Hearing Loss and Chiari Malformation Type I: A Scoping Review
Abstract
1. Introduction
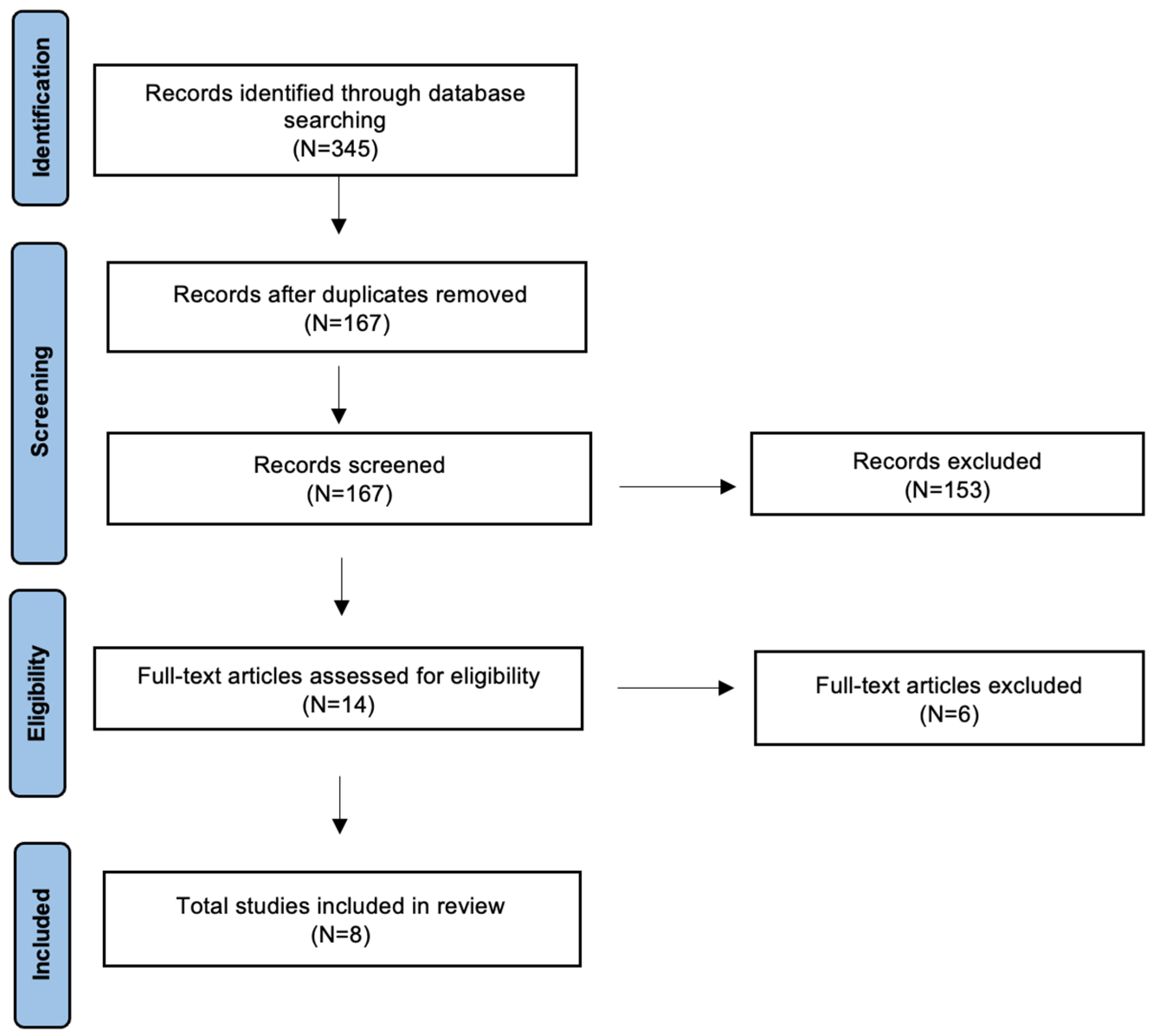
2. Materials and Methods
3. Results
3.1. Case Reports
3.2. Retrospective Study
3.3. Prospective Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aboulezz, A.O.; Sartor, K.; Geyer, C.A.; Gado, M.H. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitative approach with MR imaging. J. Comput. Assist. Tomogr. 1985, 9, 1033–1036. [Google Scholar] [CrossRef]
- Kahn, E.N.; Muraszko, K.M.; Maher, C.O. Prevalence of Chiari I Malformation and Syringomyelia. Neurosurg. Clin. N. Am. 2015, 26, 501–507. [Google Scholar] [CrossRef]
- Chiari, H. Ueber Veränderungen des Kleinhirns infolge von Hydrocephalie des Grosshirns 1. Dtsch. Med. Wochenschr. 1891, 17, 1172–1175. [Google Scholar] [CrossRef]
- Speer, M.C.; Enterline, D.S.; Mehltretter, L.; Hammock, P.; Joseph, J.; Dickerson, M.; Ellenbogen, R.G.; Milhorat, T.H.; Hauser, M.A.; George, T.M. Chiari Type I Malformation with or Without Syringomyelia: Prevalence and Genetics. J. Genet. Couns. 2003, 12, 297–311. [Google Scholar] [CrossRef]
- Heffez, D.S.; Broderick, J.; Connor, M.; Mitchell, M.; Galezowska, J.; Golchini, R.; Ghorai, J. Is there a relationship between the extent of tonsillar ectopia and the severity of the clinical Chiari syndrome? Acta Neurochir. 2020, 162, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Barkovich, A.J.; Wippold, F.J.; Sherman, J.L.; Citrin, C.M. Significance of cerebellar tonsillar position on MR. AJNR Am. J. Neuroradiol. 1986, 7, 795–799. [Google Scholar] [PubMed]
- Haktanir, A.; Yücedağ, F.; Kaçar, E.; Ulu, Ş.; Gültekin, M.A.; Ünlü, E.; Bucak, A.; Ayçiçek, A. Association of Chiari I malformation and cerebellar ectopia with sensorineural hearing loss. J. Craniofacial Surg. 2013, 24, 1153–1155. [Google Scholar] [CrossRef] [PubMed]
- Sperling, N.M.; Franco, R.A., Jr.; Milhorat, T.H. Otologic manifestations of Chiari I malformation. Otol. Neurotol. 2001, 22, 678–681. [Google Scholar] [CrossRef]
- Hatgaonkar, A.M.; Mahajan, S.M.; Hatgoankar, K.A.; Bandre, G.R. MRI Insights in Chiari Malformation Type 1 and Variations with Hydrosyringomyelia. Cureus 2024, 16, e55676. [Google Scholar] [CrossRef]
- Shaikh, A.G.; Ghasia, F.F. Neuro-ophthalmology of type 1 Chiari malformation. Expert Rev. Ophthalmol. 2015, 10, 351–357. [Google Scholar] [CrossRef]
- Goldschagg, N.; Feil, K.; Ihl, F.; Krafczyk, S.; Kunz, M.; Tonn, J.C.; Strupp, M.; Peraud, A. Decompression in Chiari Malformation: Clinical, Ocular Motor, Cerebellar, and Vestibular Outcome. Front. Neurol. 2017, 8, 292. [Google Scholar] [CrossRef]
- McClugage, S.G.; Oakes, W.J. The Chiari I Malformation. J. Neurosurg. Pediatr. 2019, 24, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Palamar, D.; Güler, H.; Hancı, M.; Sucuoğlu, H.; Sanus, G.Z.; Tüzün, Ş. Posturographic Examination of Body Balance in Patients with Chiari Type I Malformation and Correlation with the Presence of Syringomyelia and Degree of Cerebellar Ectopia. Turk. J. Phys. Med. Rehabil. 2018, 65, 74–79. [Google Scholar] [CrossRef]
- Sclafani, A.P.; DeDio, R.M.; Hendrix, R.A. The Chiari-I Malformation. Ear Nose Throat J. 1991, 70, 208–212. [Google Scholar] [PubMed]
- Kumar, A.; Patni, A.H.; Charbel, F. The Chiari I Malformation and the Neurotologist. Otol. Neurotol. 2002, 23, 727–735. [Google Scholar] [CrossRef] [PubMed]
- van Beeck Calkoen, E.A.; Engel, M.S.D.; Van De Kamp, J.M.; Yntema, H.G.; Goverts, S.; Mulder, M.; Merkus, P.; Hensen, E.F. The Etiological Evaluation of Sensorineural Hearing Loss in Children. Eur. J. Pediatr. 2019, 178, 1195–1205. [Google Scholar] [CrossRef]
- Tripathi, P.; Deshmukh, P. Sudden Sensorineural Hearing Loss: A Review. Cureus 2022, 14, e29458. [Google Scholar] [CrossRef]
- Corazzi, V.; Migliorelli, A.; Bianchini, C.; Pelucchi, S.; Ciorba, A. Hearing Loss and Blood Coagulation Disorders: A Review. Hematol. Rep. 2023, 15, 421–431. [Google Scholar] [CrossRef]
- Migliorelli, A.; Ciorba, A.; Manuelli, M.; Corazzi, V.; Stomeo, F.; Bianchini, C.; Pelucchi, S.; Monzani, D.; Genovese, E.; Palma, S. Hearing Loss and Turner Syndrome: A Scoping Review. J. Int. Adv. Otol. 2025, 21, e241723. [Google Scholar] [CrossRef]
- Manuelli, M.; Migliorelli, A.; Bianchini, C.; Stomeo, F.; Pelucchi, S.; Genovese, E.; Monzani, D.; Palma, S.; Ciorba, A. Unilateral Hearing Loss and Auditory Asymmetry in Mitochondrial Disease: A Scoping Review. J. Clin. Med. 2024, 13, 5044. [Google Scholar] [CrossRef]
- Corazzi, V.; Musumano, L.B.; Migliorelli, A.; Negossi, L.; Bianchini, C.; Stomeo, F.; Pelucchi, S.; Ciorba, A. Predictive Factors for Hearing Loss in Congenital Cytomegalovirus Infection. Audiol. Res. 2024, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.P.; Ruscetta, M.N.; Chi, D.H. Sensorineural Hearing Impairment in Children with Chiari I Malformation. Ann. Otol. Rhinol. Laryngol. 2008, 117, 443–447. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Heuer, G.G.; Gabel, B.; Lemberg, P.S.; Sutton, L.N. Chiari I Malformation Presenting with Hearing Loss: Surgical Treatment and Literature Review. Childs Nerv. Syst. 2008, 24, 1063–1066. [Google Scholar] [CrossRef]
- Dolgun, H.; Turkoglu, E.; Kertmen, H.; Yilmaz, E.R.; Sekerci, Z. Chiari Type I Malformation Presenting with Bilateral Hearing Loss. J. Clin. Neurosci. 2009, 16, 1228–1230. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Goldhagen, C.; Danner, C.; Agazzi, S. Hearing Loss and Chiari Malformation: A Clinical Pearl. Clin. Neurol. Neurosurg. 2014, 122, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Famili, H.P.; Zalewski, C.K.; Ibrahimy, A.; Mack, J.; Cantor, F.; Heiss, J.D.; Brewer, C.C. Audiovestibular Findings in a Cohort of Patients with Chiari Malformation Type I and Dizziness. J. Clin. Med. 2023, 12, 2767. [Google Scholar] [CrossRef]
- Oakes, W.J. The Chiari Malformations of the Child. In Principles of Spinal Surgery; Menezes, A.H., Sonntag, V.K.H., Eds.; McGraw-Hill: New York, NY, USA, 1996; pp. 359–377. [Google Scholar]
- Bindal, A.K.; Dunsker, S.B.; Tew, J.M. Chiari I Malformations: Classification and Management. Neurosurgery 1995, 37, 1069–1074. [Google Scholar] [CrossRef]
- Johnson, G.D.; Harbaugh, R.E.; Lenz, S.B. Surgical Decompression of Chiari I Malformation for Isolated Progressive Sensorineural Hearing Loss. Am. J. Otol. 1994, 15, 634–638. [Google Scholar]
- Ahmmed, A.U.; Mackenzie, I.; Das, V.K.; Chatterjee, S.; Lye, R.H. Audio-Vestibular Manifestations of Chiari Malformation and Outcome of Surgical Decompression: A Case Report. J. Laryngol. Otol. 1996, 110, 1060–1064. [Google Scholar] [CrossRef]
- Colpan, M.E.; Sekerci, Z. Chiari Type 1 Malformation Presenting as Hemifacial Spasm: Case Report. Neurosurgery 2005, 57, 371. [Google Scholar] [CrossRef] [PubMed]
| Author (Yrs) | Country | Type of Study | Nu CM | Aver. Yrs | Nu Adult/Nu Pediatric | Sex | Imaging Definition | Hearing Deafness | V | T | S or CPA | Other Symptoms |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sperling (2001) [8] | USA | P | 16 | 41 | 16/0 | F: 12 M: 4 | >5 mm herniation | Y | Y | Y | 0 | Headache, nausea |
| Kumar (2002) [15] | USA | R | 77 | 39 | NA | F: 56 M: 21 | >3- to 5 mm herniation | Y | Y | Y | 13 | Headache, neurological symptoms |
| Heuer (2008) [24] | USA | CR | 1 | 10 | 0/1 | F | NA | Y | N | N | 0 | Headache |
| Simons (2008) [22] | USA | R | 6 | 4.9 | 0/6 | M: 4 F: 2 | >5 mm herniation | Y | N | N | 0 | N |
| Dolgun (2009) [25] | Turkey | CR | 1 | 44 | 1/0 | F | NA | Y | N | N | 1 | Headache |
| Haktanir (2013) [7] | Turkey | R | 13 | 46.88 | NA | NA | Ectopia: herniation more than 2 mm CM 1: herniation equal or more than 5 mm | Y | NA | NA | 0 | NA |
| Sivakanthan (2014) [26] | USA | CR | 1 | 18 | 1/0 | NA | NA | Y | N | N | 0 | Headache |
| Famili (2023) [27] | USA | R | 24 | 40.88 | 24/0 | F: 21 M: 3 | >5 mm herniation | N | Y | Y | 0 | NA |
| Author (Yrs) | SNHL (Nu) | Audiometric Threshold/PTA SNHL Definition | Unilateral vs. Bilateral Definitions | Severity SNHL | Audiology Test | Surgery | Audiometric Surgical Outcome |
|---|---|---|---|---|---|---|---|
| Sperling (2001) [8] | N | 25 dB | N | Normal | ENG, Ecog, ABR, OAE | Decompression | NA |
| Kumar (2002) [15] | Y (32) Unilateral (22) Bilateral (10) | NA | NA | Mild-Moderate: 23 Profound: 9 | VFT | NA | NA |
| Heuer (2008) [24] | Y (1) | NA | NA | Mild | N | Decompression | Normal |
| Simons (2008) [22] | Y (6) Unilateral (4) Bilateral (2) | 20 dB |
Unilateral: hearing thresh-
old of greater than 20 dB for at least 1 frequency (500–4000 Hz) | Mild: 1 Moderate: 3 Profound: 2 | N | N | - |
| Dolgun (2009) [25] | Y (1) | NA | NA | Moderate | N | Shunt | Not improve |
| Haktanir (2013) [7] | Y (13) | 20 dB (PTA: 250, 500, 1000, 2000, 3000, 4000, and 6000 Hz) | NA | NA | N | N | - |
| Sivakanthan (2014) [26] | Y (1) Unilateral | NA | NA | Profound | N | Decompression | Normal |
| Famili (2023) [27] | N | 20 dB (PTA: 500, 1000, 2000, 4000 Hz) | NA | Normal | Tympanometry, ABR, Posturography, Rotational Assessment, VEMP | N | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migliorelli, A.; Manuelli, M.; Bianchini, C.; Stomeo, F.; Pelucchi, S.; Palma, S.; Ciorba, A. Hearing Loss and Chiari Malformation Type I: A Scoping Review. Diseases 2025, 13, 315. https://doi.org/10.3390/diseases13100315
Migliorelli A, Manuelli M, Bianchini C, Stomeo F, Pelucchi S, Palma S, Ciorba A. Hearing Loss and Chiari Malformation Type I: A Scoping Review. Diseases. 2025; 13(10):315. https://doi.org/10.3390/diseases13100315
Chicago/Turabian StyleMigliorelli, Andrea, Marianna Manuelli, Chiara Bianchini, Francesco Stomeo, Stefano Pelucchi, Silvia Palma, and Andrea Ciorba. 2025. "Hearing Loss and Chiari Malformation Type I: A Scoping Review" Diseases 13, no. 10: 315. https://doi.org/10.3390/diseases13100315
APA StyleMigliorelli, A., Manuelli, M., Bianchini, C., Stomeo, F., Pelucchi, S., Palma, S., & Ciorba, A. (2025). Hearing Loss and Chiari Malformation Type I: A Scoping Review. Diseases, 13(10), 315. https://doi.org/10.3390/diseases13100315







