Association between Glycosylated Hemoglobin Levels and Vaccine Preventable Diseases: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- P: Adult population (≥18 years) with or without vaccine-preventable diseases (VPDs) at the baseline;
- I: Higher HbA1c values;
- C: lower HbA1c values;
- O: Mortality and hospitalization rates in patients with VPDs and incidence of VPDs in people without VPDs at the baseline;
- S: Both prospective and retrospective observational studies.
2.3. Data Extraction and Risk of Bias
2.4. Statistical Analysis
3. Results
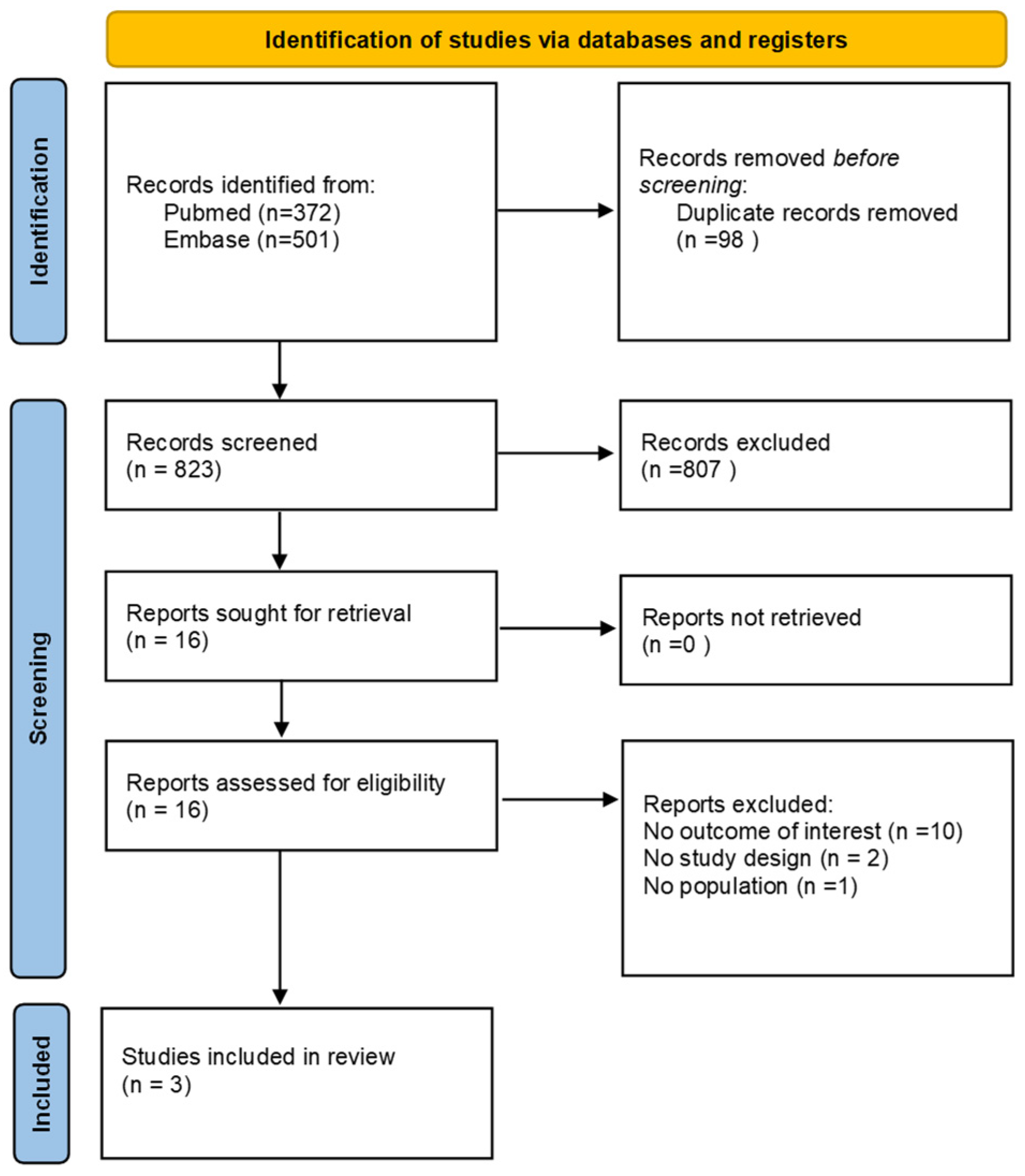
3.1. Literature Search
3.2. Descriptive Data
3.3. Main Findings
3.4. Risk of Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Abu-Ashour, W.; Twells, L.; Valcour, J.; Randell, A.; Donnan, J.; Howse, P.; Gamble, J.-M. The association between diabetes mellitus and incident infections: A systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res. Care 2017, 5, e000336. [Google Scholar] [CrossRef]
- Magliano, D.J.; Harding, J.L.; Cohen, K.; Huxley, R.R.; Davis, W.A.; Shaw, J.E. Excess Risk of Dying from Infectious Causes in Those with Type 1 and Type 2 Diabetes. Diabetes Care 2015, 38, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Stuttard, J.; Blundell, S.; Harris, T.; Cook, D.G.; Critchley, J. Diabetes and infection: Assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016, 4, 148–158. [Google Scholar] [CrossRef] [PubMed]
- McGovern, A.P.; Hine, J.; de Lusignan, S. Infection risk in elderly people with reduced glycaemic control. Lancet Diabetes Endocrinol. 2016, 4, 303–304. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, U.A.; Carey, I.M.; Critchley, J.A.; DeWilde, S.; Limb, E.S.; Bowen, L.; Panahloo, A.; Cook, D.G.; Whincup, P.H.; Harris, T. A matched cohort study evaluating the risks of infections in people with type 1 diabetes and their associations with glycated haemoglobin. Diabetes Res. Clin. Pract. 2024, 207, 111023. [Google Scholar] [CrossRef] [PubMed]
- Shohat, N.; Muhsen, K.; Gilat, R.; Rondon, A.J.; Chen, A.F.; Parvizi, J. Inadequate Glycemic Control Is Associated with Increased Surgical Site Infection in Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2018, 33, 2312–2321.e3. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Dekkers, O.M.; Nielsen, J.S.; Beck-Nielsen, H.; Sørensen, H.T.; Thomsen, R.W. Impact of Glycemic Control on Risk of Infections in Patients with Type 2 Diabetes: A Population-Based Cohort Study. Am. J. Epidemiol. 2017, 186, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Hosking, F.J.; Cook, D.G. Glycemic Control and Risk of Infections Among People with Type 1 or Type 2 Diabetes in a Large Primary Care Cohort Study. Diabetes Care 2018, 41, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Luk, A.O.; Lau, E.S.; Cheung, K.K.; Kong, A.P.; Ma, R.C.; Ozaki, R.; Chow, F.C.; So, W.Y.; Chan, J.C. Glycaemia control and the risk of hospitalisation for infection in patients with type 2 diabetes: Hong Kong Diabetes Registry. Diabetes/Metab. Res. Rev. 2017, 33, e2923. [Google Scholar] [CrossRef]
- Lederman, M.M.; Schiffman, G.; Rodman, H.M. Pneumococcal Immunization in Adult Diabetics. Diabetes 1981, 30, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Marble, A.; White, H.J.; Fernald, A.T. THE Nature of the Lowered Resistance to Infection in Diabetes Mellitus 1. J. Clin. Investig. 1938, 17, 423–430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Volti, S.L.; Caruso-Nicoletti, M.; Biazzo, F.; Sciacca, A.; Mandarà, G.; Mancuso, M.; Mollica, F. Hyporesponsiveness to intradermal administration of hepatitis B vaccine in insulin dependent diabetes mellitus. Arch. Dis. Child. 1998, 78, 54–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boroumand, A.B.; Forouhi, M.; Karimi, F.; Moghadam, A.S.; Naeini, L.G.; Kokabian, P.; Naderi, D. Immunogenicity of COVID-19 vaccines in patients with diabetes mellitus: A systematic review. Front. Immunol. 2022, 13, 940357. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, Y.; Shen, P.; Tao, Y.; Zhang, W.; Xu, B.; Bi, Y.; Han, Z.; Zhou, Y.-H. Reduced antibody response to COVID-19 vaccine composed of inactivated SARS-CoV-2 in diabetic individuals. Front. Public Health 2022, 10, 1025901. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- E Geerlings, S.; Hoepelman, A.I. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Dicembrini, I.; Silverii, G.A.; Clerico, A.; Fornengo, R.; Gabutti, G.; Sordi, V.; Tafuri, S.; Peruzzi, O.; Mannucci, E. Influenza: Diabetes as a risk factor for severe related-outcomes and the effectiveness of vaccination in diabetic population. A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Blasi, F.; Dartois, N.; Akova, M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax 2015, 70, 984–989. [Google Scholar] [CrossRef]
- Brunetti, V.C.; Ayele, H.T.; Yu, O.H.Y.; Ernst, P.; Filion, K.B. Type 2 diabetes mellitus and risk of community-acquired pneumonia: A systematic review and meta-analysis of observational studies. CMAJ Open 2021, 9, E62–E70. [Google Scholar] [CrossRef] [PubMed]
- Zoppini, G.; Fedeli, U.; Schievano, E.; Dauriz, M.; Targher, G.; Bonora, E.; Corti, M.C. Mortality from infectious diseases in diabetes. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 444–450. [Google Scholar] [CrossRef]
- Kornum, J.B.; Thomsen, R.W.; Riis, A.; Lervang, H.-H.; Schønheyder, H.C.; Sørensen, H.T. Diabetes, glycemic control, and risk of hospitalization with pneumonia: A population-based case-control study. Diabetes Care 2008, 31, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Liu, B.; Zhao, C.; Yan, J.; Pan, T.; Zhou, M.; Qu, H. Nomogram for prediction of severe community-acquired pneumonia development in diabetic patients: A multicenter study. BMC Pulm. Med. 2022, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Liao, W.-I.; Wang, Y.-C.; Chang, W.-C.; Hsu, C.-W.; Chen, Y.-H.; Tsai, S.-H. An Elevated Glycemic Gap is Associated with Adverse Outcomes in Diabetic Patients with Community-Acquired Pneumonia. Medicine 2015, 94, e1456. [Google Scholar] [CrossRef] [PubMed]
- Hartmann-Boyce, J.; Rees, K.; Perring, J.C.; Kerneis, S.A.; Morris, E.M.; Goyder, C.; Otunla, A.A.; James, O.E.; Syam, N.R.; Seidu, S. Risks of and from SARS-CoV-2 infection and COVID-19 in people with diabetes: A systematic review of reviews. Diabetes Care 2021, 44, 2790–2811. [Google Scholar] [CrossRef]
- Cai, C.; Zeng, J.; Wu, H.; Shi, R.; Wei, M.; Gao, Y.; Ma, W. Association between hepatitis B virus infection and diabetes mellitus: A meta-analysis. Exp. Ther. Med. 2015, 10, 693–698. [Google Scholar] [CrossRef]
- Huang, S.; Kao, J. The interplay between chronic hepatitis B and diabetes mellitus: A narrative and concise review. Kaohsiung J. Med. Sci. 2024, 40, 6–10. [Google Scholar] [CrossRef]
- Schmader, K.; Gnann, J.J.W.; Watson, C.P. The Epidemiological, Clinical, and Pathological Rationale for the Herpes Zoster Vaccine. J. Infect. Dis. 2008, 197 (Suppl. 2), S207–S215. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Dworkin, R.H. The management of post-herpetic neuralgia. BMJ 2000, 321, 778–779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saadatian-Elahi, M.; Bauduceau, B.; Del-Signore, C.; Vanhems, P. Diabetes as a risk factor for herpes zoster in adults: A synthetic literature review. Diabetes Res. Clin. Pract. 2019, 159, 107983. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Luchini, C.; Stubbs, B.; Solmi, M.; Veronese, N. Assessing the quality of studies in meta-analyses: Advantages and limitations of the Newcastle Ottawa Scale. World J. Meta-Anal. 2017, 5, 80–84. [Google Scholar] [CrossRef]
- Luchini, C.; Veronese, N.; Nottegar, A.; Shin, J.I.; Gentile, G.; Granziol, U.; Soysal, P.; Alexinschi, O.; Smith, L.; Solmi, M. Assessing the quality of studies in meta-research: Review/guidelines on the most important quality assessment tools. Pharm. Stat. 2021, 20, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, D.; Shimbo, T.; Noto, H.; Eto, H.; Takahashi, O.; Higuchi, T. Low level of hemoglobin A1c and the increased incidence of herpes zoster: Longitudinal study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1539–1545. [Google Scholar] [CrossRef]
- De Jong, M.; Woodward, M.; Peters, S.A. Diabetes and COVID-19–Related Mortality in Women and Men in the UK Biobank: Comparisons with Influenza/Pneumonia and Coronary Heart Disease. Diabetes Care 2020, 44, e22–e24. [Google Scholar] [CrossRef]
- Pan, B.-L.; Chou, C.-P.; Huang, K.-S.; Bin, P.-J.; Luo, K.-H.; Chuang, H.-Y. The Pattern of Hemoglobin A1C Trajectories and Risk of Herpes Zoster Infection: A Follow-Up Study. Int. J. Environ. Res. Public Health 2022, 19, 2646. [Google Scholar] [CrossRef] [PubMed]
- Goto, A.; Goto, M.; Noda, M.; Tsugane, S. Incidence of Type 2 Diabetes in Japan: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e74699. [Google Scholar] [CrossRef]
- Wu, H.; Patterson, C.C.; Zhang, X.; Ghani, R.B.A.; Magliano, D.J.; Boyko, E.J.; Ogle, G.D.; Luk, A.O. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res. Clin. Pract. 2022, 185, 109785. [Google Scholar] [CrossRef]
- Huang, C.-T.; Lee, C.-Y.; Sung, H.-Y.; Liu, S.-J.; Liang, P.-C.; Tsai, M.-C. Association Between Diabetes Mellitus and the Risk of Herpes Zoster: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2021, 107, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Heymann, A.D.; Chodick, G.; Karpati, T.; Kamer, L.; Kremer, E.; Green, M.S.; Kokia, E.; Shalev, V. Diabetes as a Risk Factor for Herpes Zoster Infection: Results of a Population-Based Study in Israel. Infection 2008, 36, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Schmader, K.E.; Dworkin, R.H. The Epidemiology and Natural History of Herpes Zoster and Postherpetic Neuralgia. In Herpes Zoster: Postherpetic Neuralgia and Other Complications; Watson, C., Gershon, A., Oxman, M., Eds.; Adis: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Czernichow, S.; Dupuy, A.; Flahault, A.; Chosidow, O. Herpes zoster: Incidence study among “sentinel” general practitioners. Ann. Dermatol. Venereol. 2001, 128, 497–501. [Google Scholar]
- Dungan, K.M.; Braithwaite, S.S.; Preiser, J.-C. Stress hyperglycaemia. Lancet 2009, 373, 1798–1807. [Google Scholar] [CrossRef]
- Suaya, J.A.; Chen, S.-Y.; Li, Q.; Burstin, S.J.; Levin, M.J. Incidence of Herpes Zoster and Persistent Post-Zoster Pain in Adults with or Without Diabetes in the United States. Open Forum Infect. Dis. 2014, 1, ofu049. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-C.; Lai, H.-C.; Lin, C.-H.; Hung, C.-J.; Chen, D.-Y.; Sheu, W.H.-H.; Lui, P.-W. Increased Risk of Herpes Zoster in Diabetic Patients Comorbid with Coronary Artery Disease and Microvascular Disorders: A Population-Based Study in Taiwan. PLoS ONE 2016, 11, e0146750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polk, C.; Sampson, M.M.; Roshdy, D.; Davidson, L.E. Skin and Soft Tissue Infections in Patients with Diabetes Mellitus. Infect. Dis. Clin. N. Am. 2020, 35, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Queenan, J.A.; Farahani, P.; Ehsani-Moghadam, B.; Birtwhistle, R.V. The Prevalence and Risk for Herpes Zoster Infection in Adult Patients with Diabetes Mellitus in the Canadian Primary Care Sentinel Surveillance Network. Can. J. Diabetes 2018, 42, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Pizzol, D.; Di Gennaro, F.; Chhaganlal, K.D.; Fabrizio, C.; Monno, L.; Putoto, G.; Saracino, A. Prevalence of diabetes mellitus in newly diagnosed pulmonary tuberculosis in Beira, Mozambique. Afr. Health Sci. 2017, 17, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Yue, T.; Xu, M.; Zhang, P.; Wang, Y.; Liu, Q.; Huang, J.; Shen, T.; Yin, Q.; Sheng, Z.; et al. Poor glycemic control in type-2 diabetic patients infected with hepatitis B: A retrospective propensity-matched study. J. Med. Virol. 2023, 95, e28635. [Google Scholar] [CrossRef] [PubMed]
- Schillie, S.F.; Xing, J.; Murphy, T.V.; Hu, D.J. Prevalence of hepatitis B virus infection among persons with diagnosed diabetes mellitus in the United States, 1999–2010*. J. Viral Hepat. 2012, 19, 674–676. [Google Scholar] [CrossRef]
- Han, B.; Liu, W.; Yang, S.; Wang, S.; Du, J.; Liu, Y.; Cui, F. Association between self-monitoring of blood glucose and hepatitis B virus infection among people with diabetes mellitus: A cross-sectional study in Gansu Province, China. BMJ Open 2021, 11, e048463. [Google Scholar] [CrossRef]
- Donadon, V.; Balbi, M.; Valent, F.; Avogaro, A. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J. Gastroenterol. WJG 2010, 16, 3025. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Cai, H.; Liu, Y.; Qin, G. Hepatitis B virus infection status and risk of type 2 diabetes mellitus: A meta-analysis. Hepatol. Res. 2015, 45, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, H.; Guan, Y.; Peng, H.; Wang, S.; Zhang, P.; Su, B. Positive hepatitis B surface antibody is associated with reduced risk of diabetes mellitus in retired female Chinese workers. J. Diabetes 2015, 8, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.C.; Schmidtgrimminger, D.; Patrick, S.; Ryschon, T.; Linz, L.; Chauhan, S.C. There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains. Gynecol. Oncol. 2007, 107, 236–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- García-Esquinas, E.; Guinó, E.; Castaño-Vinyals, G.; Pérez-Gómez, B.; Llorca, J.; Altzibar, J.M.; Peiró-Pérez, R.; Martín, V.; Moreno-Iribas, C.; Tardón, A.; et al. Association of diabetes and diabetes treatment with incidence of breast cancer. Acta Diabetol. 2015, 53, 99–107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef]
- Moghaddam Tabrizi, F.; Rasmi, Y.; Hosseinzadeh, E.; Rezaei, S.; Balvardi, M.; Kouchari, M.R.; Ebrahimi, G. Diabetes is associated with higher mortality and severity in hospitalized patients with COVID-19. EXCLI J. 2021, 20, 444–453. [Google Scholar] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Vafea, M.T.; Traboulsi, C.; Stefanovic-Racic, M. Lower HbA1c is associated with lower in-hospital mortality in patients with COVID-19: A systematic review of the literature and meta-analysis. Endocr. Pract. 2023, 30, 70–77. [Google Scholar] [CrossRef]
| Author | Year | Country | Type of Study | VPD Considered | Follow-Up (Months) | Total Sample Size | % of Females | Mean Age | Outcome | Confounders Mentioned in the Multivariate Analysis (Number) |
|---|---|---|---|---|---|---|---|---|---|---|
| Bo-Lin Pan [35] | 2022 | China | Prospective cohort study | HZ | 72 | 121,999 | 46.3 | 60.4 | Incidence of HZ | Age, sex, comorbidities (3) |
| Kobayashi D [33] | 2019 | Japan | Retrospective longitudinal study | HZ | 132 | 81,466 | 51.3 | 46.5 | Incidence of HZ | Age, sex, time variable; social histories, including smoking status, alcohol consumption, BMI, and exercise habits; history of diabetes and its pharmacological treatment status, medical histories, including cancer, autoimmune disease, hemodialysis, and chronic hepatitis/cirrhosis (13) |
| De Jong M [34] | 2021 | UK | Prospective cohort study | COVID-19 | 134 | 501,884 | 54 | Aged 40–69 years at baseline | Death from COVID-19 | Age, BMI, socioeconomic status, smoking, systolic blood pressure, total cholesterol, antihypertensivemedication, glucose-loweringmedication, lipid lowering medication (9) |
| TOTAL | Two prospective; one retrospective | 113 | 705,349 | 54 | Two: HZ; one: COVID-19 | 8 |
| Author | Year | Main Findings |
|---|---|---|
| Bo-Lin Pan et al. [35] | 2022 | Four HbA1C trajectories were identified: ‘good control’ (mean HbA1C of 6.7% or 50 mmol/mol), ‘high decreasing’ (mean HbA1C of 7.9% or 63 mmol/mol), ‘moderate control’ (mean HbA1C of 8.4% or 68 mmol/mol), and ‘poor control’ (mean HbA1C of 10.7% or 93 mmol/mol). A significantly higher risk of HZ was observed in the ‘poor control’ trajectory with an HR = 1.44 (95% CI 1.26–1.64) after adjusting for confounders and comorbidities. |
| Kobayashi D [33] | 2019 | Individuals in the lowest HbA1c group (HbA1c of <5.0%) exhibited a significantly higher risk of developing HZ (OR 1.63; 95%CI: 1.07–2.48) compared with the reference group (HbA1c of 5.0–6.4%). Individuals in the highest HbA1c group (HbA1cof ≥ 9.5%) had a higher but nonsignificant risk than the reference group (OR 2.15; 95% CI, 0.67–6.94). |
| De Jong M [34] | 2021 | There was no association between higher levels of HbA1c and increased risk of COVID-19 or influenza/pneumonia death in women. On the contrary, compared with no diabetes, an HbA1c > 7.5% (58 mmol/mol) was associated with an increased risk of COVID-19 or influenza/pneumonia death in men. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vita, E.; Limongi, F.; Veronese, N.; Di Gennaro, F.; Saracino, A.; Maggi, S. Association between Glycosylated Hemoglobin Levels and Vaccine Preventable Diseases: A Systematic Review. Diseases 2024, 12, 187. https://doi.org/10.3390/diseases12080187
De Vita E, Limongi F, Veronese N, Di Gennaro F, Saracino A, Maggi S. Association between Glycosylated Hemoglobin Levels and Vaccine Preventable Diseases: A Systematic Review. Diseases. 2024; 12(8):187. https://doi.org/10.3390/diseases12080187
Chicago/Turabian StyleDe Vita, Elda, Federica Limongi, Nicola Veronese, Francesco Di Gennaro, Annalisa Saracino, and Stefania Maggi. 2024. "Association between Glycosylated Hemoglobin Levels and Vaccine Preventable Diseases: A Systematic Review" Diseases 12, no. 8: 187. https://doi.org/10.3390/diseases12080187
APA StyleDe Vita, E., Limongi, F., Veronese, N., Di Gennaro, F., Saracino, A., & Maggi, S. (2024). Association between Glycosylated Hemoglobin Levels and Vaccine Preventable Diseases: A Systematic Review. Diseases, 12(8), 187. https://doi.org/10.3390/diseases12080187








