A 3D Bio-Printed-Based Model for Pancreatic Ductal Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Lines
2.2. Rheological Characterization
2.3. Cell Culture
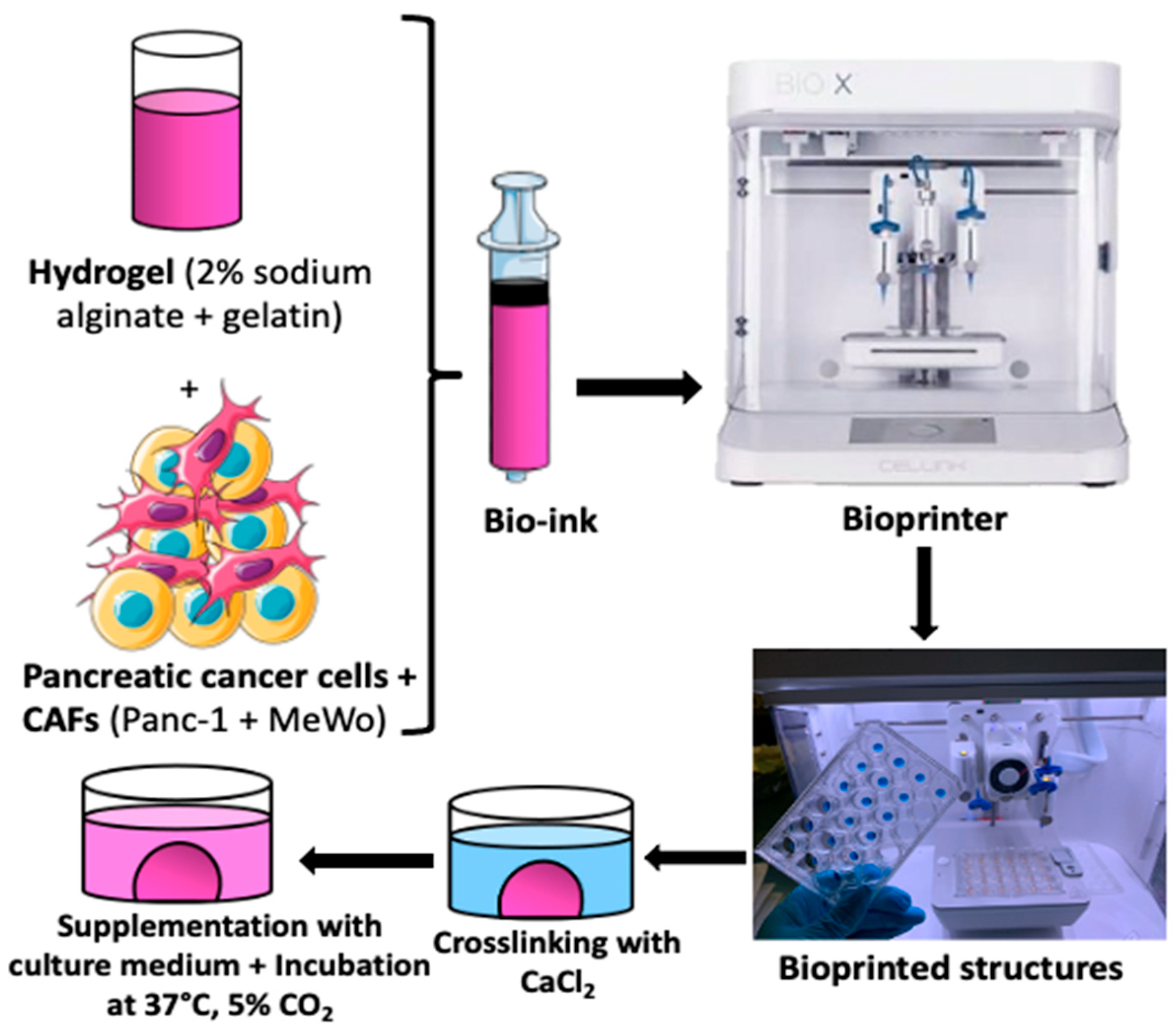
2.4. Tumoral Mass Design
2.5. Bio-Ink Preparation and Tumoral Mass 3D Bio-Printing
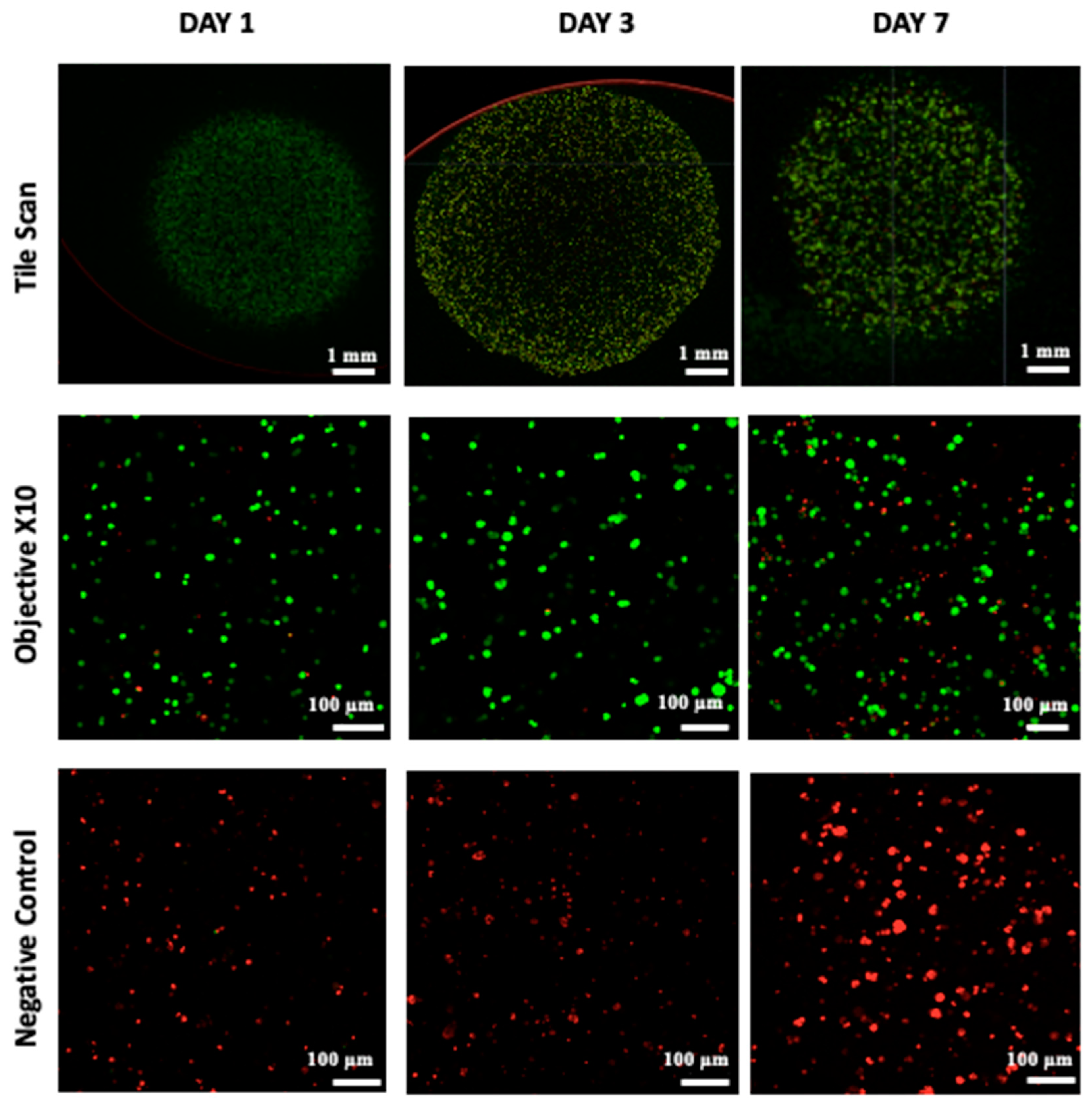
2.6. Cellular Viability
2.7. Metabolic Activity and Cell Proliferation
2.8. Histological and Immunohistochemical Analysis
- -
- The structures were first washed twice with Hank’s Balanced Salt Solution (HBSS) for 10 min at 37 °C and then fixed in a 4% paraformaldehyde solution containing 50 mM CaCl2 for 2 h at room temperature at each time point.
- -
- After fixation, the structures were washed twice with HBSS for 10 min each and then placed in a fresh HBSS solution at 4 °C for 45 min.
- -
- Afterwards, the structures were exposed to a treatment with 30% sucrose for 45 min at room temperature. The bio-printed structures were embedded in ShandonTM CryomatrixTM resin, frozen at −80 °C, and then sectioned.
- -
- The samples were cut into 10 µm thick sections using a cryostat with an enclosure set at −20 °C.
3. Results
3.1. Rheological Characterization
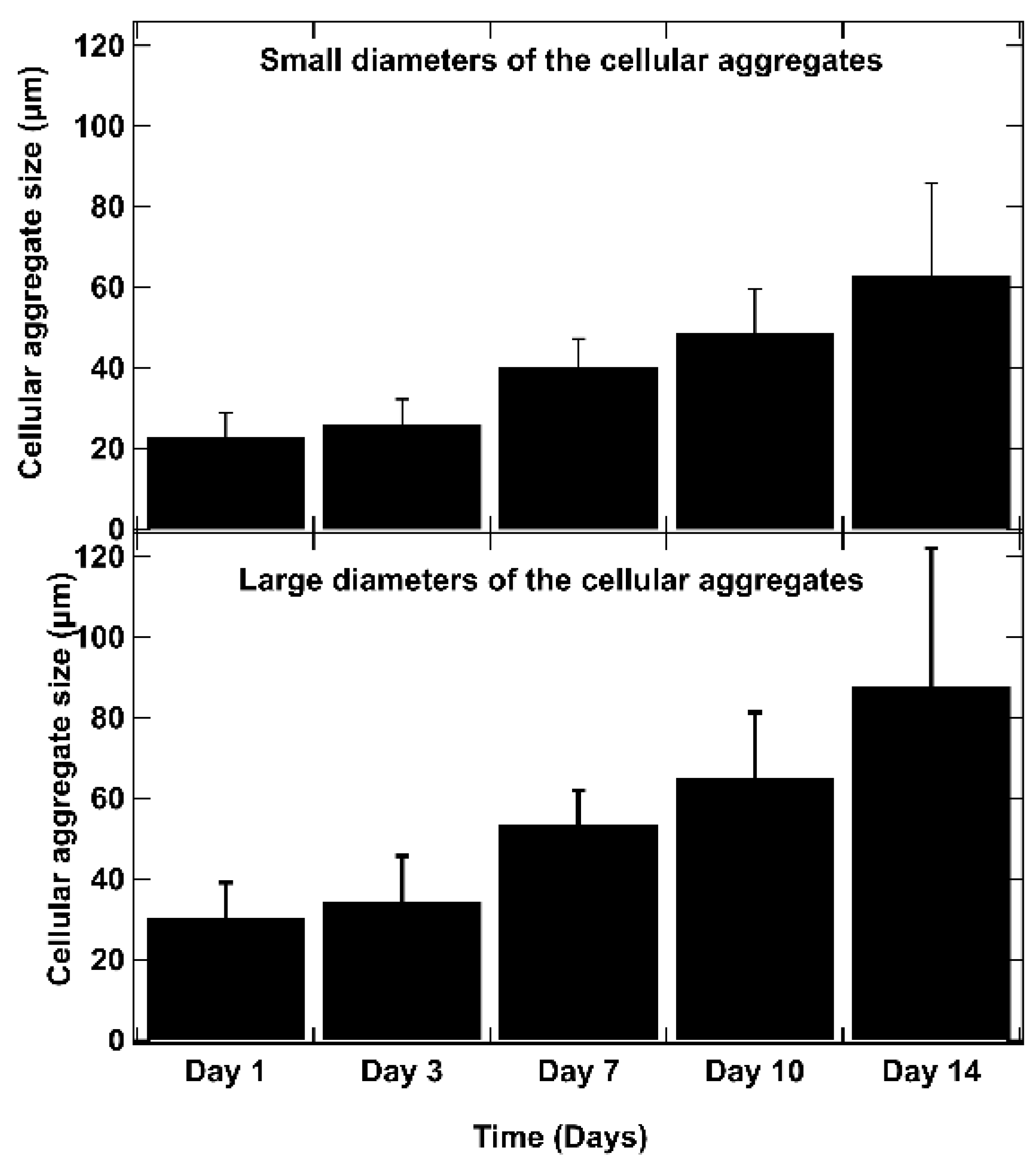
3.2. Optimization of Co-Culture (Ratios and Concentration)
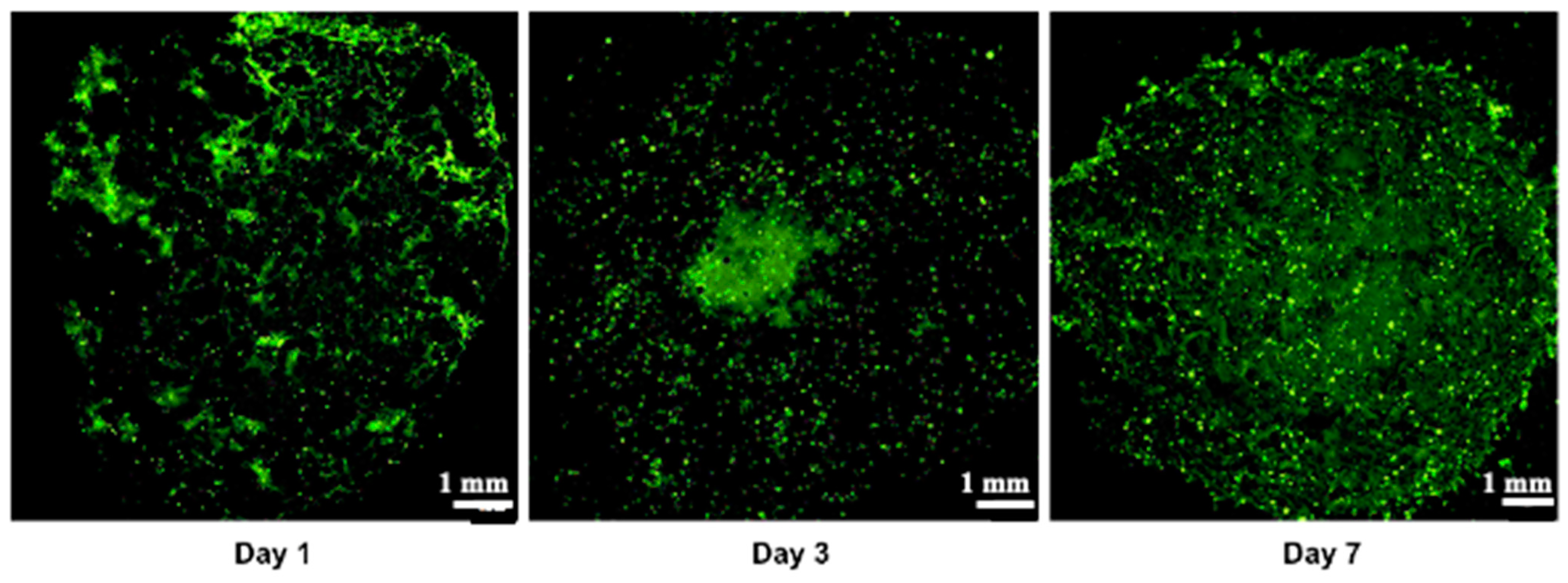
3.3. Proliferation and Cell Viability
3.4. Histological Analysis
3.5. Immunological Characterization
3.6. Creation of a Vascular Network
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buscail, L.; Bournet, B.; Cordelier, P. Le cancer du pancréas. Bull. L’acad. Natl. Méd. 2012, 196, 1819–1828. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef] [PubMed]
- Ushio, J.; Kanno, A.; Ikeda, E.; Ando, K.; Nagai, H.; Miwata, T.; Kawasaki, Y.; Tada, Y.; Yokoyama, K.; Numao, N.; et al. Pancreatic Ductal Adenocarcinoma: Epidemiology and Risk Factors. Diagnostics 2021, 11, 562. [Google Scholar] [CrossRef]
- Halbrook, C.J.; Lyssiotis, C.A.; di Magliano, M.P.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Dardare, J.; Witz, A.; Merlin, J.L.; Bochnakian, A.; Toussaint, P.; Gilson, P. Alexandre Harlé Epithelial to Mesenchymal Transition in Patients with Pancreatic Ductal Adenocarcinoma: State-of-the-Art and Therapeutic Opportunities. Pharmaceuticals 2021, 14, 740. [Google Scholar] [CrossRef]
- Ermis, M.; Falcone, N.; de Barros, N.R.; Mecwan, M.; Haghniaz, R.; Choroomi, A.; Monirizad, M.; Lee, Y.; Song, J.; Cho, H.-J.; et al. Tunable hybrid hydrogels with multicellular spheroids for modeling desmoplastic pancreatic cancer. Bioact. Mater. 2023, 25, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-D.; Song, J.; Kim, H.-A.; Im, C.-N.; Khawar, I.A.; Park, J.K.; Kuh, H.-J. Anti-Cancer Activity Profiling of Chemotherapeutic Agents in 3D Co-Cultures of Pancreatic Tumor Spheroids with Cancer-Associated Fibroblasts and Macrophages. Cancers 2021, 13, 5955. [Google Scholar] [CrossRef]
- Steinberg, E.; Orehov, N.; Tischenko, K.; Schwob, O.; Zamir, G.; Hubert, A.; Manevitch, Z.; Benny, O. Rapid Clearing for High Resolution 3D Imaging of Ex Vivo Pancreatic Cancer Spheroids. Int. J. Mol. Sci. 2020, 21, 7703. [Google Scholar] [CrossRef]
- Hakobyan, D.; Médina, C.; Dusserre, N.; Stachowicz, M.-L.; Handschin, C.; Fricain, J.-C.; Guillermet-Guibert, J.; Oliveira, H. Laser-assisted 3D bioprinting of exocrine pancreas spheroid models for cancer initiation study. Biofabrication 2020, 12, 035001. [Google Scholar] [CrossRef]
- Sokolowska, P.; Zuchowska, A.; Brzozka, Z. Why Can Organoids Improve Current Organ-on-Chip Platforms? Organoids 2022, 1, 69–84. [Google Scholar] [CrossRef]
- Szűcs, D.; Fekete, Z.; Guba, M.; Kemény, L.; Jemnitz, K.; Kis, E.; Veréb, Z. Toward better drug development: Three-dimensional bioprinting in toxicological research. Int. J. Bioprint. 2022, 9, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Dellaquila, A.; Le Bao, C.; Letourneur, D.; Simon-Yarza, T. In Vitro Strategies to Vascularize 3D Physiologically Relevant Models. Adv. Sci. 2021, 8, 2100798. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Liu, Y.; Qiu, J.; Wan, W. Bioprinting and its Use in Tumor-On-A-Chip Technology for Cancer Drug Screening: A Review. Int. J. Bioprint. 2022, 8, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Carvalho, V.; Bañobre-López, M.; Minas, G.; Teixeira, S.F.; Lima, R.; Rodrigues, R.O. The integration of spheroids and organoids into organ-on-a-chip platforms for tumour research: A review. Bioprinting 2022, 27, e00224. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Mostafa, A.M.; Morton, J.P.; Hawinkels, L.J.; Prakash, J. Translating complexity and heterogeneity of pancreatic tumor: 3D in vitro to in vivo models. Adv. Drug Deliv. Rev. 2021, 174, 265–293. [Google Scholar] [CrossRef]
- Gu, A.; Li, J.; Qiu, S.; Hao, S.; Yue, Z.-Y.; Zhai, S.; Li, M.-Y.; Liu, Y. Pancreatic cancer environment: From patient-derived models to single-cell omics. Mol. Omics 2024, 20, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Nicolas, V.; Matsusaki, M.; Akashi, M.; Couvreur, P.; Mura, S. Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity. Acta Biomater. 2018, 78, 296–307. [Google Scholar] [CrossRef]
- Kuen, J.; Darowski, D.; Kluge, T.; Majety, M. Pancreatic cancer cell/fibroblast co-culture induces M2 like macrophages that influence therapeutic response in a 3D model. PLoS ONE 2017, 12, e0182039. [Google Scholar] [CrossRef]
- Liu, X.; Gündel, B.; Li, X.; Liu, J.; Wright, A.; Löhr, M.; Arvidsson, G.; Heuchel, R. 3D heterospecies spheroids of pancreatic stroma and cancer cells demonstrate key phenotypes of pancreatic ductal adenocarcinoma. Transl. Oncol. 2021, 14, 101107. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Choi, Y.; Cho, D.-W. Application of Gelatin Bioinks and Cell-Printing Technology to Enhance Cell Delivery Capability for 3D Liver Fibrosis-on-a-Chip Development. ACS Biomater. Sci. Eng. 2020, 6, 2469–2477. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef]
- Rahmani Dabbagh, S.; Rezapour Sarabi, M.; Birtek, M.T.; Mustafaoglu, N.; Zhang, Y.S.; Tasoglu, S. 3D bioprinted organ-on-chips. Aggregate 2023, 4, e197. [Google Scholar] [CrossRef]
- Persaud, A.; Maus, A.; Strait, L.; Zhu, D. 3D Bioprinting with Live Cells. Eng. Regen. 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Zimmer, J.; Castriconi, R.; Scaglione, S. Editorial: Recent 3D Tumor Models for Testing Immune-Mediated Therapies. Front. Immunol. 2021, 12, 798493. [Google Scholar] [CrossRef] [PubMed]
- Flores-Torres, S.; Jiang, T.; Kort-Mascort, J.; Yang, Y.; Peza-Chavez, O.; Pal, S.; Mainolfi, A.; Pardo, L.A.; Ferri, L.; Bertos, N.; et al. Constructing 3D In Vitro Models of Heterocellular Solid Tumors and Stromal Tissues Using Extrusion-Based Bioprinting. ACS Biomater. Sci. Eng. 2023, 9, 542–561. [Google Scholar] [CrossRef]
- Jung, M.; Skhinas, J.N.; Du, E.Y.; Tolentino, M.A.K.; Utama, R.H.; Engel, M.; Volkerling, A.; Sexton, A.; O’Mahony, A.P.; Ribeiro, J.C.C.; et al. A high-throughput 3D bioprinted cancer cell migration and invasion model with versatile and broad biological applicability. Biomater. Sci. 2022, 10, 5876–5887. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, W.; Kuss, M.A.; Mirza, S.; Qi, D.; Krasnoslobodtsev, A.; Zeng, J.; Band, H.; Band, V.; Duan, B. 3D Bioprinting of Breast Cancer Models for Drug Resistance Study. ACS Biomater. Sci. Eng. 2018, 4, 4401–4411. [Google Scholar] [CrossRef]
- Dai, X.; Ma, C.; Lan, Q.; Xu, T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication 2016, 8, 045005. [Google Scholar] [CrossRef]
- Baka, Z.; Godier, C.; Lamy, L.; Mallick, A.; Gribova, V.; Figarol, A.; Bezdetnaya, L.; Chateau, A.; Magne, Z.; Stiefel, M.; et al. A Coculture Based, 3D Bioprinted Ovarian Tumor Model Combining Cancer Cells and Cancer Associated Fibroblasts. Macromol. Biosci. 2023, 23, e2200434. [Google Scholar] [CrossRef] [PubMed]
- Chaji, S.; Al-Saleh, J.; Gomillion, C.T. Bioprinted Three-Dimensional Cell-Laden Hydrogels to Evaluate Adipocyte-Breast Cancer Cell Interactions. Gels 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Song, J.M. 3D bioprinted drug-resistant breast cancer spheroids for quantitative in situ evaluation of drug resistance. Acta Biomater. 2022, 138, 228–239. [Google Scholar] [CrossRef]
- Horder, H.; Lasheras, M.G.; Grummel, N.; Nadernezhad, A.; Herbig, J.; Ergün, S.; Teßmar, J.; Groll, J.; Fabry, B.; Bauer-Kreisel, P.; et al. Bioprinting and Differentiation of Adipose-Derived Stromal Cell Spheroids for a 3D Breast Cancer-Adipose Tissue Model. Cells 2021, 10, 803. [Google Scholar] [CrossRef] [PubMed]
- Langer, E.M.; Allen-Petersen, B.L.; King, S.M.; Kendsersky, N.D.; Turnidge, M.A.; Kuziel, G.M.; Riggers, R.; Samatham, R.; Amery, T.S.; Steven, L.; et al. Modeling Tumor Phenotypes In Vitro with Three-Dimensional Bioprinting. Cell Rep. 2019, 26, 608–623.e6. [Google Scholar] [CrossRef]
- Sgarminato, V.; Licciardello, M.; Ciardelli, G.; Tonda-Turo, C. Melt-electrowriting of 3D anatomically relevant scaffolds to recreate a pancreatic acinar unit in vitro. Int. J. Bioprint. 2024, 10, 1975. [Google Scholar] [CrossRef]
- Abadpour, S.; Aizenshtadt, A.; Olsen, P.A.; Shoji, K.; Wilson, S.R.; Krauss, S.; Scholz, H. Pancreas-on-a-Chip Technology for Transplantation Applications. Curr. Diabetes Rep. 2020, 20, 72. [Google Scholar] [CrossRef]
- Sokolowska, P.; Zukowski, K.; Janikiewicz, J.; Jastrzebska, E.; Dobrzyn, A.; Brzozka, Z. Islet-on-a-chip: Biomimetic micropillar-based microfluidic system for three-dimensional pancreatic islet cell culture. Biosens. Bioelectron. 2021, 183, 113215. [Google Scholar] [CrossRef]
- Huang, B.; Wei, X.; Chen, K.; Wang, L.; Xu, M. Bioprinting of hydrogel beads to engineer pancreatic tumor-stroma microtissues for drug screening. Int. J. Bioprint. 2023, 9, 676. [Google Scholar] [CrossRef]
- Wei, X.; Wu, Y.; Chen, K.; Wang, L.; Xu, M. Embedded bioprinted multicellular spheroids modeling pancreatic cancer bioarchitecture towards advanced drug therapy. J. Mater. Chem. B 2024, 12, 1788–1797. [Google Scholar] [CrossRef]
- Novák, Š.; Kolář, M.; Szabó, A.; Vernerová, Z.; Lacina, L.; Strnad, H.; Šáchová, J.; Hradilová, M.; Havránek, J.; Španko, M.; et al. Desmoplastic Crosstalk in Pancreatic Ductal Adenocarcinoma Is Reflected by Different Responses of Panc-1, MIAPaCa-2, PaTu-8902, and CAPAN-2 Cell Lines to Cancer-associated/Normal Fibroblasts. Cancer Genom.—Proteom. 2021, 18, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Fulawka, L.; Halon, A. Proliferation Index Evaluation in Breast Cancer Using ImageJ and ImmunoRatio Applications. Anticancer. Res. 2016, 36, 3965–3972. [Google Scholar] [PubMed]
- Jain, R.; Fischer, S.; Serra, S.; Chetty, R. The Use of Cytokeratin 19 (CK19) Immunohistochemistry in Lesions of the Pancreas, Gastrointestinal Tract, and Liver. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Baba, H.; Yoshida, N.; Miyake, K.; Yasuda, T.; Uchihara, T.; Tan, P.; Ishimoto, T. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene 2019, 38, 4887–4901. [Google Scholar] [CrossRef] [PubMed]
- Handra-Luca, A.; Hong, S.-M.; Walter, K.; Wolfgang, C.; Hruban, R.; Goggins, M. Tumour epithelial vimentin expression and outcome of pancreatic ductal adenocarcinomas. Br. J. Cancer 2011, 104, 1296–1302. [Google Scholar] [CrossRef] [PubMed]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2019, 146, 895–905. [Google Scholar] [CrossRef]
- Labowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A Review on the Adaption of Alginate-Gelatin Hydrogels for 3D Cultures and Bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Hayashi, T.; Kunita, I.; Nakamasu, A.; Torisawa, Y.-S.; Nakayama, M.; Takigawa-Imamura, H.; Kotera, H.; Nishiyama, K.; Miura, T.; et al. Integrating perfusable vascular networks with a three-dimensional tissue in a microfluidic device. Integr. Biol. 2017, 9, 506–518. [Google Scholar] [CrossRef]
- Wang, P.; Sun, L.; Li, C.; Jin, B.; Yang, H.; Wu, B.; Mao, Y. Study on drug screening multicellular model for colorectal cancer constructed by three-dimensional bioprinting technology. Int. J. Bioprint. 2023, 9, 379–398. [Google Scholar] [CrossRef]
- Liu, J.; Chuah, Y.J.; Fu, J.; Zhu, W.; Wang, D.-A. Co-culture of human umbilical vein endothelial cells and human bone marrow stromal cells into a micro-cavitary gelatin-methacrylate hydrogel system to enhance angiogenesis. Mater. Sci. Eng. C 2019, 102, 906–916. [Google Scholar] [CrossRef]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The Pancreas Cancer Microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef] [PubMed]
- Swayden, M.; Soubeyran, P.; Iovanna, J. Upcoming Revolutionary Paths in Preclinical Modeling of Pancreatic Adenocarcinoma. Front. Oncol. 2020, 9, 1443. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T.; Reshkin, S.J.; Carvalho, T.M.A.; Di Molfetta, D.; Greco, M.R.; Alfarouk, K.O.; Cardone, R.A. Resistance to Gemcitabine in Pancreatic Ductal Adenocarcinoma: A Physiopathologic and Pharmacologic Review. Cancers 2022, 14, 2486. [Google Scholar] [CrossRef] [PubMed]
- Mollica, P.A.; Booth-Creech, E.N.; Reid, J.A.; Zamponi, M.; Sullivan, S.M.; Palmer, X.-L.; Sachs, P.C.; Bruno, R.D. 3D bioprinted mammary organoids and tumoroids in human mammary derived ECM hydrogels. Acta Biomater. 2019, 95, 201–213. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, R.; Ouyang, L.; Ding, H.; Zhang, T.; Zhang, K.; Cheng, S.; Sun, W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication 2014, 6, 035001. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Dai, X.; Wang, X.; Li, X.; Diao, J.; Tao, X. Tumor-like lung cancer model based on 3D bioprinting. 3 Biotech 2018, 8, 501. [Google Scholar] [CrossRef]
- Bojin, F.; Robu, A.; Bejenariu, M.I.; Ordodi, V.; Olteanu, E.; Cean, A.; Popescu, R.; Neagu, M.; Gavriliuc, O.; Neagu, A.; et al. 3D Bioprinting of Model Tissues That Mimic the Tumor Microenvironment. Micromachines 2021, 12, 535. [Google Scholar] [CrossRef]
- Lin, F.; Chen, Z.E.; Wang, H.L. Utility of Immunohistochemistry in the Pancreatobiliary Tract. Arch. Pathol. Lab. Med. 2015, 139, 24–38. [Google Scholar] [CrossRef]
- Yakavets, I.; Francois, A.; Benoit, A.; Merlin, J.-L.; Bezdetnaya, L.; Vogin, G. Advanced co-culture 3D breast cancer model for investigation of fibrosis induced by external stimuli: Optimization study. Sci. Rep. 2020, 10, 21273. [Google Scholar] [CrossRef]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning Alginate-Gelatin Bioink Properties by Varying Solvent and Their Impact on Stem Cell Behavior. Sci. Rep. 2018, 8, 8020. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Pang, Y.; Li, L.; Chen, Z.-N.; Sun, W. 3D bioprinting of hepatoma cells and application with microfluidics for pharmacodynamic test of Metuzumab. Biofabrication 2019, 11, 034102. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.H.T.; Lee, E.; Alimperti, S.; Norgard, R.J.; Wong, A.; Lee, J.J.K.; Eyckmans, J.; Ben, Z.; Stanger, B.Z.; Chen, C.S. A biomimetic pancreatic cancer on-chip reveals endothelial ablation via ALK7 signaling. Sci. Adv. 2019, 5, eaav6789. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.; Li, D.; Yao, Y. Tumor-on-a-chip: Perfusable vascular incorporation brings new approach to tumor metastasis research and drug development. Front. Bioeng. Biotechnol. 2022, 10, 1057913. [Google Scholar] [CrossRef] [PubMed]
- Quintard, C.; Jonsson, G.; Laporte, C.; Bissardon, C.; Pitaval, A.; Werschler, N.; Leopoldi, A.; AHagelkrüys, A.; Blandin, P.; Achard, J.-L.; et al. An automated microfluidic platform integrating functional vascularized organoids-on-chip. Nat. Commun. 2024, 15, 1452. [Google Scholar]
- O’connor, C.; Brady, E.; Zheng, Y.; Moore, E.; Stevens, K.R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 2022, 7, 702–716. [Google Scholar] [CrossRef]
- Liu, Q.; Liao, Q.; Zhao, Y. Chemotherapy and tumor microenvironment of pancreatic cancer. Cancer Cell Int. 2017, 17, 68. [Google Scholar] [CrossRef]





| Bio-Ink Designation | Cell Type | Medium |
|---|---|---|
| P | Panc-1 cells | Complete DMEM (comprising 10% FBS) |
| M | MeWo cells | Complete MEM (comprising 10% FBS) |
| PM | Panc-1 cells + MeWo cells (1:4 ratio) | Complete DMEM + MEM (1:1 ratio) |
| PMH | Panc-1 cells + MeWo cells + HUVEC (1:4:4 ratio) | Complete ECGM |
| Parameters | Corresponding Value |
|---|---|
| Internal diameter of the printed needle | 23 G (0.66 mm) |
| Printhead temperature | 37 °C |
| Printing bed temperature | 8 °C |
| Extrusion pressure | 15–30 kPa |
| Printhead movement speed | 5 mm·s−1 |
| Reagent | Calcein | Ethidium Homo-Dimer 1 |
|---|---|---|
| Excitation/emission wavelength (nm) | 494/517 nm | 528/617 nm |
| Standard set filter | Green channel: (EX/EM = 488/520 nm) | Red channel: (EX/EM = 561/596 nm) |
| Antibody | Clone | Dilution | Positivity |
|---|---|---|---|
| SOX10 | Monoclonal clone EP-268 | 1/200 | MeWo |
| Anti-CK19 | Monoclonal clone RCK108 | 1/100 | Panc-1 |
| Anti-Ki67 | Monoclonal clone Mib1 | 1/50 | MeWo Panc-1 |
| Vimentin | Clone V9 | 1/200 | Panc-1 MeWo |
| Reagent | GFP |
|---|---|
| Excitation/emission wavelength (nm) | 482/502 nm |
| Standard set filter | Green channel: (EX/EM = 460/510 nm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godier, C.; Baka, Z.; Lamy, L.; Gribova, V.; Marchal, P.; Lavalle, P.; Gaffet, E.; Bezdetnaya, L.; Alem, H. A 3D Bio-Printed-Based Model for Pancreatic Ductal Adenocarcinoma. Diseases 2024, 12, 206. https://doi.org/10.3390/diseases12090206
Godier C, Baka Z, Lamy L, Gribova V, Marchal P, Lavalle P, Gaffet E, Bezdetnaya L, Alem H. A 3D Bio-Printed-Based Model for Pancreatic Ductal Adenocarcinoma. Diseases. 2024; 12(9):206. https://doi.org/10.3390/diseases12090206
Chicago/Turabian StyleGodier, Claire, Zakaria Baka, Laureline Lamy, Varvara Gribova, Philippe Marchal, Philippe Lavalle, Eric Gaffet, Lina Bezdetnaya, and Halima Alem. 2024. "A 3D Bio-Printed-Based Model for Pancreatic Ductal Adenocarcinoma" Diseases 12, no. 9: 206. https://doi.org/10.3390/diseases12090206
APA StyleGodier, C., Baka, Z., Lamy, L., Gribova, V., Marchal, P., Lavalle, P., Gaffet, E., Bezdetnaya, L., & Alem, H. (2024). A 3D Bio-Printed-Based Model for Pancreatic Ductal Adenocarcinoma. Diseases, 12(9), 206. https://doi.org/10.3390/diseases12090206







