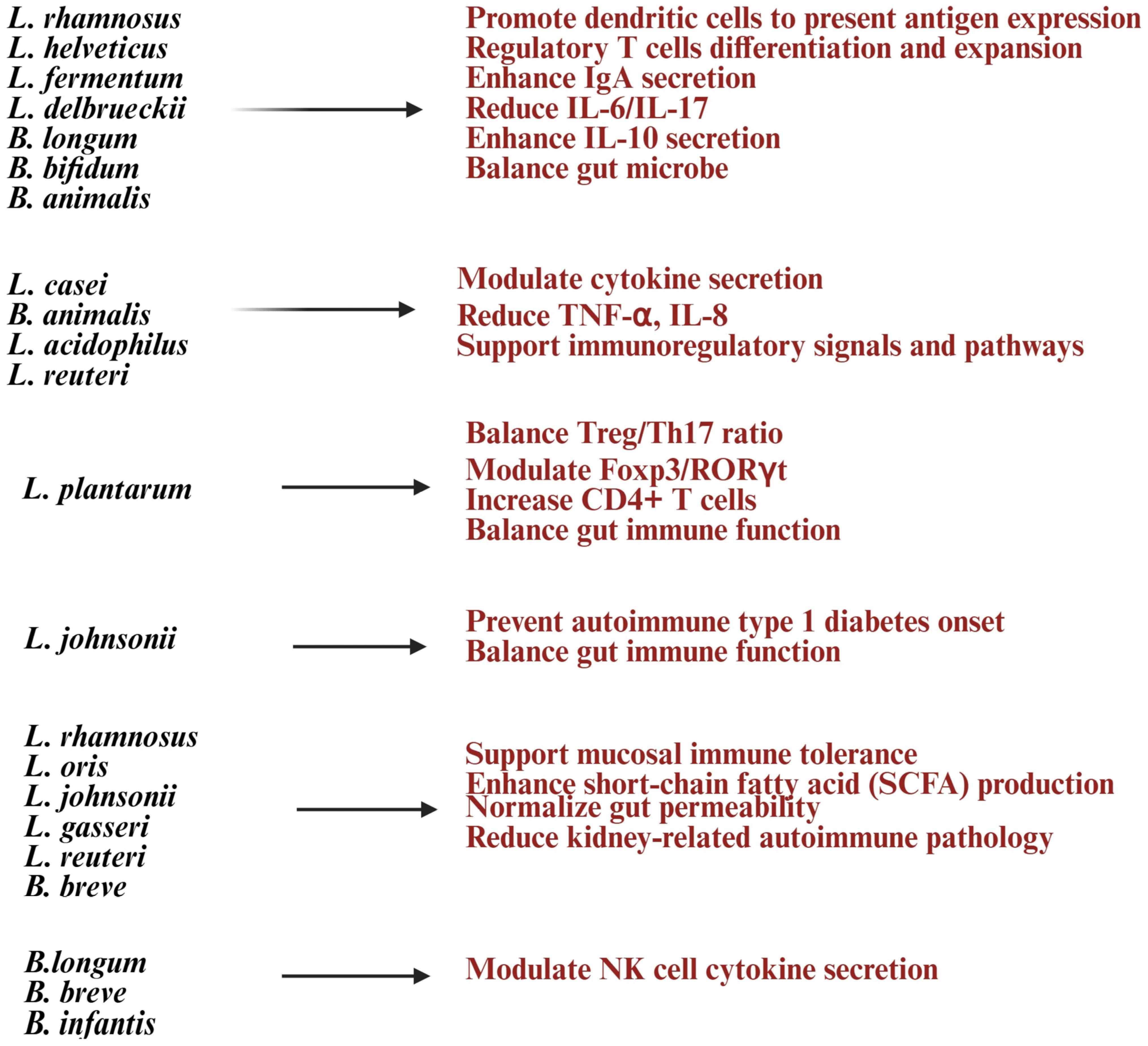
Lactobacillus and Bifidobacterium help strengthen the gut barrier by improving tight junctions, reducing gut permeability (“leaky gut”), and lowering exposure to inflammatory triggers [141,142] (Figure 4). Lactobacillus species such as Lactobacillus casei, Lactobacillus rhamnosus, Lactobacillus acidophilus, and Lactobacillus reuteri are known for their immunomodulatory and barrier-strengthening properties [27]. Lactobacillus and Bifidobacterium probiotics play a significant role in immune regulation by influencing T and B lymphocyte activity [13]. They promote Treg expansion, enhance mucosal IgA production, and balance T-helper cells by increasing anti-inflammatory Tregs while reducing Th17/Th1 inflammation. These probiotics modulate cytokines, boosting IL-10 and TGF-β while reducing TNF-α and IL-6, to maintain a balance between pro- and anti-inflammatory responses [8,16,143]. SCFAs like butyrate, acetate, and propionate support Treg differentiation, suppress pro-inflammatory Th17 cells, and alter gene expression in immune cells through histone acetylation [8]. By fostering Treg expansion and anti-inflammatory cytokines, these probiotics may improve immune tolerance and reduce autoimmune conditions like rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease [99]. Bifidobacterium species such as Bifidobacterium bifidum and Bifidobacterium animalis support Treg induction and help lower pro-inflammatory responses [144]. Molecules from Lactobacillus (like lipoteichoic acid and peptidoglycan) and Bifidobacterium (like surface proteins and polysaccharides) interact with DCs to aid their maturation and antigen presentation, maintaining immune tolerance [145,146]. NK cell function is crucial for infection control and disease progression, and dysfunction in NK cells can impact the entire immune system [147,148]. Studies have shown that probiotic formulations with specific Gram-positive bacterial strains can enhance IL-10 levels in peripheral blood mononuclear cells (PBMCs) and NK cells, providing benefits for autoimmune diseases [149].
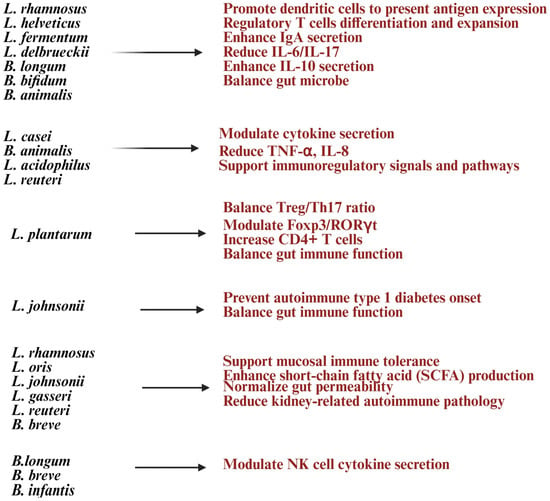
Figure 4.
List of Lactobacillus and Bifidobacterium strains and their health benefits in autoimmune diseases. Created in https://BioRender.com (accessed on 3 October 2025).
Clinical trials suggest that multi-strain probiotics containing Lactobacillus and Bifidobacterium can enhance gut barrier function, lower systemic inflammation, and improve tolerance to dietary antigens, offering relief for conditions like IBS and gut-related autoimmune disorders [117,150,151] (Figure 4). Research shows their anti-inflammatory effects and immune modulation may also help ease joint inflammation in rheumatoid arthritis (RA) [8]. These probiotics can work alongside immunosuppressive drugs, reduce side effects, and support immune balance during treatment [136]. They achieve these benefits by modulating gut bacteria, producing helpful metabolites, strengthening the gut lining, and interacting with immune cells [8,136] (Figure 4). This makes them promising, strain-specific options for restoring immune tolerance and managing chronic inflammation in autoimmune diseases.
Using Lactobacillus and Bifidobacterium to treat autoimmune diseases comes with several challenges [136]. While Lactobacillus is known for its health benefits, its role in autoimmune conditions is still unclear, reflecting the complexity of using probiotics for such treatments. These lactic acid bacteria have immunomodulatory properties and help balance gut microbiota, showing potential for conditions like type 1 diabetes, rheumatoid arthritis, lupus, Graves’ disease, and inflammatory bowel disease [152]. However, their effectiveness depends on the host’s individual background. Safety concerns, such as antibiotic resistance gene transfer, and the mechanisms of action, require thorough investigation [153,154]. Lactobacillus reuteri is beneficial for healthy individuals but might worsen autoimmune issues in predisposed hosts [136]. Host genetics, like HLA types, play a role in whether probiotics improve or exacerbate autoimmune responses, adding to the unpredictability [155]. Environmental factors like diet, antibiotics, stress, and geography further influence probiotic effectiveness by impacting gut microbiota and barrier integrity [156]. Additionally, a leaky gut in autoimmune-prone individuals raises the risk of probiotics escaping the gut [157]. Rare infections linked to some Lactobacillus strains (e.g., Lactobacillus rhamnosus, Lactobacillus paracasei) and Bifidobacterium species (e.g., Bifidobacterium longum, Bifidobacterium breve) include bacteremia, endocarditis, urinary tract infections, and neonatal sepsis in immunocompromised individuals [158,159,160]. While animal and in vitro models show promise, especially with pathways like JAK/STAT and NF-κB, human immune interactions remain complex and not fully understood [161]. Probiotics and their metabolites may also struggle to reach effective concentrations at target tissues due to metabolic competition, mucosal barriers, or rapid clearance, although techniques like microencapsulation and nano-delivery could help address these issues [162]. Autoimmune patients need personalized approaches since genetic, microbiome, and environmental differences make one-size-fits-all solutions ineffective [163]. The variability in probiotic dosage, strain formulations, and combinations makes it hard to replicate findings across studies [162].
5. Mechanism of Action, Application, and Challenges of Utilizing Lactobacillus and Bifidobacterium in Neurodegenerative Disorders
Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, Amyotrophic lateral sclerosis (ALS), and Huntington’s are progressive conditions with complex origins, including protein buildup, oxidative stress, neuroinflammation, mitochondrial dysfunction, and immune system issues [164,165]. The gut microbiome significantly influences these diseases via the microbiota–gut–brain axis, impacting neuroinflammation, gut barrier integrity, immune responses, and neurotransmitter production [166,167,168]. Dysbiosis, or an imbalance in gut bacteria, is often observed in these disorders [138,169]. It includes an increased presence of pro-inflammatory strains like Streptococcus, Alistipes, Ruminococcus, Enterococcus, and Desulfovibrio, alongside a reduction in beneficial butyrate-producing bacteria such as Faecalibacterium, Lachnospira, Roseburia, Blautia, and Prevotella [138,169]. Probiotics provide therapeutic benefits by replenishing beneficial bacteria, boosting neuroprotective metabolites like butyrate, and offering anti-inflammatory and antioxidant effects [97,166].
Lactobacillus and Bifidobacterium are well-known probiotics that play a key role in the gut–brain axis (GBA), a complex system connecting the gastrointestinal (GI) tract, immune system, and central nervous system (CNS) through neural, hormonal, and immune pathways [13] (Figure 5). Lactobacillus and Bifidobacterium also influence neurotrophic factors and neurotransmitter pathways like brain-derived neurotrophic factor (BDNF), serotonin, and gamma-aminobutyric acid (GABA), and maintain microglial balance [108,170,171]. They help reduce pro-inflammatory pathways, enhance vagus nerve signaling for better brain communication and emotional regulation, and combat oxidative stress with antioxidant defenses, which is crucial for neurodegenerative disorders [172,173]. Furthermore, they may prevent the accumulation of harmful protein aggregates [4]. These probiotics support the intestinal barrier by boosting mucin production, tight-junction proteins like occludin and claudin-1, and reducing intestinal permeability, which helps block neuroinflammatory mediators from entering the bloodstream [174]. This protective barrier limits exposure to bacterial lipopolysaccharides (LPS) and pro-inflammatory cytokines, which can trigger CNS inflammation [174,175]. Lactobacillus and Bifidobacterium secreted compounds like SCFAs, bacteriocins, and hydrogen peroxide that lower gut pH, inhibit harmful bacteria, and indirectly support CNS health by reducing systemic inflammation [174,176]. By interacting with DCs and T cells, these probiotics encourage regulatory T cell (Treg) differentiation, balance Th1/Th2 immune responses, increase anti-inflammatory cytokines like IL-10 and secretory IgA, and reduce pro-inflammatory cytokines (e.g., IL-1β, IL-18, TNF-α) in the hippocampus and other brain regions [177,178]. These actions help regulate CNS microglial activity, preventing neuronal damage caused by chronic inflammation [177] (Figure 5).
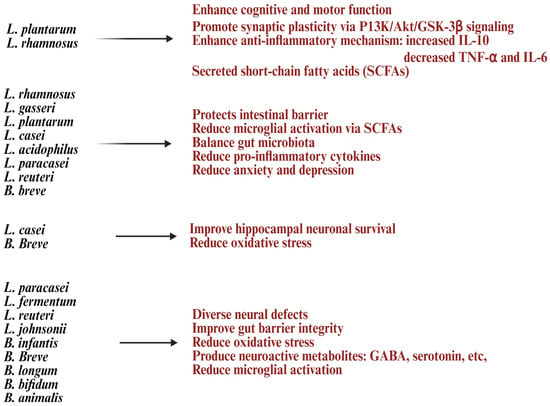
Figure 5.
List of Lactobacillus and Bifidobacterium strains and their health benefits in neurodegenerative disorders. Created in https://BioRender.com (accessed on 3 October 2025).
Combining probiotic strains like Lactobacillus and Bifidobacterium species can work together to improve motor and cognitive symptoms in Parkinson’s disease models [151,179]. Taking Lactobacillus casei and Bifidobacterium breve orally has been shown to enhance short-term memory by improving learning performance and antioxidant capacity in the demyelinated corpus callosum, highlighting a potential therapeutic approach for multiple sclerosis and other neurodegenerative disorders [180]. These strains increase levels of α-Klotho, Sirtuin1, HO-1, and Nrf2 (these pathways play a crucial role in neuroprotection) while reducing pro-inflammatory genes like IL-1β and IL-18 in the hippocampus. They also boost BDNF expression, protect hippocampal neurons, and reduce neurodegeneration [180]. They also show potential in treating depression, with both clinical and preclinical studies providing encouraging results [181]. Probiotics offer neuroprotective benefits by strengthening the gut barrier, regulating immunity, producing neuroactive compounds, and lowering oxidative stress. Multi-strain combinations are particularly effective, making them a promising addition to treatments for neurodegeneration and cognitive decline. Probiotic formulations should aim to balance the gut microbiome, increase butyrate-producing bacteria, and reduce inflammation.
Lactobacillus and Bifidobacterium are gaining attention as potential treatments for neurodegenerative disorders like Alzheimer’s, Parkinson’s, Huntington’s, multiple sclerosis, and ALS due to their effects on the microbiota–gut–brain axis. They can reduce neuroinflammation, produce neuroactive compounds like GABA and serotonin, and enhance neuronal resilience. However, challenges remain. Not all strains provide neuroprotection; for instance, Lactobacillus casei and Bifidobacterium breve perform well together in aging mouse models, but monotherapy might be less effective [182,183]. Variations in metabolic activity and neurotransmitter production make standardizing treatments difficult. Oral probiotics face survival issues in the gastrointestinal tract due to stomach acid, bile salts, and enzymes. Gut microbiota composition and colonization capacity also vary, impacting outcomes. Factors like biofilm formation and gut lining adherence, unique to each strain, influence persistence and efficacy. Advanced delivery methods like microencapsulation improve stability but face hurdles like polymer compatibility, storage, and regulatory challenges. Manufacturing, storage, and transport conditions also affect effectiveness. Personalized responses based on diet, age, genetics, medications, and diseases add complexity, as some individuals may see no benefit. While generally considered safe, risks exist. Future efforts should focus on personalized regimens, advanced targeting systems like nano-armor probiotics, and larger clinical trials.
6. Clinical Outcomes That Align with the Findings from the Preclinical Studies
Early clinical trials confirm preclinical findings, showing that Lactobacillus and Bifidobacterium strains restore intestinal microbiota diversity disrupted by chemotherapy or radiotherapy, reducing gastrointestinal issues like diarrhea and mucositis [184,185]. These probiotics enhance the immune system, with strains like Lactobacillus rhamnosus and Bifidobacterium boosting markers such as NK cell activity and anti-inflammatory cytokines [116]. In older adults, they support intestinal health, reduce systemic low-grade inflammation through beneficial fermentation, and increase SCFA production [186,187]. For autoimmune conditions like ulcerative colitis and rheumatoid arthritis, probiotics help modulate microbiome-immune interactions, shifting the Th17/Treg balance toward regulatory phenotypes and lowering inflammation markers [188]. They also improve bloating and stool regularity in autoimmune gut disorders, influence microbiota-derived metabolites, and reduce inflammatory markers linked to neuroinflammation [189]. Small clinical studies consistently report improved MMSE scores and reduced depression/anxiety symptoms [190].
7. Research Gaps Contributing to Inconsistencies Between Clinical Outcomes and the Findings from the Preclinical Studies
Research on probiotics like Lactobacillus and Bifidobacterium reveals significant gaps between animal studies and human trials due to biological differences, study designs, and translational challenges [27,191]. Preclinical studies indicate that Lactobacillus may support gut barrier protection, immune system modulation, and metabolism. Human trials partially back these findings, with results varying by strain, host, and environment [27,191,192]. Animal models like rodent colitis or germ-free mice provide insights into mechanisms but do not account for human genetic diversity, microbiota differences, or diet-related interactions [192,193] (Table 1). Preclinical studies often use probiotic dosages and durations impractical for humans [194]. This suggests mechanisms like barrier reinforcement, SCFA signaling, and immune modulation, but rarely predict the scale or variability of human responses [195]. Human outcomes are typically less pronounced, more variable, and highly context-dependent compared to preclinical findings [104,190]. Translational outcomes depend on factors like baseline microbiota, genetics (e.g., FUT2 genotype), diet, co-administered therapies, dosage, and treatment duration. Strains like Lactobacillus plantarum, Lactobacillus rhamnosus, and Lactobacillus reuteri show promise in mice, but human trials often produce inconsistent results due to factors like dosage, age, and maternal supplementation timing [196]. For instance, Lactobacillus rhamnosus reduces allergy and eczema in mice, but human trials yield mixed results [197,198]. Similarly, Lactobacillus plantarum improves lipid profiles in rodents but only modestly reduces cholesterol (5–10%) in humans, with significant variability [199]. While Lactobacillus enhances tight junctions and reduces endotoxemia in mice, its effects in humans are minimal, with biomarkers like zonulin showing inconsistent responses [200,201]. Compounds deemed safe in mice may still cause gastrointestinal or immune reactions in humans, underscoring the need for cautious translation [201].

Table 1.
Factors influencing differences in preclinical and clinical results of Lactobacillus and Bifidobacterium applications.
Research gaps include the lack of standardized outcome measures, like patient-reported versus biomarker-based, and core outcome sets (COS) across diseases, which complicate meta-analyses and evidence synthesis [202]. Strain-specific effects are often inconsistently reported, with many studies relying on combination preparations or poorly characterized formulations [139]. The mechanisms connecting probiotic use to systemic immunomodulation, anti-cancer effects, and neuroprotection remain unclear [13]. More investigation is needed into the gut–brain and gut-immune axes, especially in long-term disease models [13]. Clinical trials frequently lack large-scale, multicenter randomized clinical trials with extended follow-ups, and variability in patient populations and baseline microbiota reduces reproducibility [203]. Subgroup analyses by age, disease type, diet, and genetics are also limited. There is a lack of regulatory guidance on dosing, duration, and formulation, while safety monitoring in immunocompromised individuals and the frail elderly is underexplored. Additionally, limited studies have looked at how probiotics interact—whether synergistically or antagonistically—with chemotherapy, immunotherapies, or anti-inflammatory drugs. We have listed the most common factors interfering with consistent outcomes for probiotic use in humans, which do not align with preclinical outcomes (Table 1).
To maximize the benefits of Lactobacillus and Bifidobacterium, combining mechanistic data with well-structured, stratified clinical trials is key [190,204]. Focusing on disease-specific outcomes for probiotics will standardize reporting. Large placebo-controlled trials should account for baseline microbiota, age, and comorbidities. Advanced methods like metagenomics and metabolomics can reveal pathways, while cohort studies should link probiotics to outcomes and quality of life, particularly in cancer, aging, autoimmune, and neurodegenerative conditions. Future research should adopt precision nutrition strategies, factoring in baseline microbiota, genetics, and diet to refine probiotics [190,205]. Longitudinal randomized clinical trials stratified by microbiota, genetics, and clinical subtypes are essential [206]. Multi-omics tools can connect mechanistic insights to outcomes, while postbiotics and metabolites might act as biomarkers bridging preclinical and clinical data [207]. Ensuring safety and optimizing formulations are crucial for vulnerable groups like the elderly or immunocompromised.
8. Conclusions
Lactobacillus and Bifidobacterium probiotics are well-known for their benefits on gut health, with recent studies showcasing their broader role in health and disease management. They help restore immune function in aging and conditions like cancer, autoimmune, and neurodegenerative disorders. As an adjuvant cancer therapy, they support immune balance, balance gut microbiota, and improve intestinal function, which results in minimizing the adverse effects of chemotherapy, radiotherapy, and checkpoint inhibitors. Research indicates these probiotics activate and expand anti-cancer immune cells like NK and CD8+ T cells, enhancing their cytotoxic activity, IFN-γ secretion, and recruitment to tumors. However, challenges include strain-specific effects, infection risks, inconsistent metabolite production, and lack of standardized clinical protocols. Future research should focus on personalized microbiome profiling, optimized strains and dosages, and innovative delivery systems like microencapsulation. Combining probiotics with genetic engineering or metabolite supplementation could lead to safer, more effective treatments, but thorough clinical trials are essential. Advancing these techniques and understanding molecular mechanisms is key to unlocking probiotics’ therapeutic potential and improving health outcomes.
Funding
This research received no external funding.
Conflicts of Interest
The author declares that the work reviewed in this article was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TLR | Toll-like receptor |
| Tregs | Regulatory T cells |
| Th | T helper |
| IL | Interleukin |
| TGF-β | Transforming growth factor-beta |
| IgA | Immunoglobulin A |
| DCs | Dendritic cells |
| SCFA | Short-chain fatty acids |
| MyD88 | Myeloid differentiation primary response 88 |
| DNA | Deoxyribonucleic acid |
| NK | Natural killer |
| CD8 | Cluster of differentiation 8 |
| IFN-γ | Interferon-gamma |
| iNOS | Inducible nitric oxide synthase |
| NO | Nitric oxide |
| NKG2D | Natural killer group 2, member D |
| CCR7 | C-C chemokine receptor type 7 |
| PD-1 | Programmed death protein-1 |
| CRP | C-reactive protein |
| GABA | Gamma-aminobutyric acid |
| IBS | Irritable bowel syndrome |
| IBD | Inflammatory bowel disease |
| RA | Rheumatoid arthritis |
| ALS | Amyotrophic lateral sclerosis |
| GBA | Gut–brain axis |
| CNS | Central nervous system |
| BDNF | Brain-derived neurotrophic factor |
References
- Metchnikoff, E. Essais Optimistes; A. Maloine: Paris, France, 1907; p. iii. 438p. [Google Scholar]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Hemati, M.; Nejad, Z.R.; Arabkari, V.; Namdar, A. Importance of probiotics in the prevention and treatment of colorectal cancer. J. Cell Physiol. 2019, 234, 17127–17143. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Modulation of gut health using probiotics: The role of probiotic effector molecules. J. Future Foods 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Nogueira, D.S.; de Oliveira, L.M.; Amorim, C.C.O.; Gazzinelli-Guimarães, A.C.; Barbosa, F.S.; Oliveira, F.M.S.; Kraemer, L.; Mattos, M.; Cardoso, M.S.; Resende, N.M.; et al. Eosinophils mediate SIgA production triggered by TLR2 and TLR4 to control Ascaris suum infection in mice. PLoS Pathog. 2021, 17, e1010067. [Google Scholar] [CrossRef]
- Dikiy, S.; Rudensky, A.Y. Principles of regulatory T cell function. Immunity 2023, 56, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Lee, C.-G.; Cha, K.H.; Kim, G.-C.; Im, S.-H.; Kwon, H.-K. Exploring probiotic effector molecules and their mode of action in gut–immune interactions. FEMS Microbiol. Rev. 2023, 47, fuad046. [Google Scholar] [CrossRef] [PubMed]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Yu, L.; Zhao, J.; Tian, F.; Chen, W.; Zhai, Q. Capsular polysaccarides of probiotics and their immunomodulatory roles. Food Sci. Hum. Wellness 2022, 11, 1111–1120. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.F.; Yu, W.R.; Sun, S.Y.; Lei, Y.M.; Li, Y.X.; Lu, C.X.; Zhai, J.N.; Bai, F.R.; Ren, F.; et al. Roles of Probiotics, Prebiotics, and Postbiotics in B-Cell-Mediated Immune Regulation. J. Nutr. 2025, 155, 37–51. [Google Scholar] [CrossRef]
- Abbaszadeh, S.H.; Hosseini, S.R.A.; Mahmoodpoor, A.; Yousefi, M.; Lotfi-Dizaji, L.; Mameghani, M.E. Investigating the Role of Probiotics in Modulating T Cells and the Immune Response: A Systematic Review. Indian J. Microbiol. 2024. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Saito, S.; Cao, D.-Y.; Maekawa, T.; Tsuji, N.M.; Okuno, A. Lactococcus lactis subsp. cremoris C60 Upregulates Macrophage Function by Modifying Metabolic Preference in Enhanced Anti-Tumor Immunity. Cancers 2024, 16, 1928. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Okuno, A.; Peng, Z.; Cao, D.Y.; Tsuji, N.M. Probiotic lactic acid bacteria promote anti-tumor immunity through enhanced major histocompatibility complex class I-restricted antigen presentation machinery in dendritic cells. Front. Immunol. 2024, 15, 1335975. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Xia, F.; Lin, R. Global burden of cancer and associated risk factors in 204 countries and territories, 1980–2021: A systematic analysis for the GBD 2021. J. Hematol. Oncol. 2024, 17, 119. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Smith, D.P. Global cancer burden: Progress, projections, and challenges. Lancet 2025, 406, 1536–1537. [Google Scholar] [CrossRef]
- Bowie, K. Cancer deaths expected to reach 18 million by 2050, major study forecasts. BMJ 2025, 390, r2022. [Google Scholar] [CrossRef]
- Force, L.M.; Kocarnik, J.M.; May, M.L.; Bhangdia, K.; Crist, A.; Penberthy, L.; Pritchett, N.; Acheson, A.; Deitesfeld, L.; A, B.; et al. The global, regional, and national burden of cancer, 1990–2023, with forecasts to 2050: A systematic analysis for the Global Burden of Disease Study 2023. Lancet 2025, 406, 1565–1586. [Google Scholar] [CrossRef]
- Calvert, M.; Wilson, R.; Peipert, J.D. Symptoms, side effects, quality of life and financial toxicity matter to patients with cancer, we need to do a better job of capturing and reporting these concepts in trials and clinical care. BMJ Oncol. 2025, 4, e000879. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, M.; Xia, X.; Liang, J.; Yin, X.; Ju, Q.; Hao, J. Effect of dietary probiotics intake on cancer mortality: A cohort study of NHANES 1999–2018. Sci. Rep. 2025, 15, 959. [Google Scholar] [CrossRef] [PubMed]
- Pyo, Y.; Kwon, K.H.; Jung, Y.J. Probiotic Functions in Fermented Foods: Anti-Viral, Immunomodulatory, and Anti-Cancer Benefits. Foods 2024, 13, 2386. [Google Scholar] [CrossRef]
- Shah, A.B.; Baiseitova, A.; Zahoor, M.; Ahmad, I.; Ikram, M.; Bakhsh, A.; Shah, M.A.; Ali, I.; Idress, M.; Ullah, R.; et al. Probiotic significance of Lactobacillus strains: A comprehensive review on health impacts, research gaps, and future prospects. Gut Microbes 2024, 16, 2431643. [Google Scholar] [CrossRef] [PubMed]
- Faghfoori, Z.; Faghfoori, M.H.; Saber, A.; Izadi, A.; Yari Khosroushahi, A. Anticancer effects of bifidobacteria on colon cancer cell lines. Cancer Cell Int. 2021, 21, 258. [Google Scholar] [CrossRef]
- Aburjaile, F.F.; de Jesus, L.C.L.; da Silva, T.F.; Drumond, M.M.; de Oliveira Carvalho, R.D.; Azevedo, V.; Mancha-Agresti, P.D.C. Chapter 12—Lactic acid bacteria in gut microbiota, probiotics and disease prevention. In Lactic Acid Bacteria in Food Biotechnology; Ray, R.C., Paramithiotis, S., de Carvalho Azevedo, V.A., Montet, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 207–219. [Google Scholar]
- Dempsey, E.; Corr, S.C. Lactobacillus spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Pei, B.; Peng, S.; Huang, C.; Zhou, F. Bifidobacterium modulation of tumor immunotherapy and its mechanism. Cancer Immunol. Immunother. 2024, 73, 94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Gajjar, D.; Seshadri, S. Understanding the role of gut microfloral bifidobacterium in cancer and its potential therapeutic applications. Microbiome Res. Rep. 2024, 3, 3. [Google Scholar] [CrossRef]
- Amonkar, R.; Uy, A.A.; Ramirez, P.; Patel, H.; Jeong, J.J.; Shoyele, N.O.; Vaghela, V.; Lakshmikuttyamma, A. Engineered Bifidobacterium Strains Colonization at Tumor Sites: A Novel Approach to the Delivery of Cancer Treatments. Cancers 2025, 17, 2487. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef]
- Kahouli, I.; Malhotra, M.; Westfall, S.; Alaoui-Jamali, M.A.; Prakash, S. Design and validation of an orally administrated active L. fermentum-L. acidophilus probiotic formulation using colorectal cancer ApcMin/+mouse model. Appl. Microbiol. Biotechnol. 2017, 101, 1999–2019. [Google Scholar] [CrossRef]
- Hou, M.; Yu, Q.Q.; Yang, L.; Zhao, H.; Jiang, P.; Qin, L.; Zhang, Q. The role of short-chain fatty acid metabolism in the pathogenesis, diagnosis and treatment of cancer. Front. Oncol. 2024, 14, 1451045. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Z. A functional and genetic overview of exopolysaccharides produced by Lactobacillus plantarum. J. Funct. Foods 2018, 47, 229–240. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Z.; Wang, Y.; Zeng, H.; Wang, B. Proteomics and metabolomics elucidate the biosynthetic pathway of acid stress-induced exopolysaccharides and its impact on growth phenotypes in Lactiplantibacillus plantarum HMX2. Food Chem. 2025, 476, 143431. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Sun, T.; Xu, J. Bacteriocins in Cancer Treatment: Mechanisms and Clinical Potentials. Biomolecules 2024, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Thoda, C.; Touraki, M. Molecular Mechanisms of Probiotic Action Against Gastrointestinal Cancers. Int. J. Mol. Sci. 2025, 26, 7857. [Google Scholar] [CrossRef]
- Sankarapandian, V.; Venmathi Maran, B.A.; Rajendran, R.L.; Jogalekar, M.P.; Gurunagarajan, S.; Krishnamoorthy, R.; Gangadaran, P.; Ahn, B.C. An Update on the Effectiveness of Probiotics in the Prevention and Treatment of Cancer. Life 2022, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Yi, T.; Li, H.; Tang, X.; Liu, D.; Wu, D.; Li, Y. Decoding tumor angiogenesis: Pathways, mechanisms, and future directions in anti-cancer strategies. Biomark. Res. 2025, 13, 62. [Google Scholar] [CrossRef]
- Liu, Z.-L.; Chen, H.-H.; Zheng, L.-L.; Sun, L.-P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Poggi, A.; Benelli, R.; Venè, R.; Costa, D.; Ferrari, N.; Tosetti, F.; Zocchi, M.R. Human Gut-Associated Natural Killer Cells in Health and Disease. Front. Immunol. 2019, 10, 961. [Google Scholar] [CrossRef]
- Aziz, N.; Bonavida, B. Activation of Natural Killer Cells by Probiotics. Immunopathol. Dis. Ther. 2016, 7, 41–55. [Google Scholar] [CrossRef]
- Saleena, L.A.K.; Teo, M.Y.M.; How, Y.H.; In, L.L.A.; Pui, L.P. Immunomodulatory action of Lactococcus lactis. J. Biosci. Bioeng. 2023, 135, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oerlemans, M.M.P.; Akkerman, R.; Ferrari, M.; Walvoort, M.T.C.; de Vos, P. Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health. J. Funct. Foods 2021, 76, 104289. [Google Scholar] [CrossRef]
- Górska, A.; Przystupski, D.; Niemczura, M.J.; Kulbacka, J. Probiotic Bacteria: A Promising Tool in Cancer Prevention and Therapy. Curr. Microbiol. 2019, 76, 939–949. [Google Scholar] [CrossRef]
- Tarrah, A.; de Castilhos, J.; Rossi, R.C.; Duarte, V.d.S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In vitro Probiotic Potential and Anti-cancer Activity of Newly Isolated Folate-Producing Streptococcus thermophilus Strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef]
- Jacouton, E.; Torres Maravilla, E.; Boucard, A.S.; Pouderous, N.; Pessoa Vilela, A.P.; Naas, I.; Chain, F.; Azevedo, V.; Langella, P.; Bermúdez-Humarán, L.G. Anti-tumoral Effects of Recombinant Lactococcus lactis Strain Secreting IL-17A Cytokine. Front. Microbiol. 2018, 9, 3355. [Google Scholar] [CrossRef]
- Zaharuddin, L.; Mokhtar, N.M.; Muhammad Nawawi, K.N.; Raja Ali, R.A. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Vallejos, O.P.; Bueno, S.M.; Kalergis, A.M. Probiotics in inflammatory bowel disease: Microbial modulation and therapeutic prospects. Trends Mol. Med. 2025, 31, 731–742. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Bui, V.T.; Tseng, H.C.; Kozlowska, A.; Maung, P.O.; Kaur, K.; Topchyan, P.; Jewett, A. Augmented IFN-gamma and TNF-alpha Induced by Probiotic Bacteria in NK Cells Mediate Differentiation of Stem-Like Tumors Leading to Inhibition of Tumor Growth and Reduction in Inflammatory Cytokine Release; Regulation by IL-10. Front. Immunol. 2015, 6, 576. [Google Scholar] [CrossRef]
- Rosenberg, J.; Huang, J. CD8(+) T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Dunai, C.; Collins, C.P.; Barao, I.; Murphy, W.J. Chapter 1—NK cells and CD8 T cells in cancer immunotherapy: Similar functions by different mechanisms. In Successes and Challenges of NK Immunotherapy; Bonavida, B., Jewett, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–31. [Google Scholar]
- Viel, S.; Vivier, E.; Walzer, T.; Marçais, A. Targeting metabolic dysfunction of CD8 T cells and natural killer cells in cancer. Nat. Rev. Drug Discov. 2025, 24, 190–208. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Cook, J.; Park, S.H.; Topchyan, P.; Kozlowska, A.; Ohanian, N.; Fang, C.; Nishimura, I.; Jewett, A. Novel Strategy to Expand Super-Charged NK Cells with Significant Potential to Lyse and Differentiate Cancer Stem Cells: Differences in NK Expansion and Function between Healthy and Cancer Patients. Front. Immunol. 2017, 8, 297. [Google Scholar] [CrossRef]
- Kaur, K.; Kozlowska, A.K.; Topchyan, P.; Ko, M.W.; Ohanian, N.; Chiang, J.; Cook, J.; Maung, P.O.; Park, S.H.; Cacalano, N.; et al. Probiotic-Treated Super-Charged NK Cells Efficiently Clear Poorly Differentiated Pancreatic Tumors in Hu-BLT Mice. Cancers 2019, 12, 63. [Google Scholar] [CrossRef]
- Kaur, K.; Topchyan, P.; Kozlowska, A.K.; Ohanian, N.; Chiang, J.; Maung, P.O.; Park, S.H.; Ko, M.W.; Fang, C.; Nishimura, I.; et al. Super-charged NK cells inhibit growth and progression of stem-like/poorly differentiated oral tumors in vivo in humanized BLT mice; effect on tumor differentiation and response to chemotherapeutic drugs. Oncoimmunology 2018, 7, e1426518. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cobo, S.; Pieper, N.; Campos-Silva, C.; Garcia-Cuesta, E.M.; Reyburn, H.T.; Paschen, A.; Vales-Gomez, M. Impaired NK cell recognition of vemurafenib-treated melanoma cells is overcome by simultaneous application of histone deacetylase inhibitors. Oncoimmunology 2018, 7, e1392426. [Google Scholar] [CrossRef]
- Kaur, K.; Ko, M.-W.; Ohanian, N.; Cook, J.; Jewett, A. Osteoclast-expanded super-charged NK-cells preferentially select and expand CD8+ T cells. Sci. Rep. 2020, 10, 20363. [Google Scholar] [CrossRef]
- Leivas, A.; Perez-Martinez, A.; Blanchard, M.J.; Martin-Clavero, E.; Fernandez, L.; Lahuerta, J.J.; Martinez-Lopez, J. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. Oncoimmunology 2016, 5, e1250051. [Google Scholar] [CrossRef]
- Kaur, K.; Topchyan, P.; Jewett, A. Supercharged Natural Killer (sNK) Cells Inhibit Melanoma Tumor Progression and Restore Endogenous NK Cell Function in Humanized BLT Mice. Cancers 2025, 17, 2430. [Google Scholar] [CrossRef]
- Kaur, K.; Reese, P.; Chiang, J.; Jewett, A. Natural Killer Cell Therapy Combined with Probiotic Bacteria Supplementation Restores Bone Integrity in Cancer by Promoting IFN-γ Production. Cells 2025, 14, 1347. [Google Scholar] [CrossRef]
- Agah, S.; Alizadeh, A.M.; Mosavi, M.; Ranji, P.; Khavari-Daneshvar, H.; Ghasemian, F.; Bahmani, S.; Tavassoli, A. More Protection of Lactobacillus acidophilus Than Bifidobacterium bifidum Probiotics on Azoxymethane-Induced Mouse Colon Cancer. Probiotics Antimicrob. Proteins 2019, 11, 857–864. [Google Scholar] [CrossRef]
- Gui, Q.F.; Lu, H.F.; Zhang, C.X.; Xu, Z.R.; Yang, Y.H. Well-balanced commensal microbiota contributes to anti-cancer response in a lung cancer mouse model. Genet. Mol. Res. 2015, 14, 5642–5651. [Google Scholar] [CrossRef]
- Urbanska, A.M.; Bhathena, J.; Cherif, S.; Prakash, S. Orally delivered microencapsulated probiotic formulation favorably impacts polyp formation in APC (Min/+) model of intestinal carcinogenesis. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Imani Fooladi, A.A.; Yazdi, M.H.; Pourmand, M.R.; Mirshafiey, A.; Hassan, Z.M.; Azizi, T.; Mahdavi, M.; Soltan Dallal, M.M. Th1 Cytokine Production Induced by Lactobacillus acidophilus in BALB/c Mice Bearing Transplanted Breast Tumor. Jundishapur J. Microbiol. 2015, 8, e17354. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Suzutani, T. Intake of Bifidobacterium longum and Fructo-oligosaccharides prevents Colorectal Carcinogenesis. Euroasian J. Hepatogastroenterol. 2018, 8, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, C.A.; Gamal-Eldeen, A.M.; El-Hussieny, E.A.; Raafat, B.M.; Mehanna, N.S.; Talaat, R.M.; Shaaban, M.T. Bifidobacterium longum Suppresses Murine Colorectal Cancer through the Modulation of oncomiRs and Tumor Suppressor miRNAs. Nutr. Cancer 2019, 71, 688–700. [Google Scholar] [CrossRef]
- Kuugbee, E.D.; Shang, X.; Gamallat, Y.; Bamba, D.; Awadasseid, A.; Suliman, M.A.; Zang, S.; Ma, Y.; Chiwala, G.; Xin, Y.; et al. Structural Change in Microbiota by a Probiotic Cocktail Enhances the Gut Barrier and Reduces Cancer via TLR2 Signaling in a Rat Model of Colon Cancer. Dig. Dis. Sci. 2016, 61, 2908–2920. [Google Scholar] [CrossRef]
- Walia, S.; Kamal, R.; Kanwar, S.S.; Dhawan, D.K. Cyclooxygenase as a target in chemoprevention by probiotics during 1,2-dimethylhydrazine induced colon carcinogenesis in rats. Nutr. Cancer 2015, 67, 603–611. [Google Scholar] [CrossRef]
- Mendes, M.C.S.; Paulino, D.S.; Brambilla, S.R.; Camargo, J.A.; Persinoti, G.F.; Carvalheira, J.B.C. Microbiota modification by probiotic supplementation reduces colitis associated colon cancer in mice. World J. Gastroenterol. 2018, 24, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, C.; Ye, L.; Yang, W.; Huang, H.; Meng, F.; Shi, S.; Ding, Z. Anti-tumour immune effect of oral administration of Lactobacillus plantarum to CT26 tumour-bearing mice. J. Biosci. 2015, 40, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kassayová, M.; Bobrov, N.; Strojný, L.; Orendáš, P.; Demečková, V.; Jendželovský, R.; Kubatka, P.; Kisková, T.; Kružliak, P.; Adamkov, M.; et al. Anticancer and Immunomodulatory Effects of Lactobacillus plantarum LS/07, Inulin and Melatonin in NMU-induced Rat Model of Breast Cancer. Anticancer. Res. 2016, 36, 2719–2728. [Google Scholar]
- Wan Mohd Kamaluddin, W.N.F.; Rismayuddin, N.A.R.; Ismail, A.F.; Mohamad Aidid, E.; Othman, N.; Mohamad, N.A.H.; Arzmi, M.H. Probiotic inhibits oral carcinogenesis: A systematic review and meta-analysis. Arch. Oral Biol. 2020, 118, 104855. [Google Scholar] [CrossRef]
- Kita, A.; Fujiya, M.; Konishi, H.; Tanaka, H.; Kashima, S.; Iwama, T.; Ijiri, M.; Murakami, Y.; Takauji, S.; Goto, T.; et al. Probiotic-derived ferrichrome inhibits the growth of refractory pancreatic cancer cells. Int. J. Oncol. 2020, 57, 721–732. [Google Scholar] [CrossRef]
- Shin, R.; Itoh, Y.; Kataoka, M.; Iino-Miura, S.; Miura, R.; Mizutani, T.; Fujisawa, T. Anti-tumor activity of heat-killed Lactobacillus plantarum BF-LP284 on Meth-A tumor cells in BALB/c mice. Int. J. Food Sci. Nutr. 2016, 67, 641–649. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yao, N.; Wei, K.K.; Jiang, L.; Hanif, S.; Wang, Z.X.; Pei, C.X. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016, 70, 1246–1253. [Google Scholar] [CrossRef]
- Schlienger de Alba, B.N.; Espinosa Andrews, H. Benefits and Challenges of Encapsulating Bifidobacterium Probiotic Strains with Bifidogenic Prebiotics. Probiotics Antimicrob. Proteins 2024, 16, 1790–1800. [Google Scholar] [CrossRef]
- Mejía-Caballero, A.; Salas-Villagrán, V.A.; Jiménez-Serna, A.; Farrés, A. Challenges in the production and use of probiotics as therapeuticals in cancer treatment or prevention. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab052. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Cantabrana, C.; Delgado, S.; Ruiz, L.; Ruas-Madiedo, P.; Sánchez, B.; Margolles, A. Bifidobacteria and Their Health-Promoting Effects. Microbiol. Spectr. 2017, 5, 73–98. [Google Scholar] [CrossRef]
- Xie, A.; Gao, M.; Du, H.; Pan, X. Next-generation probiotics delivery: Innovations and applications of single-cell encapsulation. Curr. Opin. Food Sci. 2025, 61, 101234. [Google Scholar] [CrossRef]
- Goyani, P.; Christodoulou, R.; Vassiliou, E. Immunosenescence: Aging and Immune System Decline. Vaccines 2024, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.T.; Cho, K.A. Targeting immunosenescence and inflammaging: Advancing longevity research. Exp. Mol. Med. 2025, 57, 1881–1892. [Google Scholar] [CrossRef]
- Gounder, S.S.; Abdullah, B.J.J.; Radzuanb, N.; Zain, F.; Sait, N.B.M.; Chua, C.; Subramani, B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal. Cell. Pathol. 2018, 2018, 7871814. [Google Scholar] [CrossRef]
- Qi, C.; Liu, Q. Natural killer cells in aging and age-related diseases. Neurobiol. Dis. 2023, 183, 106156. [Google Scholar] [CrossRef]
- Brauning, A.; Rae, M.; Zhu, G.; Fulton, E.; Admasu, T.D.; Stolzing, A.; Sharma, A. Aging of the Immune System: Focus on Natural Killer Cells Phenotype and Functions. Cells 2022, 11, 1017. [Google Scholar] [CrossRef]
- Fasbender, F.; Widera, A.; Hengstler, J.G.; Watzl, C. Natural Killer Cells and Liver Fibrosis. Front. Immunol. 2016, 7, 19. [Google Scholar] [CrossRef]
- Kaur, K.; Jewett, A. Decreased surface receptors, function, and suboptimal osteoclasts-induced cell expansion in natural killer (NK) cells of elderly subjects. Aging 2025, 17, 798–821. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef] [PubMed]
- Bosco, N.; Noti, M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021, 22, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wu, M.; Huang, B.; Zhao, R.; Jin, L.; Fu, B.; Wang, P.; Wang, D.; Zheng, M.; Fang, J.; et al. Single-cell transcriptomics reveal a unique memory-like NK cell subset that accumulates with ageing and correlates with disease severity in COVID-19. Genome Med. 2022, 14, 46. [Google Scholar] [CrossRef]
- Mafe, A.N.; Edo, G.I.; Majeed, O.S.; Gaaz, T.S.; Akpoghelie, P.O.; Isoje, E.F.; Igbuku, U.A.; Owheruo, J.O.; Opiti, R.A.; Garba, Y.; et al. A review on probiotics and dietary bioactives: Insights on metabolic well-being, gut microbiota, and inflammatory responses. Food Chem. Adv. 2025, 6, 100919. [Google Scholar] [CrossRef]
- Boichuk, S.; Galembikova, A.; Vollmer, D. Enhancement of NK Cell Cytotoxic Activity and Immunoregulatory Effects of a Natural Product Supplement Across a Wide Age Span: A 30-Day In Vivo Human Study. Int. J. Mol. Sci. 2025, 26, 2897. [Google Scholar] [CrossRef]
- Virk, M.S.; Virk, M.A.; He, Y.; Tufail, T.; Gul, M.; Qayum, A.; Rehman, A.; Rashid, A.; Ekumah, J.-N.; Han, X.; et al. The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs. Nutrients 2024, 16, 546. [Google Scholar] [CrossRef]
- Zhao, Y.; Hong, K.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Lactobacillus fermentum and its potential immunomodulatory properties. J. Funct. Foods 2019, 56, 21–32. [Google Scholar] [CrossRef]
- Qin, D.; Ma, Y.; Wang, Y.; Hou, X.; Yu, L. Contribution of Lactobacilli on Intestinal Mucosal Barrier and Diseases: Perspectives and Challenges of Lactobacillus casei. Life 2022, 12, 1910. [Google Scholar] [CrossRef]
- Zeng, Z.; Huang, Z.; Yue, W.; Nawaz, S.; Chen, X.; Liu, J. Lactobacillus plantarum modulate gut microbiota and intestinal immunity in cyclophosphamide-treated mice model. Biomed. Pharmacother. 2023, 169, 115812. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhao, Z.; Wang, W.; Liu, X. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. J. Immunol. Res. 2021, 2021, 8030297. [Google Scholar] [CrossRef]
- Maftei, N.M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Q.; Sun, J.; Wu, X.; Song, Y.; Xu, Y.; Nie, H.; Huang, J.; Mu, G. Probiotic potential of lactic acid bacteria with antioxidant properties in modulating health: Mechanisms, applications, and future directions. Food Biosci. 2025, 66, 106181. [Google Scholar] [CrossRef]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Dell’Anno, M.; Giromini, C.; Reggi, S.; Cavalleri, M.; Moscatelli, A.; Onelli, E.; Rebucci, R.; Sundaram, T.S.; Coranelli, S.; Spalletta, A.; et al. Evaluation of Adhesive Characteristics of L. plantarum and L. reuteri Isolated from Weaned Piglets. Microorganisms 2021, 9, 1587. [Google Scholar] [CrossRef]
- Kumar, A.; Sivamaruthi, B.S.; Dey, S.; Kumar, Y.; Malviya, R.; Prajapati, B.G.; Chaiyasut, C. Probiotics as modulators of gut-brain axis for cognitive development. Front. Pharmacol. 2024, 15, 1348297. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Moloney, G.M.; Keane, L.; Clarke, G.; Cryan, J.F. The gut microbiota-immune-brain axis: Therapeutic implications. Cell Rep. Med. 2025, 6, 101982. [Google Scholar] [CrossRef]
- Ansari, F.; Neshat, M.; Pourjafar, H.; Jafari, S.M.; Samakkhah, S.A.; Mirzakhani, E. The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 2023, 10, 1173660. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Wachamo, S.; Gaultier, A. The emerging role of microbiota derived SCFAs in neurodegenerative disorders. Brain Behav. Immun.—Health 2025, 46, 101012. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, J.; Zhang, W.; Chen, F.; Liu, B. Gut microbiota-derived short chain fatty acids act as mediators of the gut-brain axis targeting age-related neurodegenerative disorders: A narrative review. Crit. Rev. Food Sci. Nutr. 2025, 65, 265–286. [Google Scholar] [CrossRef]
- Caetano-Silva, M.E.; Rund, L.; Hutchinson, N.T.; Woods, J.A.; Steelman, A.J.; Johnson, R.W. Inhibition of inflammatory microglia by dietary fiber and short-chain fatty acids. Sci. Rep. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Li, S.C.; Hsu, W.F.; Chang, J.S.; Shih, C.K. Combination of Lactobacillus acidophilus and Bifidobacterium animalis subsp. lactis Shows a Stronger Anti-Inflammatory Effect than Individual Strains in HT-29 Cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef]
- Aghamohammad, S.; Sepehr, A.; Miri, S.T.; Najafi, S.; Pourshafie, M.R.; Rohani, M. The Role of Combining Probiotics in Preventing and Controlling Inflammation: A Focus on the Anti-Inflammatory and Immunomodulatory Effects of Probiotics in an In Vitro Model of IBD. Can. J. Gastroenterol. Hepatol. 2022, 2022, 2045572. [Google Scholar] [CrossRef]
- Ku, S.; Haque, M.A.; Jang, M.J.; Ahn, J.; Choe, D.; Jeon, J.I.; Park, M.S. The role of Bifidobacterium in longevity and the future of probiotics. Food Sci. Biotechnol. 2024, 33, 2097–2110. [Google Scholar] [CrossRef]
- Setbo, E.; Campbell, K.; O’Cuiv, P.; Hubbard, R. Utility of Probiotics for Maintenance or Improvement of Health Status in Older People—A Scoping Review. J. Nutr. Health Aging 2019, 23, 364–372. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Bergh, C.; Kruger, K.; Sűsserová, M.; Allen, J.; Améen, S.; Tingö, L. The Effect of Probiotics on Health Outcomes in the Elderly: A Systematic Review of Randomized, Placebo-Controlled Studies. Microorganisms 2021, 9, 1344. [Google Scholar] [CrossRef]
- Huang, X.; Huang, L.; Lu, J.; Cheng, L.; Wu, D.; Li, L.; Zhang, S.; Lai, X.; Xu, L. The relationship between telomere length and aging-related diseases. Clin. Exp. Med. 2025, 25, 72. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Zhang, J.; Li, L. Links between telomere dysfunction and hallmarks of aging. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2023, 888, 503617. [Google Scholar] [CrossRef]
- Shi, F.; Peng, J.; Li, H.; Liu, D.; Han, L.; Wang, Y.; Liu, Q.; Liu, Q. Probiotics as a targeted intervention in anti-ageing: A review. Biomarkers 2024, 29, 577–585. [Google Scholar] [CrossRef]
- Choudhary, P.; Kathuria, D.; Suri, S.; Bahndral, A.; Kanthi Naveen, A. Probiotics- its functions and influence on the ageing process: A comprehensive review. Food Biosci. 2023, 52, 102389. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, L.; Zhao, J.; Chen, W.; Lu, W. The gut core microbial species Bifidobacterium longum: Colonization, mechanisms, and health benefits. Microbiol. Res. 2025, 290, 127966. [Google Scholar] [CrossRef]
- Kanimozhi, N.V.; Sukumar, M. Aging through the lens of the gut microbiome: Challenges and therapeutic opportunities. Arch. Gerontol. Geriatr. Plus 2025, 2, 100142. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Oneca, M.; Torre, P.; Díaz, J.V.; Encio, I.J.; Barajas, M.; Araña, M. Influence of Storage Temperature and Packaging on Bacteria and Yeast Viability in a Plant-Based Fermented Food. Foods 2020, 9, 302. [Google Scholar] [CrossRef]
- Castro-Herrera, V.M.; Fisk, H.L.; Wootton, M.; Lown, M.; Owen-Jones, E.; Lau, M.; Lowe, R.; Hood, K.; Gillespie, D.; Hobbs, F.D.R.; et al. Combination of the Probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis, BB-12 Has Limited Effect on Biomarkers of Immunity and Inflammation in Older People Resident in Care Homes: Results From the Probiotics to Reduce Infections iN CarE home reSidentS Randomized, Controlled Trial. Front. Immunol. 2021, 12, 643321. [Google Scholar] [CrossRef]
- Andrade, J.C.; Almeida, D.; Domingos, M.; Seabra, C.L.; Machado, D.; Freitas, A.C.; Gomes, A.M. Commensal Obligate Anaerobic Bacteria and Health: Production, Storage, and Delivery Strategies. Front. Bioeng. Biotechnol. 2020, 8, 550. [Google Scholar] [CrossRef]
- Jain, P.; Joshi, N.; Sahu, V.; Dominic, A.; Aggarwal, S. Autoimmune diseases and microbiome targeted therapies. Int. Rev. Cell Mol. Biol. 2025, 395, 133–156. [Google Scholar] [CrossRef]
- Smolinska, S.; Popescu, F.-D.; Zemelka-Wiacek, M. A Review of the Influence of Prebiotics, Probiotics, Synbiotics, and Postbiotics on the Human Gut Microbiome and Intestinal Integrity. J. Clin. Med. 2025, 14, 3673. [Google Scholar] [CrossRef]
- Giri, P.S.; Shah, F.; Dwivedi, M.K. Chapter 12—Probiotics and prebiotics in the suppression of autoimmune diseases. In Probiotics in the Prevention and Management of Human Diseases; Dwivedi, M.K., Amaresan, N., Sankaranarayanan, A., Kemp, E.H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 161–186. [Google Scholar]
- Mirfeizi, Z.; Mahmoudi, M.; Faridzadeh, A. Probiotics as a complementary treatment in systemic lupus erythematosus: A systematic review. Health Sci. Rep. 2023, 6, e1640. [Google Scholar] [CrossRef]
- Mohamed, A.a.H.; Shafie, A.; Al-Samawi, R.I.; Jamali, M.C.; Ashour, A.A.; Felemban, M.F.; Alqarni, A.; Ahmad, I.; Mansuri, N.; Ahmad, F.; et al. The role of probiotics in promoting systemic immune tolerance in systemic lupus erythematosus. Gut Pathog. 2025, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Kesavelu Sr, D.; Krishnamurty, A.Y.; Chander, A. Single Strain vs Multiple Strain Probiotics: The Clinician’s Choice. Cureus 2025, 17, e86353. [Google Scholar] [CrossRef]
- de Salis, L.V.V.; Martins, L.S.; Rodrigues, G.S.P.; de Oliveira, G.L.V. Chapter 13—Dysbiosis and probiotic applications in autoimmune diseases. In Translational Autoimmunity; Rezaei, N., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 2, pp. 269–294. [Google Scholar]
- McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Strain-Specificity and Disease-Specificity of Probiotic Efficacy: A Systematic Review and Meta-Analysis. Front. Med. 2018, 5, 124. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Hollander, D.; Kaunitz, J.D. The “Leaky Gut”: Tight Junctions but Loose Associations? Dig. Dis. Sci. 2020, 65, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef]
- Dwivedi, M.; Kumar, P.; Laddha, N.C.; Kemp, E.H. Induction of regulatory T cells: A role for probiotics and prebiotics to suppress autoimmunity. Autoimmun. Rev. 2016, 15, 379–392. [Google Scholar] [CrossRef]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Pyclik, M.; Srutkova, D.; Schwarzer, M.; Górska, S. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins—Their chemical structure and biological attributes. Int. J. Biol. Macromol. 2020, 147, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Li, M.; Han, X.; Fan, Y.-T.; Yang, J.-J.; Long, Y.; Yu, J.; Ji, H.-Y. Lactobacilli-Mediated Regulation of the Microbial–Immune Axis: A Review of Key Mechanisms, Influencing Factors, and Application Prospects. Foods 2025, 14, 1763. [Google Scholar] [CrossRef]
- Yang, Y.; Day, J.; Souza-Fonseca Guimaraes, F.; Wicks, I.P.; Louis, C. Natural killer cells in inflammatory autoimmune diseases. Clin. Transl. Immunol. 2021, 10, e1250. [Google Scholar] [CrossRef]
- Camous, X.; Pera, A.; Solana, R.; Larbi, A. NK cells in healthy aging and age-associated diseases. J. Biomed. Biotechnol. 2012, 2012, 195956. [Google Scholar] [CrossRef]
- Chen, P.-C.; Kaur, K.; Ko, M.-W.; Huerta-Yepez, S.; Jain, Y.; Jewett, A. Regulation of Cytotoxic Immune Effector Function by AJ3 Probiotic Bacteria in Amyotrophic Lateral Sclerosis (ALS). Crit. Rev. Immunol. 2023, 43, 13–26. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Z.; Tang, P.; Wu, Y.; Zhang, A.; Li, D.; Wang, C.-Z.; Wan, J.-Y.; Yao, H.; Yuan, C.-S. Probiotics fortify intestinal barrier function: A systematic review and meta-analysis of randomized trials. Front. Immunol. 2023, 14, 1143548. [Google Scholar] [CrossRef] [PubMed]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-Strain Probiotics: Synergy among Isolates Enhances Biological Activities. Biology 2021, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Heczko, P.B.; Giemza, M.; Ponikiewska, W.; Strus, M. Importance of Lactobacilli for Human Health. Microorganisms 2024, 12, 2382. [Google Scholar] [CrossRef]
- Shahali, A.; Soltani, R.; Akbari, V. Probiotic Lactobacillus and the potential risk of spreading antibiotic resistance: A systematic review. Res. Pharm. Sci. 2023, 18, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A critical review of antibiotic resistance in probiotic bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef]
- Uusitalo, U.; Mramba, L.K.; Aronsson, C.A.; Vehik, K.; Yang, J.; Hummel, S.; Lernmark, Å.; Rewers, M.; Hagopian, W.; McIndoe, R.; et al. HLA Genotype and Probiotics Modify the Association Between Timing of Solid Food Introduction and Islet Autoimmunity in the TEDDY Study. Diabetes Care 2023, 46, 1839–1847. [Google Scholar] [CrossRef]
- Karahan, F. Environmental Factors Affecting the Gut Microbiota and Their Consequences. Nat. Cell Sci. 2024, 2, 133–140. [Google Scholar] [CrossRef]
- Paray, B.A.; Albeshr, M.F.; Jan, A.T.; Rather, I.A. Leaky Gut and Autoimmunity: An Intricate Balance in Individuals Health and the Diseased State. Int. J. Mol. Sci. 2020, 21, 9770. [Google Scholar] [CrossRef]
- Rossi, F.; Amadoro, C.; Gasperi, M.; Colavita, G. Lactobacilli Infection Case Reports in the Last Three Years and Safety Implications. Nutrients 2022, 14, 1178. [Google Scholar] [CrossRef]
- Radcliffe, C.V.; Anahtar, M.N.; Hohmann, E.L.; Turbett, S.E.; Dugdale, C.M.; Paras, M.L. Lactobacillus Bacteremia and Endovascular Infections: A Retrospective Study of 100 Patients. Open Forum Infect. Dis. 2025, 12, ofaf466. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; El-Masry, S.S.; El-Dougdoug, N.K. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019, 10, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Palasca, O.; Yarani, R.; Litman, T.; Anthon, C.; Groenen, M.A.M.; Stadler, P.F.; Pociot, F.; Jensen, L.J.; Gorodkin, J. Human pathways in animal models: Possibilities and limitations. Nucleic Acids Res. 2021, 49, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Shen, X.; Shi, X.; Sakandar, H.A.; Quan, K.; Li, Y.; Jin, H.; Kwok, L.-Y.; Zhang, H.; Sun, Z. Targeting gut microbiota and metabolism as the major probiotic mechanism—An evidence-based review. Trends Food Sci. Technol. 2023, 138, 178–198. [Google Scholar] [CrossRef]
- Díaz-Peña, R. Personalized Medicine in Autoimmune Diseases. J. Pers. Med. 2021, 11, 1181. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, M.; Pandini, C.; Bordoni, M.; Pansarasa, O.; Rey, F.; Costa, A.; Minafra, B.; Diamanti, L.; Zucca, S.; Carelli, S.; et al. Alzheimer’s, Parkinson’s Disease and Amyotrophic Lateral Sclerosis Gene Expression Patterns Divergence Reveals Different Grade of RNA Metabolism Involvement. Int. J. Mol. Sci. 2020, 21, 9500. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huh, J.R.; Shah, K. Microbiota and the gut-brain-axis: Implications for new therapeutic design in the CNS. eBioMedicine 2022, 77, 103908. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Origüela, V.; Lopez-Zaplana, A. Gut Microbiota: An Immersion in Dysbiosis, Associated Pathologies, and Probiotics. Microorganisms 2025, 13, 1084. [Google Scholar] [CrossRef]
- Beikmohammadi, M.; Halimi, S.; Fard, N.A.; Wen, W. Therapeutic Potential of Probiotics: A Review of Their Role in Modulating Inflammation. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Meher, A.K.; Acharya, B.; Sahu, P.K. Probiotics: Bridging the interplay of a healthy gut and psychoneurological well-being. Food Bioeng. 2024, 3, 126–147. [Google Scholar] [CrossRef]
- Ong, J.S.; Lew, L.C.; Hor, Y.Y.; Liong, M.T. Probiotics: The Next Dietary Strategy against Brain Aging. Prev. Nutr. Food Sci. 2022, 27, 1–13. [Google Scholar] [CrossRef]
- Sharma, R.; Gupta, D.; Mehrotra, R.; Mago, P. Psychobiotics: The Next-Generation Probiotics for the Brain. Curr. Microbiol. 2021, 78, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- di Vito, R.; Conte, C.; Traina, G. A Multi-Strain Probiotic Formulation Improves Intestinal Barrier Function by the Modulation of Tight and Adherent Junction Proteins. Cells 2022, 11, 2617. [Google Scholar] [CrossRef] [PubMed]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Yamazaki, S. Diverse roles of dendritic cell and regulatory T cell crosstalk in controlling health and disease. Int. Immunol. 2024, 37, 5–14. [Google Scholar] [CrossRef]
- Pittet, M.J.; Di Pilato, M.; Garris, C.; Mempel, T.R. Dendritic cells as shepherds of T cell immunity in cancer. Immunity 2023, 56, 2218–2230. [Google Scholar] [CrossRef]
- Gu, Q.; Yin, Y.; Yan, X.; Liu, X.; Liu, F.; McClements, D.J. Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: A review. Adv. Colloid. Interface Sci. 2022, 309, 102781. [Google Scholar] [CrossRef]
- Hasaniani, N.; Ghasemi-Kasman, M.; Halaji, M.; Rostami-Mansoor, S. Bifidobacterium breve Probiotic Compared to Lactobacillus casei Causes a Better Reduction in Demyelination and Oxidative Stress in Cuprizone-Induced Demyelination Model of Rat. Mol. Neurobiol. 2024, 61, 498–509. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Guo, S.; Liu, S.; Du, Y.; Wang, L.; He, D.; Mo, X.; Zhang, H.; Cheng, Q.; et al. The emerging role of the gut microbiome in depression: Implications for precision medicine. Mol. Psychiatry 2025. [Google Scholar] [CrossRef]
- Abd Mutalib, N.; Syed Mohamad, S.A.; Jusril, N.A.; Hasbullah, N.I.; Mohd Amin, M.C.I.; Ismail, N.H. Lactic Acid Bacteria (LAB) and Neuroprotection, What Is New? An Up-To-Date Systematic Review. Pharmaceuticals 2023, 16, 712. [Google Scholar] [CrossRef]
- Lim, S.-M.; Zaki Ramli, M.; Ahmad Alwi, N.A.; Mani, V.; Majeed, A.B.A.; Ramasamy, K. Chapter 79—Probiotics and Neuroprotection. In Diet and Nutrition in Dementia and Cognitive Decline; Martin, C.R., Preedy, V.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 859–868. [Google Scholar]
- Blake, S.J.; Wolf, Y.; Boursi, B.; Lynn, D.J. Role of the microbiota in response to and recovery from cancer therapy. Nat. Rev. Immunol. 2024, 24, 308–325. [Google Scholar] [CrossRef]
- Mahooti, M.; Safaei, F.; Firuzpour, F.; Abdolalipour, E.; Zare, D.; Sanami, S.; Safavi, M.; Mirdamadi, S. Exploring the role of inflammatory regulatory effects of probiotics as adjuvants in cancer development management with considering possible challenges: A comprehensive review. Inflammopharmacology 2025, 33, 3823–3841. [Google Scholar] [CrossRef]
- Zhuang, K.; Luo, H.; Zeng, M.; Chan, S.C.L.; Gong, M.; Wang, Y. Effects of probiotics, prebiotics, and synbiotics on gut microbiota in older adults: A systematic review and meta-analysis of randomized controlled trials. Nutr. J. 2025, 24, 147. [Google Scholar] [CrossRef]
- Ale, E.C.; Binetti, A.G. Role of Probiotics, Prebiotics, and Synbiotics in the Elderly: Insights Into Their Applications. Front. Microbiol. 2021, 12, 631254. [Google Scholar] [CrossRef]
- Jouriani, F.H.; Torkamaneh, M.; Torfeh, M.; Ashrafian, F.; Aghamohammad, S.; Rohani, M. Native Lactobacillus and Bifidobacterium probiotics modulate autophagy genes and exert anti-inflammatory effect. Sci. Rep. 2025, 15, 25006. [Google Scholar] [CrossRef]
- Ringel-Kulka, T.; Palsson, O.S.; Maier, D.; Carroll, I.; Galanko, J.A.; Leyer, G.; Ringel, Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: A double-blind study. J. Clin. Gastroenterol. 2011, 45, 518–525. [Google Scholar] [CrossRef]
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A comprehensive review of probiotics and human health-current prospective and applications. Front. Microbiol. 2024, 15, 1487641. [Google Scholar] [CrossRef]
- Jena, R.; Singh, N.A.; Ahmed, N.; Choudhury, P.K. Bifidobacteria in antibiotic-associated dysbiosis: Restoring balance in the gut microbiome. World J. Microbiol. Biotechnol. 2025, 41, 297. [Google Scholar] [CrossRef]
- Butel, M.J. Probiotics, gut microbiota and health. Med. Mal. Infect. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Kamareddine, L.; Najjar, H.; Sohail, M.U.; Abdulkader, H.; Al-Asmakh, M. The Microbiota and Gut-Related Disorders: Insights from Animal Models. Cells 2020, 9, 2401. [Google Scholar] [CrossRef]
- Whelan, K.; Alexander, M.; Gaiani, C.; Lunken, G.; Holmes, A.; Staudacher, H.M.; Theis, S.; Marco, M.L. Design and reporting of prebiotic and probiotic clinical trials in the context of diet and the gut microbiome. Nat. Microbiol. 2024, 9, 2785–2794. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Azad, M.B.; Bäckhed, F.; Blaser, M.J.; Byndloss, M.; Chiu, C.Y.; Chu, H.; Dugas, L.R.; Elinav, E.; Gibbons, S.M.; et al. Clinical translation of microbiome research. Nat. Med. 2025, 31, 1099–1113. [Google Scholar] [CrossRef]
- Wickens, K.; Black, P.; Stanley, T.V.; Mitchell, E.; Barthow, C.; Fitzharris, P.; Purdie, G.; Crane, J. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin. Exp. Allergy 2012, 42, 1071–1079. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, X.; Ye, W.; Liu, Y.; Luo, J.; Tang, X.; Wang, J.; Liu, C.; Zhou, H. Effect of Lactobacillus rhamnosus LZ260E on allergic symptoms and intestinal microbiota in β-lactoglobulin–sensitized mice. J. Funct. Foods 2024, 113, 106045. [Google Scholar] [CrossRef]
- Yamasaki, M.; Minesaki, M.; Iwakiri, A.; Miyamoto, Y.; Ogawa, K.; Nishiyama, K.; Tsend-Ayush, C.; Oyunsuren, T.; Li, Y.; Nakano, T.; et al. Lactobacillus plantarum 06CC2 reduces hepatic cholesterol levels and modulates bile acid deconjugation in Balb/c mice fed a high-cholesterol diet. Food Sci. Nutr. 2020, 8, 6164–6173. [Google Scholar] [CrossRef]
- Al-Sadi, R.; Nighot, P.; Nighot, M.; Haque, M.; Rawat, M.; Ma, T.Y. Lactobacillus acidophilus Induces a Strain-specific and Toll-Like Receptor 2-Dependent Enhancement of Intestinal Epithelial Tight Junction Barrier and Protection Against Intestinal Inflammation. Am. J. Pathol. 2021, 191, 872–884. [Google Scholar] [CrossRef]
- Wang, G.; Xu, Q.; Jin, X.; Hang, F.; Liu, Z.; Zhao, J.; Zhang, H.; Chen, W. Effects of lactobacilli with different regulatory behaviours on tight junctions in mice with dextran sodium sulphate-induced colitis. J. Funct. Foods 2018, 47, 107–115. [Google Scholar] [CrossRef]
- Liao, Z.; Quintana, Y. Challenges to Global Standardization of Outcome Measures. AMIA Jt. Summits Transl. Sci. Proc. 2021, 2021, 404–409. [Google Scholar]
- Chung, K.C.; Malay, S.; Shauver, M.J. The Complexity of Conducting a Multicenter Clinical Trial: Taking It to the Next Level Stipulated by the Federal Agencies. Plast. Reconstr. Surg. 2019, 144, 1095e–1103e. [Google Scholar] [CrossRef]
- Matera, M. Bifidobacteria, Lactobacilli... when, how and why to use them. Glob. Pediatr. 2024, 8, 100139. [Google Scholar] [CrossRef]
- Livingstone, K.M.; Ramos-Lopez, O.; Pérusse, L.; Kato, H.; Ordovas, J.M.; Martínez, J.A. Precision nutrition: A review of current approaches and future endeavors. Trends Food Sci. Technol. 2022, 128, 253–264. [Google Scholar] [CrossRef]
- Lyu, R.; Qu, Y.; Divaris, K.; Wu, D. Methodological Considerations in Longitudinal Analyses of Microbiome Data: A Comprehensive Review. Genes 2023, 15, 51. [Google Scholar] [CrossRef]
- Mohr, A.E.; Ortega-Santos, C.P.; Whisner, C.M.; Klein-Seetharaman, J.; Jasbi, P. Navigating Challenges and Opportunities in Multi-Omics Integration for Personalized Healthcare. Biomedicines 2024, 12, 1496. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).