A Multi-Pathogen Retrospective Study in Patients Hospitalized for Acute Gastroenteritis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, T.; Klamer, S.; Jacquinet, S.; Catry, B.; Litzroth, A.; Mortgat, L.; Mamouris, P.; Rebolledo, J.; Vaes, B.; Van Cauteren, D.; et al. The health and economic impact of acute gastroenteritis in Belgium, 2010–2014. Epidemiol. Infect. 2019, 147, e146. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.F.; Zomer, E.; O’Toole, J.; Sinclair, M.; Gibney, K.; Liew, D.; Leder, K. Cost of gastroenteritis in Australia: A healthcare perspective. PLoS ONE 2018, 13, e0195759. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the global burden of disease study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Groom, H.C.; Rawlings, A.M.; Mattison, C.P.; Salas, S.B.; Burke, R.M.; Hallowell, B.D.; Calderwood, L.E.; Donald, J.; Balachandran, N.; et al. Incidence, Etiology, and Healthcare Utilization for Acute Gastroenteritis in the Community, United States. Emerg. Infect. Dis. 2022, 28, 2234–2242. [Google Scholar] [CrossRef]
- Graves, N.S. Acute gastroenteritis. Prim. Care 2013, 40, 727–741. [Google Scholar] [CrossRef]
- Raboni, S.M.; Damasio, G.A.C.; Ferreira, C.E.O.; Pereira, L.A.; Nogueira, M.B.; Vidal, L.R.; Cruz, C.R.; Almeida, S.M. Acute Gastroenteritis and Enteric Viruses in Hospitalised Children in Southern Brazil: Aetiology, Seasonality and Clinical Outcomes. Mem. Inst. Oswaldo Cruz 2014, 109, 428–435. [Google Scholar] [CrossRef]
- Guido, M.; Zizza, A.; Bredl, S.; Lindner, J.; De Donno, A.; Quattrocchi, M.; Grima, P.; Modrow, S.; Seroepidemiology Group. Seroepidemiology of human bocavirus in Apulia, Italy. Clin. Microbiol. Infect. 2012, 18, E74–E76. [Google Scholar] [CrossRef]
- Rose, T.C.; Adams, N.; Taylor-Robinson, D.C.; Barr, B.; Hawker, J.; O’Brien, S.; Violato, M.; Whitehead, M. Relationship between socioeconomic status and gastrointestinal infections in developed countries: A systematic review protocol. Syst. Rev. 2016, 5, 13. [Google Scholar] [CrossRef]
- Tate, J.E.; Burton, A.H.; Boschi-Pinto, C.; Steele, A.D.; Duque, J.; Parashar, U.D.; WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2012, 12, 136–141. [Google Scholar] [CrossRef]
- Lanata, C.F.; Fischer-Walker, C.L.; Olascoaga, A.C.; Torres, C.X.; Aryee, M.J.; Black, R.E.; Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children <5 years of age: A systematic review. PLoS ONE 2013, 8, e72788. [Google Scholar]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.P.; Zhou, S.X.; Wang, X.; Lu, Q.B.; Shi, L.S.; Ren, X.; Zhang, H.Y.; Wang, Y.F.; Lin, S.H.; Zhang, C.H. Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat. Commun. 2021, 12, 2464. [Google Scholar] [CrossRef] [PubMed]
- Costantino, C.; Amodio, E.; Vitale, F. Impact on Rotavirus Gastro-Enteritis Hospitalisation during the First Year of Universal Vaccination in Sicily. Paediatr. Int. Child. Health 2015, 35, 342–343. [Google Scholar] [CrossRef]
- Khagayi, S.; Omore, R.; Otieno, G.P.; Ogwel, B.; Ochieng, J.B.; Juma, J.; Apondi, E.; Bigogo, G.; Onyango, C.; Ngama, M.; et al. Effectiveness of Monovalent Rotavirus Vaccine Against Hospitalization with Acute Rotavirus Gastroenteritis in Kenyan Children. Clin. Infect. Dis. 2020, 70, 2298–2305. [Google Scholar] [CrossRef]
- Kobayashi, M.; Miyazaki, M.; Ogawa, A.; Tatsumi, M. Sustained reduction in rotavirus-coded hospitalizations in children aged <5 years after introduction of self-financed rotavirus vaccines in Japan. Hum. Vaccines Immunother. 2020, 16, 132–137. [Google Scholar]
- Muhsen, K.; Rubenstein, U.; Kassem, E.; Goren, S.; Schachter, Y.; Kremer, A.; Shulman, L.M.; Ephros, M.; Cohen, D. A significant and consistent reduction in rotavirus gastroenteritis hospitalization of children under 5 years of age, following the introduction of universal rotavirus immunization in Israel. Hum. Vaccines Immunother. 2015, 11, 2475–2482. [Google Scholar] [CrossRef]
- Baker, J.M.; Tate, J.E.; Steiner, C.A.; Haber, M.J.; Parashar, U.D.; Lopman, B.A. Longer-Term Direct and Indirect Effects of Infant Rotavirus Vaccination Across All Ages in the United States in 2000–2013: Analysis of a Large Hospital Discharge Data Set. Clin. Infect. Dis. 2019, 68, 976–983. [Google Scholar] [CrossRef]
- Operario, D.J.; Platts-Mills, J.A.; Nadan, S.; Page, N.; Seheri, M.; Mphahlele, J.; Praharaj, I.; Kang, G.; Araujo, I.T.; Leite, J.P.G.; et al. Etiology of Severe Acute Watery Diarrhea in Children in the Global Rotavirus Surveillance Network Using Quantitative Polymerase Chain Reaction. J. Infect. Dis. 2017, 216, 220–227. [Google Scholar] [CrossRef]
- Payne, D.C.; Vinjé, J.; Szilagyi, P.G.; Edwards, K.M.; Staat, M.A.; Weinberg, G.A.; Hall, C.B.; Chappell, J.; Bernstein, D.I.; Curns, A.T.; et al. Norovirus and medically attended gastroenteritis in U.S. children. N. Engl. J. Med. 2013, 368, 1121–1130. [Google Scholar] [CrossRef]
- Kotloff, K.L. Bacterial diarrhoea. Curr. Opin. Pediatr. 2022, 34, 147–155. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar]
- WHO. PDVAC Executive Summary 2020: Update on Development of Enterotoxigenic E. coli (ETEC) Vaccines. Available online: https://www.who.int/publications/m/item/pdvac-agenda-2020-update-on-development-of-etec-vaccines (accessed on 6 May 2024).
- WHO. WHO Preferred Product Characteristics for Vaccines against Shigella. Available online: https://www.who.int/publications/i/item/9789240036741 (accessed on 6 May 2024).
- Giersing, B.K.; Vekemans, J.; Nava, S.; Kaslow, D.C.; Moorthy, V. Report from the World Health Organization’s third Product Development for Vaccines Advisory Committee (PDVAC) meeting, Geneva, 8–10th June 2016. Vaccine 2019, 37, 7315–7327. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Duan, Q.; Zhang, W. Vaccines against gastroenteritis, current progress and challenges. Gut Microbes 2020, 11, 1486–1517. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Kaminski, R.W.; Porter, C.; Choy, R.K.M.; White, J.A.; Fleckenstein, J.M.; Cassels, F.; Bourgeois, L. Vaccines for Protecting Infants from Bacterial Causes of Diarrheal Disease. Microorganisms 2021, 9, 1382. [Google Scholar] [CrossRef]
- Luo, L.F.; Qiao, K.; Wang, X.G.; Ding, K.Y.; Su, H.L.; Li, C.Z.; Yan, H.J. Acute gastroenteritis outbreak caused by a GII.6 norovirus. World J. Gastroenterol. 2015, 21, 5295–5302. [Google Scholar] [CrossRef]
- Zhao, X.; Ni, B.; Wang, Y.; Shen, X.; Zhang, C.; Liu, J.; Li, S. Aetiological characteristics of adult acute diarrhoea in a general hospital of Shanghai. Epidemiol. Infect. 2017, 145, 545–552. [Google Scholar] [CrossRef]
- Patel, M.M.; Pitzer, V.E.; Alonso, W.J.; Vera, D.; Lopman, B.; Tate, J.; Viboud, C.; Parashar, U.D. Global seasonality of rotavirus disease. Pediatr. Infect. Dis. J. 2013, 32, e134–e147. [Google Scholar] [CrossRef]
- Patel, M.M.; Hall, A.J.; Vinjé, J.; Parashar, U.D. Noroviruses: A comprehensive review. J. Clin. Virol. 2009, 44, 1–8. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Lopman, B.A.; Levy, K. A systematic review and meta-analysis of the global seasonality of norovirus. PLoS ONE 2013, 8, e75922. [Google Scholar] [CrossRef]
- Elliott, E.J. Acute gastroenteritis in children. BMJ 2007, 334, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Duzova, A.; Bakkaloglu, A.; Kalyoncu, M.; Poyrazoglu, H.; Delibas, A.; Ozkaya, O.; Peru, H.; Alpay, H.; Soylemezoglu, O.; Gur-Guven, A.; et al. Etiology and outcome of acute kidney injury in children. Pediatr. Nephrol. 2010, 25, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.M.; Ji, J.; Sheikhi, F.H.; Widen, E.; Tian, L.; Alexander, S.R.; Ling, X.B. AKI in hospitalized children: Epidemiology and clinical associations in a national cohort. Clin. J. Am. Soc. Nephrol. 2013, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Mammen, C.; Al Abbas, A.; Skippen, P.; Nadel, H.; Levine, D.; Collet, J.P.; Matsell, D.G. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am. J. Kidney Dis. 2012, 59, 523–530. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef]
- Stanyevic, B.; Sepich, M.; Biondi, S.; Baroncelli, G.I.; Peroni, D.; Di Cicco, M. The evolving epidemiology of acute gastroenteritis in hospitalized children in Italy. Eur. J. Pediatr. 2022, 181, 349–358. [Google Scholar] [CrossRef]
- Biscaro, V.; Piccinelli, G.; Gargiulo, F.; Ianiro, G.; Caruso, A.; Caccuri, F.; De Francesco, M.A. Detection and molecular characterization of enteric viruses in children with acute gastroenteritis in Northern Italy. Infect. Genet. Evol. 2018, 60, 35–41. [Google Scholar] [CrossRef]
- Regione Puglia. Protocollo Operativo “Sorveglianza delle Gastroenteriti Emorragiche in Età Pediatrica” Prot. AOO_005_000221 del 21 Giugno 2018. Available online: https://www.sanita.puglia.it/documents/20182/26606314/Protocollo+Operativo_Sorveglianza+Gastroenteriti+emorragiche+in+et%C3%A0+pediatrica.pdf/dc372a68-bcf3-465e-bf25-cee20a892da8 (accessed on 13 June 2024).
- Schnadower, D.; Finkelstein, Y.; Freedman, S.B. Ondansetron and probiotics in the management of pediatric acute gastroenteritis in developed countries. Curr. Opin. Gastroenterol. 2015, 31, 1–6. [Google Scholar] [CrossRef]
- Mattei, A.; Sbarbati, M.; Fiasca, F.; Angelone, A.M.; Mazzei, M.C.; di Orio, F. Temporal trends in hospitalization for rotavirus gastroenteritis: A nationwide study in Italy, 2005–2012. Hum. Vaccines Immunother. 2016, 12, 534–539. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. Deaths Diarrheal Diseases. Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 6 March 2024).
- Chironna, M.; Loconsole, D.; Centrone, F.; Giordano, M. Sorveglianza Regionale delle Gastroenteriti Emorragiche in età Pediatrica. Report sulle Attività Svolte dal 21/06/2018 30/11/2021. Report Regione Puglia 2021. Available online: https://www.sanita.puglia.it/documents/20182/26606314/Report+GE+Puglia+2021_DEF.pdf/a3f002e6-c54a-4a9f-850b-217f74de1fe0 (accessed on 19 June 2024).
- Zizza, A.; Recchia, V.; Aloisi, A.; Guido, M. Clinical features of COVID-19 and SARS epidemics. A literature review. J. Prev. Med. Hyg. 2021, 62, E13–E24. [Google Scholar]
- Di Martino, G.; Cedrone, F.; Di Giovanni, P.; Tognaccini, L.; Trebbi, E.; Romano, F.; Staniscia, T. The Burden of HPV-Related Hospitalizations: Analysis of Hospital Discharge Records from the Years 2015–2021 from a Southern Italian Region. Pathogens 2023, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Cedrone, F.; Montagna, V.; Del Duca, L.; Camplone, L.; Mazzocca, R.; Carfagnini, F.; Fortunato, V.; Di Martino, G. The Burden of Streptococcus pneumoniae-Related Admissions and In-Hospital Mortality: A Retrospective Observational Study between the Years 2015 and 2022 from a Southern Italian Province. Vaccines 2023, 11, 1324. [Google Scholar] [CrossRef] [PubMed]
- Thwiny, H.T.; Alsalih, N.J.; Saeed, Z.F.; Al-Yasari, A.M.R.; Al-Saadawe, M.A.A.; Alsaadawi, M.A.E. Prevalence and seasonal pattern of enteric viruses among hospitalized children with acute gastroenteritis in Samawah, Iraq. J. Med. Life 2022, 15, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Gu, Y.; Wang, X.; Zhang, Y.; Zhan, L.; Liu, J.; Yan, H.; Liu, Y.; Zhen, S.; Chen, X.; et al. Epidemiological and clinical differences between sexes and pathogens in a three-year surveillance of acute infectious gastroenteritis in Shanghai. Sci. Rep. 2019, 9, 9993. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Taybeh, A.O.; Al-Nabulsi, A.; Al-Holy, M.; Hatmal, M.M.; Alzyoud, J.; Aolymat, I.; Abughoush, M.H.; Shahbaz, H.; Alzyoud, A.; et al. Common and Potential Emerging Foodborne Viruses: A Comprehensive Review. Life 2024, 14, 190. [Google Scholar] [CrossRef]
- Summa, M.; Tuutti, E.; Al-Hello, H.; Huttunen, L.M.; Rimhanen-Finne, R. Norovirus GII.17 Caused Five Outbreaks Linked to Frozen Domestic Bilberries in Finland, 2019. Food Environ. Virol. 2024, 16, 180–187. [Google Scholar] [CrossRef]
- Li, F.; Guo, L.; Li, Q.; Xu, H.; Fu, Y.; Huang, L.; Feng, G.; Liu, G.; Chen, X.; Xie, Z. Changes in the epidemiology and clinical characteristics of viral gastroenteritis among hospitalized children in the Mainland of China: A retrospective study from 2016 to 2020. BMC Pediatr. 2024, 24, 303. [Google Scholar] [CrossRef]
- Farfán-García, A.E.; Imdad, A.; Zhang, C.; Arias-Guerrero, M.Y.; Sánchez-Álvarez, N.T.; Iqbal, J.; Hernández-Gamboa, A.E.; Slaughter, J.C.; Gómez-Duarte, O.G. Etiology of acute gastroenteritis among children less than 5 years of age in Bucaramanga, Colombia: A case-control study. PLoS Negl. Trop. Dis. 2020, 14, e0008375. [Google Scholar] [CrossRef]
- Amodio, E.; De Grazia, S.; Genovese, D.; Bonura, F.; Filizzolo, C.; Collura, A.; Di Bernardo, F.; Giammanco, G.M. Clinical and Epidemiologic Features of Viral Gastroenteritis in Hospitalized Children: An 11-Year Surveillance in Palermo (Sicily). Viruses 2022, 15, 41. [Google Scholar] [CrossRef]
- Posovszky, C.; Buderus, S.; Classen, M.; Lawrenz, B.; Keller, K.M.; Koletzko, S. Acute Infectious Gastroenteritis in Infancy and Childhood. Dtsch. Arztebl. Int. 2020, 117, 615–624. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/ (accessed on 7 June 2024).
- Gauchan, E.; Malla, K.K. Relationship of Renal Function Tests and Electrolyte Levels with Severity of Dehydration in Acute Diarrhea. J. Nepal Health Res. Counc. 2015, 13, 84–89. [Google Scholar] [PubMed]
- Tutay, G.J.; Capraro, G.; Spirko, B.; Garb, J.; Smithline, H. Electrolyte profile of pediatric patients with hypertrophic pyloric stenosis. Pediatr. Emerg. Care 2013, 29, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Grassi, T.; De Donno, A.; Guido, M.; Gabutti, G.; Collaborative Group for the Surveillance of Rotavirus Infection. The epidemiology and disease burden of rotavirus infection in the Salento peninsula, Italy. Turk. J. Pediatr. 2008, 50, 132–136. [Google Scholar] [PubMed]
- Soares-Weiser, K.; Bergman, H.; Henschke, N.; Pitan, F.; Cunliffe, N. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2019, 2019, CD008521. [Google Scholar] [PubMed]
- Pindyck, T.; Tate, J.E.; Parashar, U.D. A decade of experience with rotavirus vaccination in the United States—Vaccine uptake, effectiveness, and impact. Expert Rev. Vaccines 2018, 17, 593–606. [Google Scholar] [CrossRef]
- Shah, M.P.; Dahl, R.M.; Parashar, U.D.; Lopman, B.A. Annual changes in rotavirus hospitalization rates before and after rotavirus vaccine implementation in the United States. PLoS ONE 2018, 13, e0191429. [Google Scholar] [CrossRef]
- Italian Ministry of Health. Vaccinazioni Dell’età Pediatrica e Dell’adolescenza—Coperture Vaccinali. Available online: https://www.salute.gov.it/imgs/C_17_tavole_20_11_0_file.pdf (accessed on 12 June 2024).
- Velasquez-Portocarrero, D.E.; Wang, X.; Cortese, M.M.; Snider, C.J.; Anand, A.; Costantini, V.P.; Yunus, M.; Aziz, A.B.; Haque, W.; Parashar, U.; et al. Head-to-head comparison of the immunogenicity of RotaTeq and Rotarix rotavirus vaccines and factors associated with seroresponse in infants in Bangladesh: A randomised, controlled, open-label, parallel, phase 4 trial. Lancet Infect. Dis. 2022, 22, 1606–1616. [Google Scholar] [CrossRef]
- Osborne, C.M.; Montano, A.C.; Robinson, C.C.; Schultz-Cherry, S.; Dominguez, S.R. Viral gastroenteritis in children in Colorado 2006–2009. J. Med. Virol. 2015, 87, 931–939. [Google Scholar] [CrossRef]
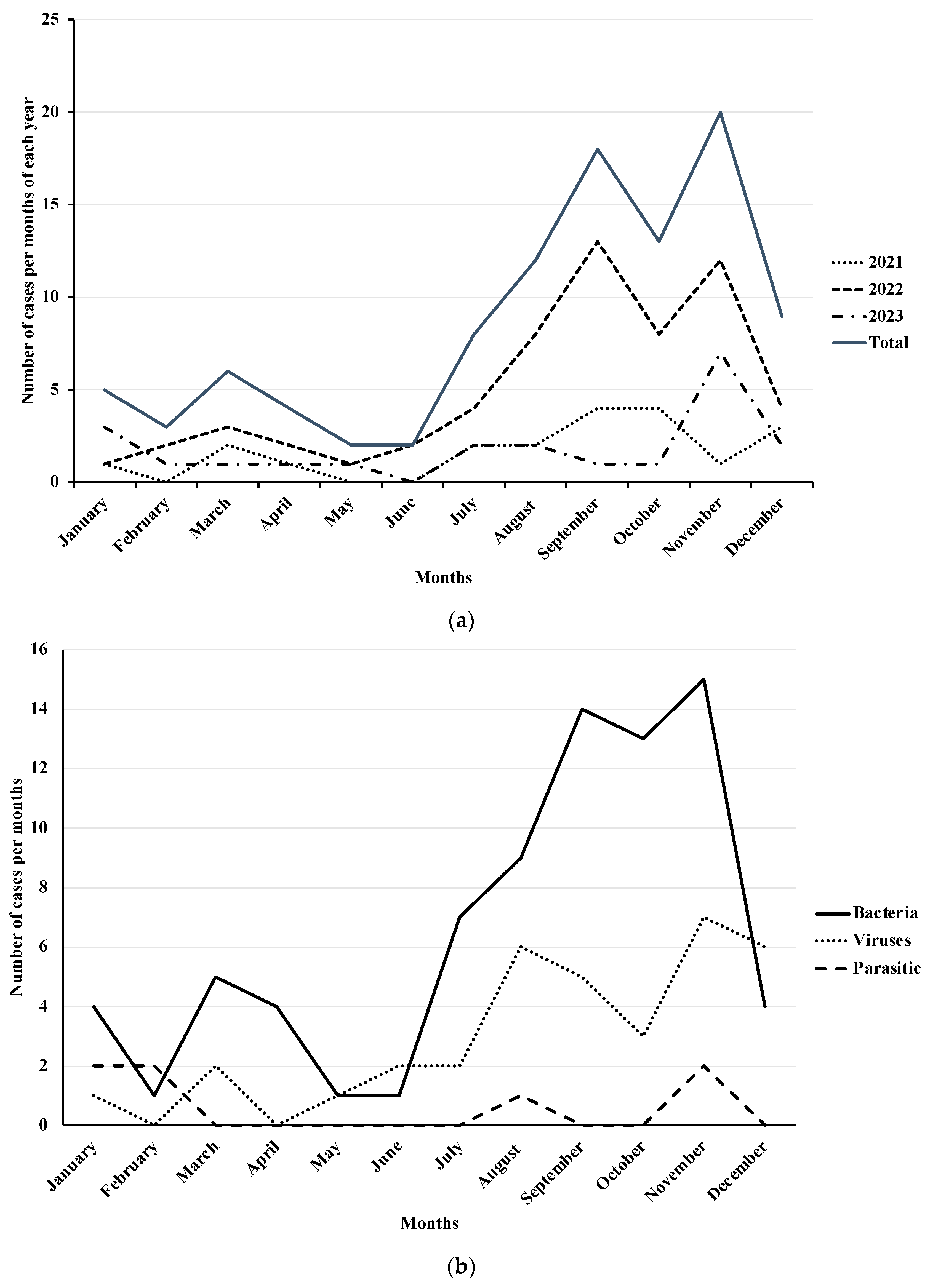
| Characteristics | All Patients (n = 103) | Group 1 (<5 Years) (n = 59) | Group 2 (≥5 Years) (n = 44) | p |
|---|---|---|---|---|
| Age, median years (IQR) | 3 (1–8) | 1 (1–2) | 9 (6–12.25) | 0.0001 ^ |
| Male, n. (%) | 60 (58.25) | 33 (55.9) | 27 (61.36) | 0.5803 ** |
| Hospital admission year, n. (%) | ||||
| - 2021, n. (%) | 20 (18.69) | 12 (20.34) | 8 (18.18) | |
| - 2022, n. (%) | 61 (57.01) | 39 (66.10) | 22 (40.00) | |
| - 2023, n. (%) | 26 (24.30) | 8 (13.56) | 14 (31.82) | 0.0782 ** |
| Hospital admission season (2021–2023), n. (%) | ||||
| - Summer, n. (%) | 37 (35.92) | 20 (33.90) | 17 (38.64) | |
| - Autumn, n. (%) | 39 (37.86) | 26 (44.07) | 13 (29.55) | |
| - Winter, n. (%) | 19 (18.45) | 10 (16.95) | 9 (20.45) | |
| - Spring, n. (%) | 8 (7.77) | 3 (5.08) | 5 (11.36) | 0.3903 ** |
| Hospital length of stay in days, median (IQR) | 5 (4–6) | 5 (4–6) | 5 (4–7) | 0.2762 ^ |
| Diagnosis | Group 1 (<5 Years) (n = 59) | Group 2 (≥5 Years) (n = 44) | p |
|---|---|---|---|
| Method | |||
| Coproculture, n. (%) | 4 (6.78) | 1 (4.54) | |
| Multiplex PCR, n. (%) | 55 (93.22) | 43 (97.72) | 0.3898 * |
| Microbiological pathogen detected | |||
| Bacterial infection, n. (%) | 40 (67.80) | 42 (95.45) | 0.0004 * |
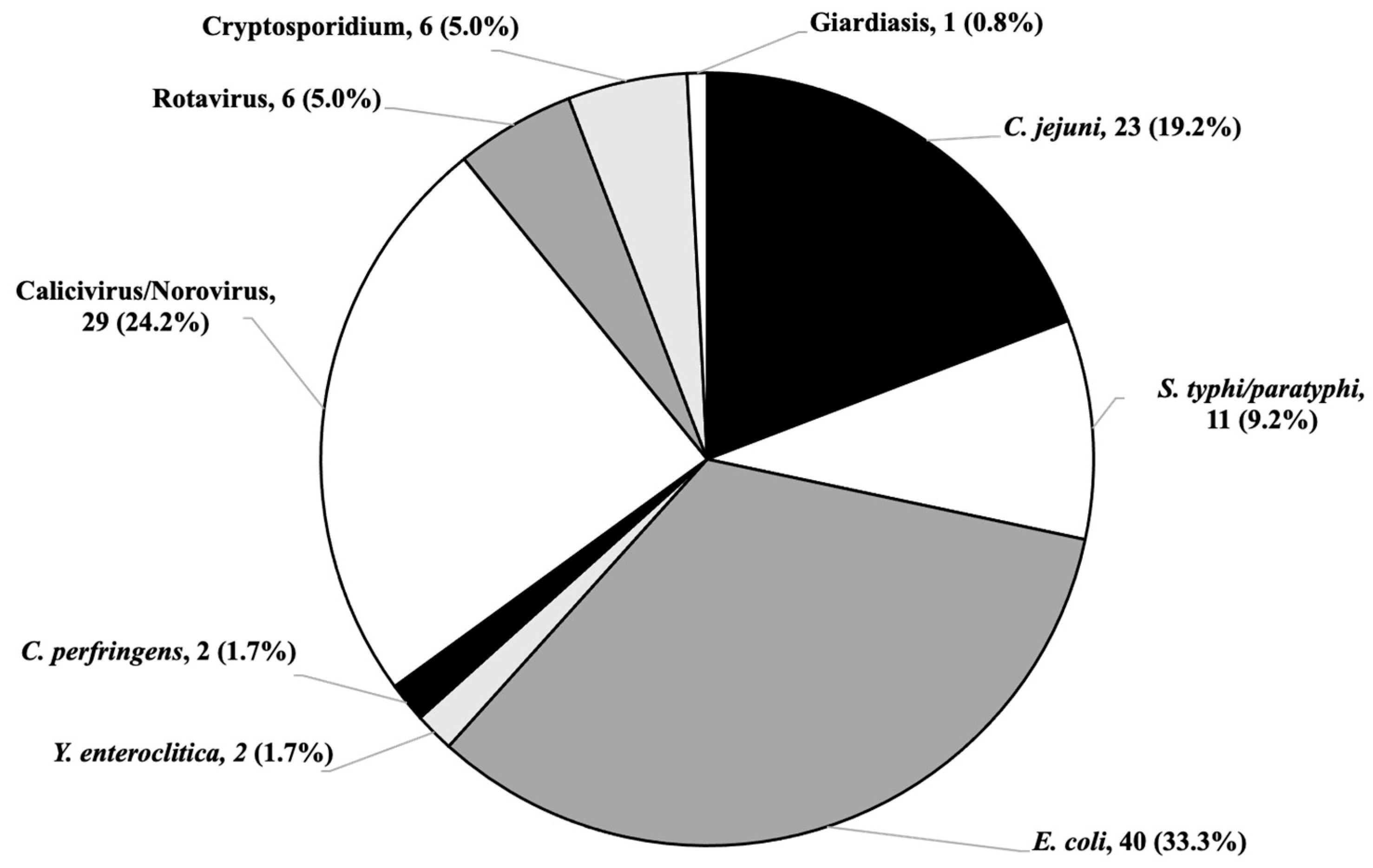
| C. jejuni, n. (%) | 8 (13.56) | 15 (34.09) | 0.1332 ** |
| S. typhi/Paratyphi, n. (%) | 5 (8.47) | 6 (13.64) | 0.4014 ** |
| E. coli, n. (%) | 25 (42.37) | 15 (34.09) | 0.3936 ** |
| Y. enterocolitica, n. (%) | 1 (1.69) | 1 (2.27) | 1.0000 * |
| C. perfringens, n. (%) | 1 (1.69) | 1 (2.27) | 1.0000 * |
| Viral infection, n. (%) | 26 (44.07) | 9 (20.45) | 0.0123 ** |
| Norovirus, n. (%) | 23 (38.98) | 6 (13.63) | 0.0047 ** |
| Rotavirus, n. (%) | 3 (5.08) | 3 (6.81) | 0.6989 * |
| Parasitic infection, n. (%) | 3 (5.08) | 5 (11.36) | 0.2820 * |
| Cryptosporidium, n. (%) | 3 (5.08) | 4 (9.09) | 0.4567 * |
| Giardiasis, n. (%) | 0 (-) | 1 (2.27) | 0.4272 * |
| Co-infections, n. (%) | 10 (16.95) | 7 (15.91) | 0.8881 ** |
| Hematology and Serum Chemistry Value | Group 1 (<5 Years) (n = 59) | Group 2 (≥5 Years) (n = 44) | p |
|---|---|---|---|
| Decreased Hematocrit | 22 (37.29) | 17 (38.63) | 0.8890 ** |
| Increased Lymphocytes | 22 (37.29) | 27 (61.36) | 0.0155 ** |
| Increased Neutrophils | 40 (67.80) | 29 (65.90) | 0.9918 ** |
| Increased CRP | 36 (61.02) | 29 (65.90) | 0.3605 ** |
| Decreased Sodium | 23 (38.98) | 22 (50.00) | 0.3605 ** |
| Decreased Potassium | 9 (15.25) | 3 (6.82) | 0.2274 * |
| Increased Creatinine | 20 (33.90) | 4 (8.33) | 0.0000 * |
| Increased AST | 3 (5.08) | 0 (-) | 0.2587 * |
| Increased ALT | 1 (1.69) | 0 (-) | 1.0000 * |
| Increased γ-GT | 0 (-) | 0 (-) | 1.0000 * |
| Increased Total bilirubin | 0 (-) | 0 (-) | 1.0000 * |
| Mixed Pathogens | All Patients (n = 103) | Group 1 (<5 Years) (n = 59) | Group 2 (≥5 Years) (n = 44) |
|---|---|---|---|
| Bacterial | |||
| E. coli + S. typhi/Paratyphi, n. (%) | 2 (1.94) | 1 (1.69) | 1 (2.27) |
| E. coli + C. jejuni, n. (%) | 3 (2.91) | (-) | 3 (6.81) |
| Bacterial + viral | |||
| E. coli + Norovirus, n. (%) | 6 (5.82) | 5 (0.72) | 1 (2.27) |
| C. jejuni + Rotavirus, n. (%) | 1 (0.97) | 1 (1.69) | (-) |
| C. jejuni + Norovirus, n. (%) | 1 (0.97) | (-) | 1 (2.27) |
| Y. enterocolitica + Rotavirus, n. (%) | 1 (0.97) | 1 (1.69) | (-) |
| Bacterial + parasitic | |||
| E. coli + Cryptosporidium, n. (%) | 2 (1.94) | 2 (3.39) | (-) |
| S. typhi/Paratyphi + Cryptosporidium, n. (%) | 1 (0.97) | (-) | 1 (2.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zizza, A.; Guido, M.; Sedile, R.; Benelli, M.; Nuzzo, M.; Paladini, P.; Romano, A.; Grima, P. A Multi-Pathogen Retrospective Study in Patients Hospitalized for Acute Gastroenteritis. Diseases 2024, 12, 213. https://doi.org/10.3390/diseases12090213
Zizza A, Guido M, Sedile R, Benelli M, Nuzzo M, Paladini P, Romano A, Grima P. A Multi-Pathogen Retrospective Study in Patients Hospitalized for Acute Gastroenteritis. Diseases. 2024; 12(9):213. https://doi.org/10.3390/diseases12090213
Chicago/Turabian StyleZizza, Antonella, Marcello Guido, Raffaella Sedile, Marzia Benelli, Milva Nuzzo, Pasquale Paladini, Anacleto Romano, and Pierfrancesco Grima. 2024. "A Multi-Pathogen Retrospective Study in Patients Hospitalized for Acute Gastroenteritis" Diseases 12, no. 9: 213. https://doi.org/10.3390/diseases12090213
APA StyleZizza, A., Guido, M., Sedile, R., Benelli, M., Nuzzo, M., Paladini, P., Romano, A., & Grima, P. (2024). A Multi-Pathogen Retrospective Study in Patients Hospitalized for Acute Gastroenteritis. Diseases, 12(9), 213. https://doi.org/10.3390/diseases12090213









