Evaluation of the Use of Cell Lines in Studies of Selenium-Dependent Glutathione Peroxidase 2 (GPX2) Involvement in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
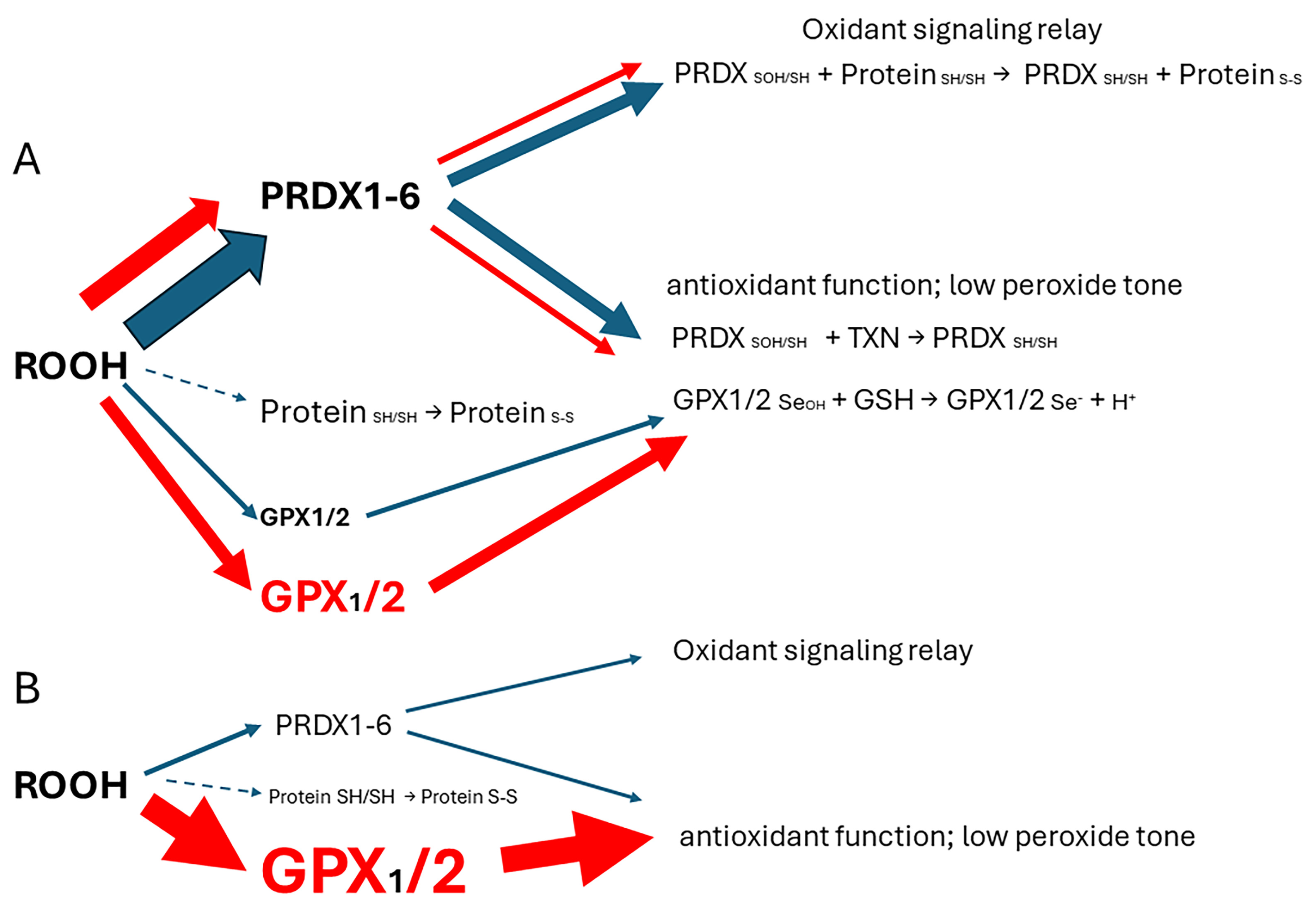
3. Results and Discussion
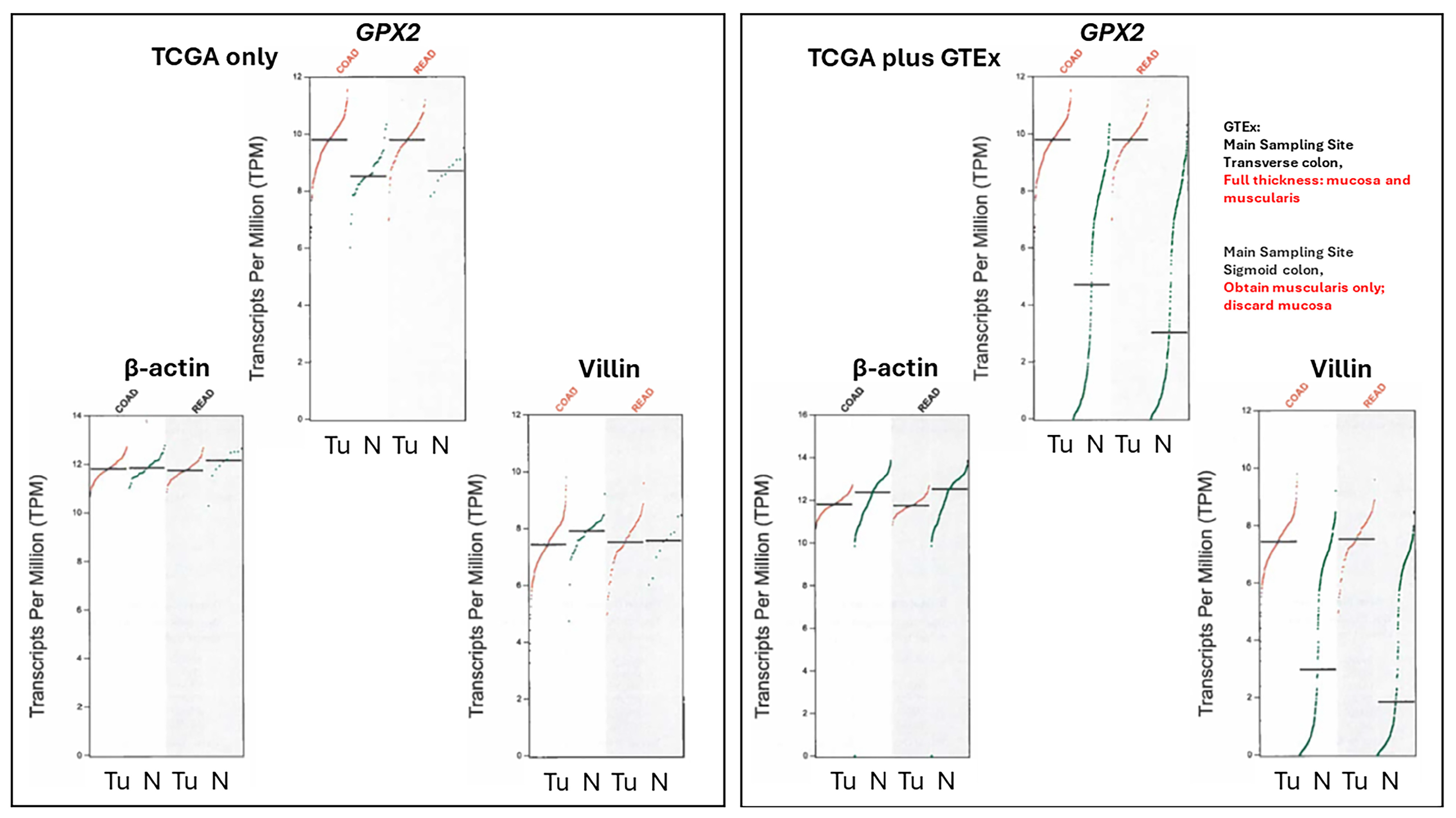
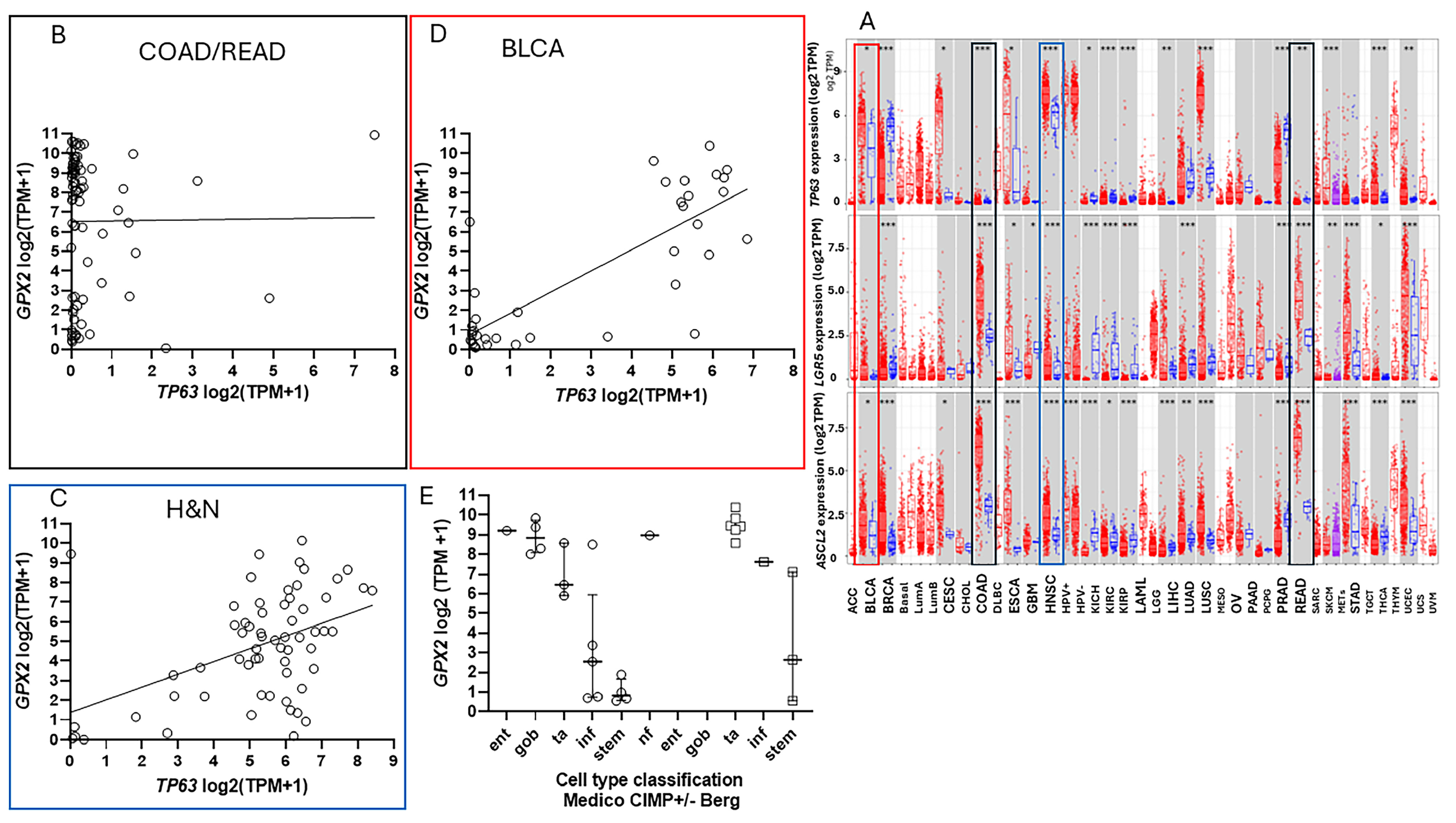
3.1. Correlation of GPX2 Levels in Tumors and Cell Lines
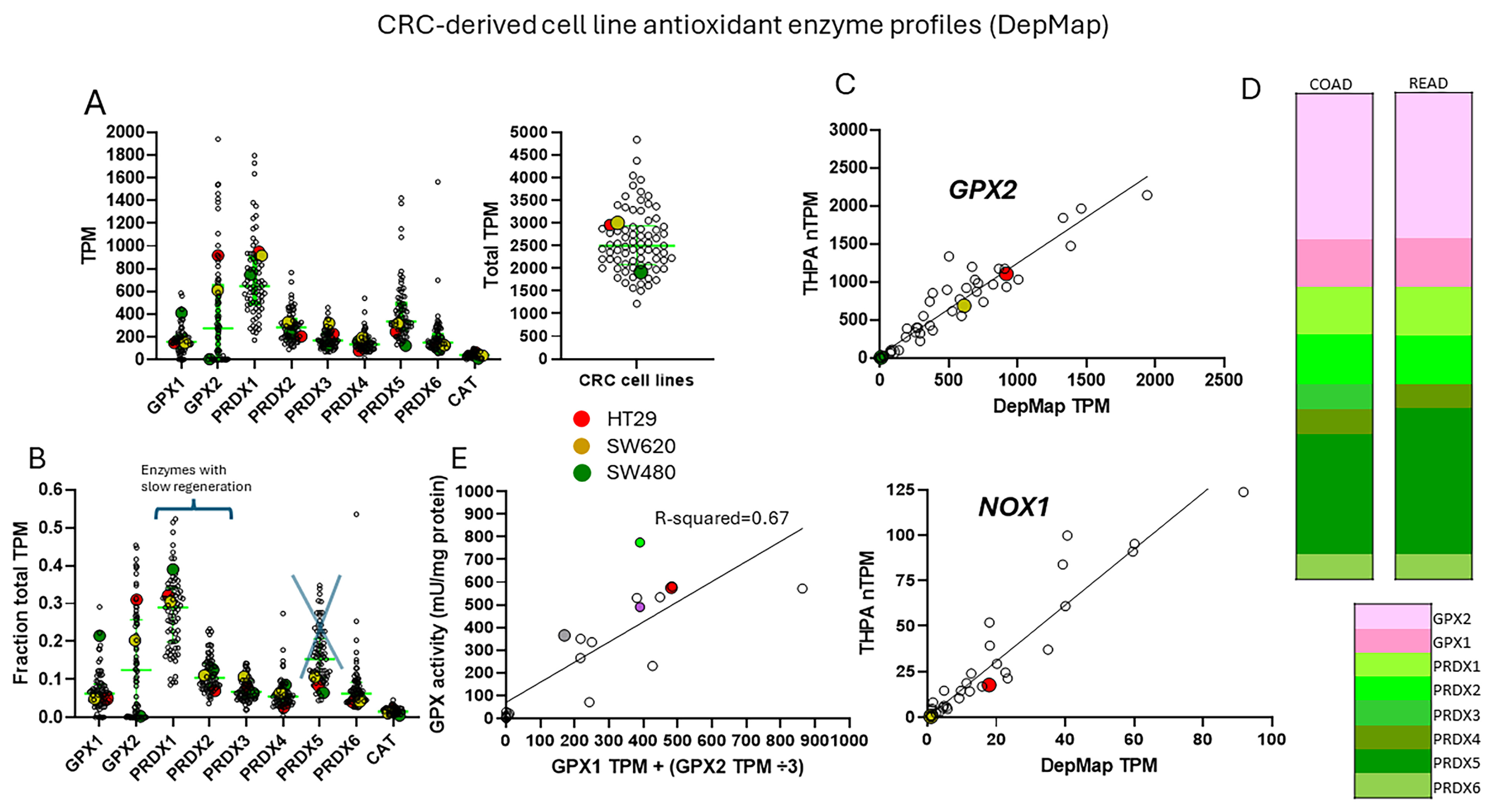
3.2. The Choice of CRC-Derived Cell Lines for a Case Study: GPX2 and NOX1
3.3. Remaining Uncertainties in GPX2 Expression in Normal Colon/Rectum
3.4. The Problems with Databases Combined with the Issues of Normal Cell Expression
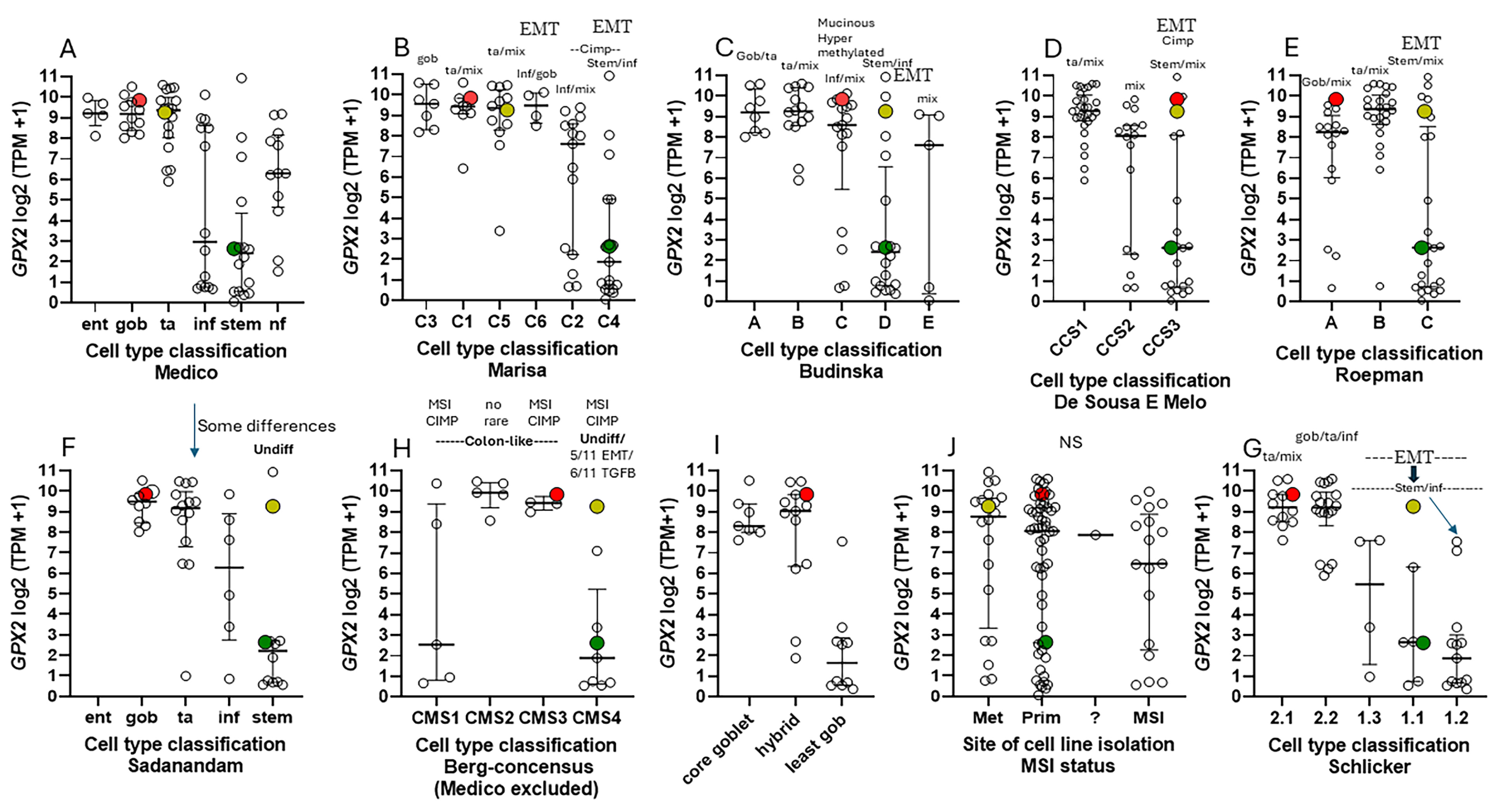
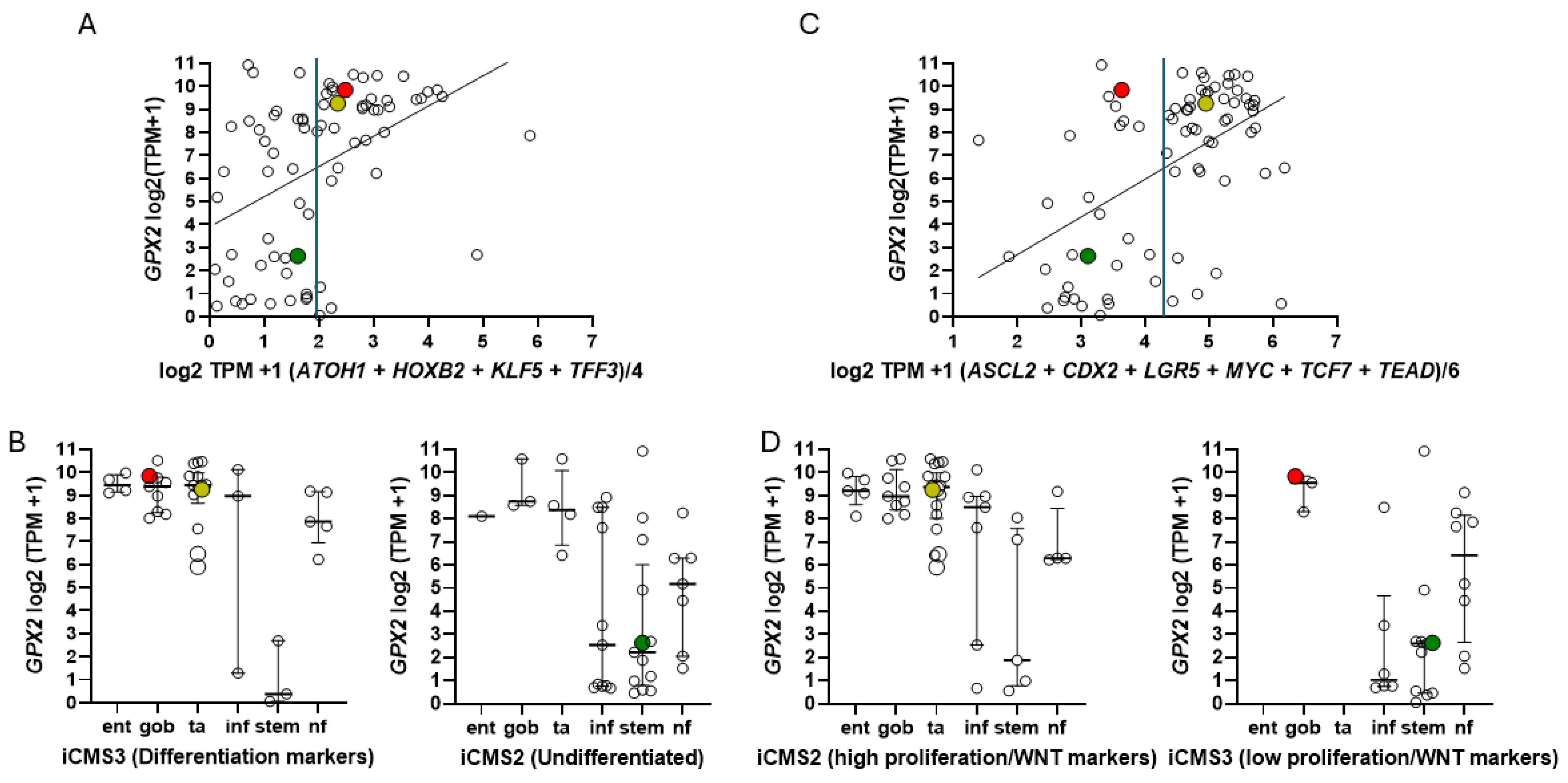
3.5. Efforts to Assist Investigators in Selection of Cell Lines
3.6. The Issue of Low GPX2 Expressing CRC-Derived Cell Lines and Circumvention
3.7. How Different Are CRC-Derived Cell Lines Sorted into Classes by Medico et al. for Expression Levels of Genes Encoding Proteins with Reactive Sulfhydryl Groups?
3.8. Intratumor Variation, Plasticity of Cell Components, Stromal Cell Interactions, and GPX2
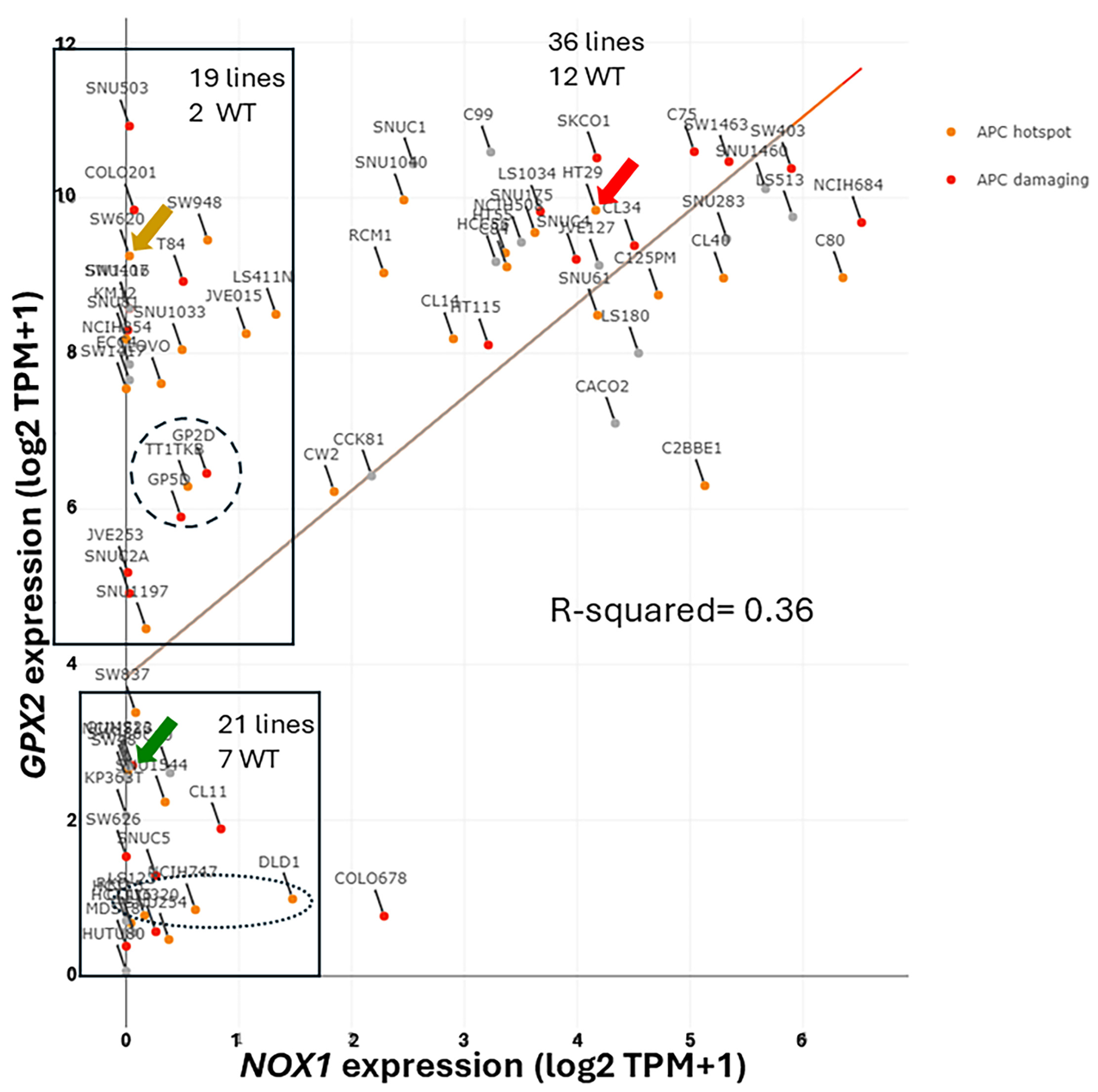
3.9. NOX1 in CRC and Links to GPX2
3.10. Low GPX2 Expression in Cell Lines, Redux
4. Summary
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esworthy, R.S.; Chu, F.F. Using Information from Public Databases to Critically Evaluate Studies Linking the Antioxidant Enzyme Selenium-Dependent Glutathione Peroxidase 2 (GPX2) to Cancer. BioMedInformatics 2023, 3, 985–1014. [Google Scholar] [CrossRef]
- Esworthy, R.S.; Doroshow, J.H.; Chu, F.F. The beginning of GPX2 and 30 years later. Free Radic. Biol. Med. 2022, 188, 419–433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef] [PubMed]
- Villar, S.F.; Corrales-González, L.; Márquez de Los Santos, B.; Dalla Rizza, J.; Zeida, A.; Denicola, A.; Ferrer-Sueta, G. Kinetic and structural assessment of the reduction of human 2-Cys peroxiredoxins by thioredoxins. FEBS J. 2024, 291, 778–794. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, C.; Wang, Y.; Shi, Y.; Yuan, L.; Xu, J.; Zhang, Y.; Chen, J.; Wei, Q.; Qin, J.; et al. Glutathione peroxidase 2 knockdown suppresses gastric cancer progression and metastasis via regulation of kynurenine metabolism. Oncogene 2023, 42, 1994–2006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Medico, E.; Russo, M.; Picco, G.; Cancelliere, C.; Valtorta, E.; Corti, G.; Buscarino, M.; Isella, C.; Lamba, S.; Martinoglio, B.; et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat. Commun. 2015, 6, 7002. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, A.; Lyssiotis, C.A.; Homicsko, K.; Collisson, E.A.; Gibb, W.J.; Wullschleger, S.; Ostos, L.C.; Lannon, W.A.; Grotzinger, C.; Del Rio, M.; et al. A colorectal cancer classification system that associates cellular phenotype and responses to therapy. Nat. Med. 2013, 19, 619–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marisa, L.; de Reyniès, A.; Duval, A.; Selves, J.; Gaub, M.P.; Vescovo, L.; Etienne-Grimaldi, M.C.; Schiappa, R.; Guenot, D.; Ayadi, M.; et al. Gene expression classification of colon cancer into molecular subtypes: Characterization, validation, and prognostic value. PLoS Med. 2013, 10, e1001453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Budinska, E.; Popovici, V.; Tejpar, S.; D’Ario, G.; Lapique, N.; Sikora, K.O.; Di Narzo, A.F.; Yan, P.; Hodgson, J.G.; Weinrich, S.; et al. Gene expression patterns unveil a new level of molecular heterogeneity in colorectal cancer. J. Pathol. 2013, 231, 63–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Sousa EMelo, F.; Wang, X.; Jansen, M.; Fessler, E.; Trinh, A.; de Rooij, L.P.; de Jong, J.H.; de Boer, O.J.; van Leersum, R.; Bijlsma, M.F.; et al. Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions. Nat. Med. 2013, 19, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Roepman, P.; Schlicker, A.; Tabernero, J.; Majewski, I.; Tian, S.; Moreno, V.; Snel, M.H.; Chresta, C.M.; Rosenberg, R.; Nitsche, U.; et al. Colorectal cancer intrinsic subtypes predict chemotherapy benefit, deficient mismatch repair and epithelial-to-mesenchymal transition. Int. J. Cancer 2014, 134, 552–562. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schlicker, A.; Beran, G.; Chresta, C.M.; McWalter, G.; Pritchard, A.; Weston, S.; Runswick, S.; Davenport, S.; Heathcote, K.; Castro, D.A.; et al. Subtypes of primary colorectal tumors correlate with response to targeted treatment in colorectal cell lines. BMC Med. Genom. 2012, 5, 66. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, F.J.; Li, Y.J.; Zhang, L.; Ji, D.B.; Liu, X.Z.; Chen, Y.J.; Wang, L.; Wu, A.W. Single-cell profiling reveals differences between human classical adenocarcinoma and mucinous adenocarcinoma. Commun. Biol. 2023, 6, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Joanito, I.; Wirapati, P.; Zhao, N.; Nawaz, Z.; Yeo, G.; Lee, F.; Eng, C.L.P.; Macalinao, D.C.; Kahraman, M.; Srinivasan, H.; et al. Single-cell and bulk transcriptome sequencing identifies two epithelial tumor cell states and refines the consensus molecular classification of colorectal cancer. Nat. Genet. 2022, 54, 963–975. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ranganathan, P.; Agrawal, A.; Bhushan, R.; Chavalmane, A.K.; Kalathur, R.K.; Takahashi, T.; Kondaiah, P. Expression profiling of genes regulated by TGF-beta: Differential regulation in normal and tumour cells. BMC Genom. 2007, 8, 98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peidl, A.; Perbal, B.; Leask, A. Yin/Yang expression of CCN family members: Transforming growth factor beta 1, via ALK5/FAK/MEK, induces CCN1 and CCN2, yet suppresses CCN3, expression in human dermal fibroblasts. PLoS ONE. 2019, 14, e0218178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oshi, M.; Roy, A.M.; Yan, L.; Kinoshita, S.; Tamura, Y.; Kosaka, T.; Akiyama, H.; Kunisaki, C.; Takabe, K.; Endo, I. Enhanced epithelial-mesenchymal transition signatures are linked with adverse tumor microenvironment, angiogenesis and worse survival in gastric cancer. Cancer Gene Ther. 2024, 31, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.F.; Doroshow, J.H.; Esworthy, R.S. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J. Biol. Chem. 1993, 268, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.W.; Cowley, G.S.; Weir, B.A.; Boehm, J.S.; Rusin, S.; Scott, J.A.; East, A.; Ali, L.D.; Lizotte, P.H.; Wong, T.C.; et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 12372–12377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leist, M.; Raab, B.; Maurer, S.; Rösick, U.; Brigelius-Flohé, R. Conventional cell culture media do not adequately supply cells with antioxidants and thus facilitate peroxide-induced genotoxicity. Free Radic. Biol. Med. 1996, 21, 297–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Touat-Hamici, Z.; Bulteau, A.L.; Bianga, J.; Jean-Jacques, H.; Szpunar, J.; Lobinski, R.; Chavatte, L. Selenium-regulated hierarchy of human selenoproteome in cancerous and immortalized cells lines. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Parant, F.; Mure, F.; Maurin, J.; Beauvilliers, L.; Chorfa, C.; El Jamali, C.; Ohlmann, T.; Chavatte, L. Selenium Discrepancies in Fetal Bovine Serum: Impact on Cellular Selenoprotein Expression. Int. J. Mol. Sci. 2024, 25, 7261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esworthy, R.S.; Kim, B.W.; Chow, J.; Shen, B.; Doroshow, J.H.; Chu, F.F. Nox1 causes ileocolitis in mice deficient in glutathione peroxidase-1 and -2. Free Radic. Biol. Med. 2014, 68, 315–325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, F.F.; Esworthy, R.S.; Doroshow, J.H.; Grasberger, H.; Donko, A.; Leto, T.L.; Gao, Q.; Shen, B. Deficiency in Duox2 activity alleviates ileitis in GPx1- and GPx2-knockout mice without affecting apoptosis incidence in the crypt epithelium. Redox Biol. 2017, 11, 144–156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jones, R.M.; Luo, L.; Ardita, C.S.; Richardson, A.N.; Kwon, Y.M.; Mercante, J.W.; Alam, A.; Gates, C.L.; Wu, H.; Swanson, P.A.; et al. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 2013, 32, 3017–3028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kato, M.; Marumo, M.; Nakayama, J.; Matsumoto, M.; Yabe-Nishimura, C.; Kamata, T. The ROS-generating oxidase Nox1 is required for epithelial restitution following colitis. Exp. Anim. 2016, 65, 197–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsumoto, M.; Katsuyama, M.; Iwata, K.; Ibi, M.; Zhang, J.; Zhu, K.; Nauseef, W.M.; Yabe-Nishimura, C. Characterization of N-glycosylation sites on the extracellular domain of NOX1/NADPH oxidase. Free Radic. Biol. Med. 2014, 68, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Brzozowa-Zasada, M.; Ianaro, A.; Piecuch, A.; Michalski, M.; Matysiak, N.; Stęplewska, K. Immunohistochemical Expression of Glutathione Peroxidase-2 (Gpx-2) and Its Clinical Relevance in Colon Adenocarcinoma Patients. Int. J. Mol. Sci. 2023, 24, 14650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komatsu, H.; Okayasu, I.; Mitomi, H.; Imai, H.; Nakagawa, Y.; Obata, F. Immunohistochemical detection of human gastrointestinal glutathione peroxidase in normal tissues and cultured cells with novel mouse monoclonal antibodies. J. Histochem. Cytochem. 2001, 49, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Florian, S.; Wingler, K.; Schmehl, K.; Jacobasch, G.; Kreuzer, O.J.; Meyerhof, W.; Brigelius-Flohé, R. Cellular and subcellular localization of gastrointestinal glutathione peroxidase in normal and malignant human intestinal tissue. Free Radic. Res. 2001, 35, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.R.; Nevins, S.A.; Chen, D.C.; Chiu, R.; Horning, A.M.; Guha, T.K.; Laquindanum, R.; Mills, M.; Chaib, H.; Ladabaum, U.; et al. Single-cell analyses define a continuum of cell state and composition changes in the malignant transformation of polyps to colorectal cancer. Nat. Genet. 2022, 54, 985–995. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasaki, N.; Sachs, N.; Wiebrands, K.; Ellenbroek, S.I.; Fumagalli, A.; Lyubimova, A.; Begthel, H.; van den Born, M.; van Es, J.H.; Karthaus, W.R.; et al. Reg4+ deep crypt secretory cells function as epithelial niche for Lgr5+ stem cells in colon. Proc. Natl. Acad. Sci. USA 2016, 113, E5399–E5407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pilat, J.M.; Brown, R.E.; Chen, Z.; Berle, N.J.; Othon, A.P.; Washington, M.K.; Anant, S.A.; Kurokawa, S.; Ng, V.H.; Thompson, J.J.; et al. SELENOP modifies sporadic colorectal carcinogenesis and WNT signaling activity through LRP5/6 interactions. J. Clin. Investig. 2023, 133, e165988. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmitt, M.; Schewe, M.; Sacchetti, A.; Feijtel, D.; van de Geer, W.S.; Teeuwssen, M.; Sleddens, H.F.; Joosten, R.; van Royen, M.E.; van de Werken, H.J.G.; et al. Paneth Cells Respond to Inflammation and Contribute to Tissue Regeneration by Acquiring Stem-like Features through SCF/c-Kit Signaling. Cell Rep. 2018, 24, 2312–2328.e7. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tong, K.; Zhao, Y.; Balasubramanian, I.; Yap, G.S.; Ferraris, R.P.; Bonder, E.M.; Verzi, M.P.; Gao, N. Paneth Cell Multipotency Induced by Notch Activation following Injury. Cell Stem Cell 2018, 23, 46–59.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mei, X.; Gu, M.; Li, M. Plasticity of Paneth cells and their ability to regulate intestinal stem cells. Stem Cell Res. Ther. 2020, 11, 349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fumagalli, A.; Oost, K.C.; Kester, L.; Morgner, J.; Bornes, L.; Bruens, L.; Spaargaren, L.; Azkanaz, M.; Schelfhorst, T.; Beerling, E.; et al. Plasticity of Lgr5-Negative Cancer Cells Drives Metastasis in Colorectal Cancer. Cell Stem Cell 2020, 26, 569–578.e7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, Y.; Liew, S.C.; Thean, L.F.; Tang, C.L.; Cheah, P.Y. Human colorectal cancer initiation is bidirectional, and cell growth, metabolic genes and transporter genes are early drivers of tumorigenesis. Cancer Lett. 2018, 431, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Liu, Y.; Chen, H.N.; Zhang, L.; Lan, J.; Gao, W.; Dou, Q.; Nice, E.C.; Huang, C. Thiol-based redox proteomics in cancer research. Proteomics 2015, 15, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Huu, T.; Park, J.; Zhang, Y.; Park, I.; Yoon, H.J.; Woo, H.A.; Lee, S.R. Redox Regulation of PTEN by Peroxiredoxins. Antioxidants 2021, 10, 302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Esworthy, R.S.; Swiderek, K.M.; Ho, Y.S.; Chu, F.F. Selenium-dependent glutathione peroxidase-GI is a major glutathione peroxidase activity in the mucosal epithelium of rodent intestine. Biochim. Biophys. Acta 1998, 1381, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Xu, Q.; Jing, X.; Chi, X.; Zhang, Z.; Meng, X.; Liu, X.; Yan, J.; Liu, X.; Shao, S. GPX2 promotes EMT and metastasis in non-small cell lung cancer by activating PI3K/AKT/mTOR/Snail signaling axis. FASEB Bioadv. 2023, 5, 233–250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haider, S.; Tyekucheva, S.; Prandi, D.; Fox, N.S.; Ahn, J.; Xu, A.W.; Pantazi, A.; Park, P.J.; Laird, P.W.; Sander, C.; et al. Systematic Assessment of Tumor Purity and Its Clinical Implications. JCO Precis. Oncol. 2020, 4, 995–1005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hiller, F.; Besselt, K.; Deubel, S.; Brigelius-Flohé, R.; Kipp, A.P. GPx2 Induction Is Mediated Through STAT Transcription Factors During Acute Colitis. Inflamm. Bowel Dis. 2015, 21, 2078–2089. [Google Scholar] [CrossRef] [PubMed]
- Florian, S.; Krehl, S.; Loewinger, M.; Kipp, A.; Banning, A.; Esworthy, S.; Chu, F.F.; Brigelius-Flohé, R. Loss of GPx2 increases apoptosis, mitosis, and GPx1 expression in the intestine of mice. Free Radic. Biol. Med. 2010, 49, 1694–1702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mouradov, D.; Sloggett, C.; Jorissen, R.N.; Love, C.G.; Li, S.; Burgess, A.W.; Arango, D.; Strausberg, R.L.; Buchanan, D.; Wormald, S.; et al. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 2014, 74, 3238–3247. [Google Scholar] [CrossRef] [PubMed]
- Isella, C.; Brundu, F.; Bellomo, S.E.; Galimi, F.; Zanella, E.; Porporato, R.; Petti, C.; Fiori, A.; Orzan, F.; Senetta, R.; et al. Selective analysis of cancer-cell intrinsic transcriptional traits defines novel clinically relevant subtypes of colorectal cancer. Nat. Commun. 2017, 8, 15107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ziskin, J.L.; Dunlap, D.; Yaylaoglu, M.; Fodor, I.K.; Forrest, W.F.; Patel, R.; Ge, N.; Hutchins, G.G.; Pine, J.K.; Quirke, P.; et al. In situ validation of an intestinal stem cell signature in colorectal cancer. Gut 2013, 62, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Kipp, A.P.; Müller, M.F.; Göken, E.M.; Deubel, S.; Brigelius-Flohé, R. The selenoproteins GPx2, TrxR2 and TrxR3 are regulated by Wnt signalling in the intestinal epithelium. Biochim. Biophys. Acta 2012, 1820, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.M.; Veeramachaneni, R.; Deng, D.; Putluri, N.; Putluri, V.; Cardenas, M.F.; Wheeler, D.A.; Decker, W.K.; Frederick, A.I.; Kazi, S.; et al. Glutathione peroxidase 2 is a metabolic driver of the tumor immune microenvironment and immune checkpoint inhibitor response. J. Immunother. Cancer 2022, 10, e004752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Smiraglia, D.J.; Rush, L.J.; Frühwald, M.C.; Dai, Z.; Held, W.A.; Costello, J.F.; Lang, J.C.; Eng, C.; Li, B.; Wright, F.A.; et al. Excessive CpG island hypermethylation in cancer cell lines versus primary human malignancies. Hum. Mol. Genet. 2001, 10, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Suter, C.M.; Norrie, M.; Ku, S.L.; Cheong, K.F.; Tomlinson, I.; Ward, R.L. CpG island methylation is a common finding in colorectal cancer cell lines. Br. J. Cancer 2003, 88, 413–419. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Auman, J.T.; McLeod, H.L. Colorectal cancer cell lines lack the molecular heterogeneity of clinical colorectal tumors. Clin. Color. Cancer 2010, 9, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Emmink, B.L.; Laoukili, J.; Kipp, A.P.; Koster, J.; Govaert, K.M.; Fatrai, S.; Verheem, A.; Steller, E.J.; Brigelius-Flohé, R.; Jimenez, C.R.; et al. GPx2 suppression of H2O2 stress links the formation of differentiated tumor mass to metastatic capacity in colorectal cancer. Cancer Res. 2014, 74, 6717–6730. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loeser, H.; Scholz, M.; Fuchs, H.; Essakly, A.; Damanakis, A.I.; Zander, T.; Büttner, R.; Schröder, W.; Bruns, C.; Quaas, A.; et al. Integrin alpha V (ITGAV) expression in esophageal adenocarcinoma is associated with shortened overall-survival. Sci. Rep. 2020, 10, 18411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurundkar, A.R.; Kurundkar, D.; Rangarajan, S.; Locy, M.L.; Zhou, Y.; Liu, R.M.; Zmijewski, J.; Thannickal, V.J. The matricellular protein CCN1 enhances TGF-β1/SMAD3-dependent profibrotic signaling in fibroblasts and contributes to fibrogenic responses to lung injury. FASEB J. 2016, 30, 2135–2150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huet, C.; Sahuquillo-Merino, C.; Coudrier, E.; Louvard, D. Absorptive and mucus-secreting subclones isolated from a multipotent intestinal cell line (HT-29) provide new models for cell polarity and terminal differentiation. J. Cell Biol. 1987, 105, 345–357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bravard, A.; Beaumatin, J.; Dussaulx, E.; Lesuffleur, T.; Zweibaum, A.; Luccioni, C. Modifications of the antioxidant metabolism during proliferation and differentiation of colon tumor cell lines. Int. J. Cancer 1994, 59, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Godtliebsen, G.; Larsen, K.B.; Bhujabal, Z.; Opstad, I.S.; Nager, M.; Punnakkal, A.R.; Kalstad, T.B.; Olsen, R.; Lund, T.; Prasad, D.K.; et al. High-resolution visualization and assessment of basal and OXPHOS-induced mitophagy in H9c2 cardiomyoblasts. Autophagy 2023, 19, 2769–2788. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewitt, R.E.; McMarlin, A.; Kleiner, D.; Wersto, R.; Martin, P.; Tsokos, M.; Stamp, G.W.; Stetler-Stevenson, W.G. Validation of a model of colon cancer progression. J. Pathol. 2000, 192, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, L.; Wang, Q.; Zheng, M. Comparison of Different Colorectal Cancer With Liver Metastases Models Using Six Colorectal Cancer Cell Lines. Pathol. Oncol. Res. 2020, 26, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.A.; Wang, Y.; Cheng, H.; Zhang, Q.; Ge, W.; Guo, D. RedoxDB--a curated database for experimentally verified protein oxidative modification. Bioinformatics 2012, 28, 2551–2552. [Google Scholar] [CrossRef] [PubMed]
- Merkley, E.D.; Metz, T.O.; Smith, R.D.; Baynes, J.W.; Frizzell, N. The succinated proteome. Mass. Spectrom. Rev. 2014, 33, 98–109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dustin, C.M.; Heppner, D.E.; Lin, M.J.; van der Vliet, A. Redox regulation of tyrosine kinase signalling: More than meets the eye. J. Biochem. 2020, 167, 151–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welsh, C.L.; Madan, L.K. Protein Tyrosine Phosphatase regulation by Reactive Oxygen Species. Adv. Cancer Res. 2024, 162, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, S.; Wei, C.; Fang, Y.; Huang, S.; Yin, T.; Xiong, B.; Yang, C. Tumour microenvironment: A non-negligible driver for epithelial-mesenchymal transition in colorectal cancer. Expert. Rev. Mol. Med. 2021, 23, e16. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23 Pt B, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Ramsay, R.G. Colorectal cancer mouse models: Integrating inflammation and the stroma. J. Gastroenterol. Hepatol. 2012, 27, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.; Seyfried, S.; Staib, T.; Fiedler, D.; Sauer, C.; Ried, T.; Witt, S.; Rueckert, F.; Gaiser, T. Newly established gastrointestinal cancer cell lines retain the genomic and immunophenotypic landscape of their parental cancers. Sci. Rep. 2020, 10, 17895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tompkins, W.A.; Watrach, A.M.; Schmale, J.D.; Schultz, R.M.; Harris, J.A. Cultural and antigenic properties of newly established cell strains derived from adenocarcinomas of the human colon and rectum. J. Natl. Cancer Inst. 1974, 52, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Céspedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fagundes, R.R.; Belt, S.C.; Bakker, B.M.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N. Beyond butyrate: Microbial fiber metabolism supporting colonic epithelial homeostasis. Trends Microbiol. 2024, 32, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Tomé, D. The Roles of Dietary Glutamate in the Intestine. Ann. Nutr. Metab. 2018, 73 (Suppl. S5), 15–20. [Google Scholar] [CrossRef] [PubMed]
- Gomes, S.; Baltazar, F.; Silva, E.; Preto, A. Microbiota-Derived Short-Chain Fatty Acids: New Road in Colorectal Cancer Therapy. Pharmaceutics 2022, 14, 2359. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Teeli, A.S.; Łuczyńska, K.; Haque, E.; Gayas, M.A.; Winiarczyk, D.; Taniguchi, H. Disruption of Tumor Suppressors HNF4α/HNF1α Causes Tumorigenesis in Liver. Cancers 2021, 13, 5357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Angelo, A.; Bluteau, O.; Garcia-Gonzalez, M.A.; Gresh, L.; Doyen, A.; Garbay, S.; Robine, S.; Pontoglio, M. Hepatocyte nuclear factor 1alpha and beta control terminal differentiation and cell fate commitment in the gut epithelium. Development 2010, 137, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Yan, X.; Kong, C.; Zhu, Y.; Li, H.; Pan, D.; Zhang, X.; Liu, Y.; Yin, F.; Qin, H. c-Myb promotes growth and metastasis of colorectal cancer through c-fos-induced epithelial-mesenchymal transition. Cancer Sci. 2019, 110, 3183–3196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.L.; Luo, T.; Luo, M. The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, W.; Pan, Y.; Gao, Y.; Deng, L.; Li, F.; Li, F.; Ma, X.; Hou, S.; Xu, J.; et al. YAP Suppresses Lung Squamous Cell Carcinoma Progression via Deregulation of the DNp63-GPX2 Axis and ROS Accumulation. Cancer Res. 2017, 77, 5769–5781. [Google Scholar] [CrossRef] [PubMed]
- Soo, H.C.; Chung, F.F.; Lim, K.H.; Yap, V.A.; Bradshaw, T.D.; Hii, L.W.; Tan, S.H.; See, S.J.; Tan, Y.F.; Leong, C.O.; et al. Cudraflavone C Induces Tumor-Specific Apoptosis in Colorectal Cancer Cells through Inhibition of the Phosphoinositide 3-Kinase (PI3K)-AKT Pathway. PLoS ONE 2017, 12, e0170551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coant, N.; Ben Mkaddem, S.; Pedruzzi, E.; Guichard, C.; Tréton, X.; Ducroc, R.; Freund, J.N.; Cazals-Hatem, D.; Bouhnik, Y.; Woerther, P.L.; et al. NADPH oxidase 1 modulates WNT and NOTCH1 signaling to control the fate of proliferative progenitor cells in the colon. Mol. Cell. Biol. 2010, 30, 2636–2650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, F.F.; Esworthy, R.S.; Shen, B.; Doroshow, J.H. Role of the microbiota in ileitis of a mouse model of inflammatory bowel disease-Glutathione peroxide isoenzymes 1 and 2-double knockout mice on a C57BL background. Microbiologyopen 2020, 9, e1107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chu, F.F.; Esworthy, R.S.; Chu, P.G.; Longmate, J.A.; Huycke, M.M.; Wilczynski, S.; Doroshow, J.H. Bacteria-induced intestinal cancer in mice with disrupted Gpx1 and Gpx2 genes. Cancer Res. 2004, 64, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jiang, G.; Wu, Y.; Antony, S.; Meitzler, J.L.; Juhasz, A.; Liu, H.; Roy, K.; Makhlouf, H.; Chuaqui, R.; et al. NADPH oxidase 1 is highly expressed in human large and small bowel cancers. PLoS ONE 2020, 15, e0233208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunne, P.D.; McArt, D.G.; Bradley, C.A.; O’Reilly, P.G.; Barrett, H.L.; Cummins, R.; O’Grady, T.; Arthur, K.; Loughrey, M.B.; Allen, W.L.; et al. Challenging the Cancer Molecular Stratification Dogma: Intratumoral Heterogeneity Undermines Consensus Molecular Subtypes and Potential Diagnostic Value in Colorectal Cancer. Clin. Cancer Res. 2016, 22, 4095–4104. [Google Scholar] [CrossRef] [PubMed]
- Leibovitz, A.; Stinson, J.C.; McCombs, W.B., 3rd; McCoy, C.E.; Mazur, K.C.; Mabry, N.D. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976, 36, 4562–4569. [Google Scholar] [PubMed]
- Solic, N.; Collins, J.E.; Richter, A.; Holt, S.J.; Campbell, I.; Alexander, P.; Davies, D.E. Two newly established cell lines derived from the same colonic adenocarcinoma exhibit differences in EGF-receptor ligand and adhesion molecule expression. Int. J. Cancer 1995, 62, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ohata, H.; Shiokawa, D.; Obata, Y.; Sato, A.; Sakai, H.; Fukami, M.; Hara, W.; Taniguchi, H.; Ono, M.; Nakagama, H.; et al. NOX1-Dependent mTORC1 Activation via S100A9 Oxidation in Cancer Stem-like Cells Leads to Colon Cancer Progression. Cell Rep. 2019, 28, 1282–1295. [Google Scholar] [CrossRef] [PubMed]
- van der Post, S.; Birchenough, G.M.H.; Held, J.M. NOX1-dependent redox signaling potentiates colonic stem cell proliferation to adapt to the intestinal microbiota by linking EGFR and TLR activation. Cell Rep. 2021, 35, 108949. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Mainardi, S.; Lieftink, C.; Velds, A.; de Rink, I.; Yang, C.; Kuiken, H.J.; Morris, B.; Edwards, F.; Jochems, F.; et al. Targeting of vulnerabilities of drug-tolerant persisters identified through functional genetics delays tumor relapse. Cell Rep. Med. 2024, 5, 101471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Oren, Y.; Tsabar, M.; Cuoco, M.S.; Amir-Zilberstein, L.; Cabanos, H.F.; Hütter, J.C.; Hu, B.; Thakore, P.I.; Tabaka, M.; Fulco, C.P.; et al. Cycling cancer persister cells arise from lineages with distinct programs. Nature 2021, 596, 576–582. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barry, C.J.; Pillay, C.S.; Rohwer, J.M. Modelling the Decamerisation Cycle of PRDX1 and the Inhibition-like Effect on Its Peroxidase Activity. Antioxidants 2023, 12, 1707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Portillo-Ledesma, S.; Randall, L.M.; Parsonage, D.; Dalla Rizza, J.; Karplus, P.A.; Poole, L.B.; Denicola, A.; Ferrer-Sueta, G. Differential Kinetics of Two-Cysteine Peroxiredoxin Disulfide Formation Reveal a Novel Model for Peroxide Sensing. Biochemistry 2018, 57, 3416–3424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Low, F.M.; Hampton, M.B.; Peskin, A.V.; Winterbourn, C.C. Peroxiredoxin 2 functions as a noncatalytic scavenger of low-level hydrogen peroxide in the erythrocyte. Blood 2007, 109, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Buetler, T.M.; Krauskopf, A.; Ruegg, U.T. Role of superoxide as a signaling molecule. News Physiol. Sci. 2004, 19, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Barbáchano, A.; Fernández-Barral, A.; Bustamante-Madrid, P.; Prieto, I.; Rodríguez-Salas, N.; Larriba, M.J.; Muñoz, A. Organoids and Colorectal Cancer. Cancers 2021, 13, 2657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, M.F.; Florian, S.; Pommer, S.; Osterhoff, M.; Esworthy, R.S.; Chu, F.F.; Brigelius-Flohé, R.; Kipp, A.P. Deletion of glutathione peroxidase-2 inhibits azoxymethane-induced colon cancer development. PLoS ONE 2013, 8, e72055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banning, A.; Kipp, A.; Schmitmeier, S.; Löwinger, M.; Florian, S.; Krehl, S.; Thalmann, S.; Thierbach, R.; Steinberg, P.; Brigelius-Flohé, R. Glutathione Peroxidase 2 Inhibits Cyclooxygenase-2-Mediated Migration and Invasion of HT-29 Adenocarcinoma Cells but Supports Their Growth as Tumors in Nude Mice. Cancer Res. 2008, 68, 9746–9753. [Google Scholar] [CrossRef] [PubMed]




| Sadanandam | Marisa | Budinska | de Sausa | Roepman | Schlicker | MSS | MET | APC mut |
| SNU503 | T84 | SNU1040 | SNU1040 | SNU1040 | CACO2 | SNU1033 | SNU503 | SNU1033 |
| SW620 | SNU407 | SNO503 | HT29 | CL40 | LOVO | SNU503 | LOVO | SNU503 |
| LOVO | CACO2 | SNU81 | SKCO1 | SW1417 | CACO2 | SW620 | LOVO | |
| LS180 | LOVO | SNU1033 | SNU1033 | SW620 | SW620 | SKCO1 | SW620 | |
| HT115 | C84 | SNU503 | SNU503 | CL40 | SKCO1 | |||
| LS411N | SW620 | SW620 | LS180 | SKCO1 | CL40 | |||
| SNUC41 | SW620 | SNU1040 | ||||||
| SNU1033 | COLO201 | |||||||
| SNU503 | ||||||||
| CACO2 | ||||||||
| CL40 | ||||||||
| SNU61 | ||||||||
| SNU1460 | ||||||||
| SKCO1 | ||||||||
| SNUC4 | ||||||||
| CL34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esworthy, R.S. Evaluation of the Use of Cell Lines in Studies of Selenium-Dependent Glutathione Peroxidase 2 (GPX2) Involvement in Colorectal Cancer. Diseases 2024, 12, 207. https://doi.org/10.3390/diseases12090207
Esworthy RS. Evaluation of the Use of Cell Lines in Studies of Selenium-Dependent Glutathione Peroxidase 2 (GPX2) Involvement in Colorectal Cancer. Diseases. 2024; 12(9):207. https://doi.org/10.3390/diseases12090207
Chicago/Turabian StyleEsworthy, R. Steven. 2024. "Evaluation of the Use of Cell Lines in Studies of Selenium-Dependent Glutathione Peroxidase 2 (GPX2) Involvement in Colorectal Cancer" Diseases 12, no. 9: 207. https://doi.org/10.3390/diseases12090207
APA StyleEsworthy, R. S. (2024). Evaluation of the Use of Cell Lines in Studies of Selenium-Dependent Glutathione Peroxidase 2 (GPX2) Involvement in Colorectal Cancer. Diseases, 12(9), 207. https://doi.org/10.3390/diseases12090207







