An Alternative Non-Invasive Screening Model for Liver Fibrosis among US Adults at Risk of MASLD
Abstract
1. Introduction
2. Data and Method
3. Outcome and Statistical Analyses
4. Results
4.1. Introduction of the Liver Stiffness Score (LSS) Model
4.2. Fibrosis Probabilities with the New LSS Model
4.3. Assessing Model Validity: Testing with Data from 2017–2018 Sample Cycle of 1562 at-Risk Participants
5. Discussion
5.1. FIB-4 and NFS Models: Screening for Fibrosis and Concerns
5.2. Comparison of LSS Model with FIB-4, NFS, SAFE, and Waist Circumference for Fibrosis Screening
5.3. Possible Clinical Applications of LSS Model
6. Limitation
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AASLD | American Association for the Study of Liver Diseases |
| AGA | American Gastroenterological Association |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| AUROC | Area under the receiver operating characteristic curves FIB-4: Fibrosis-4 |
| FIB-3 | Fibrosis-3 Index |
| FIB-4 | Fibrosis-4 (FIB-4) |
| HDL | High-density lipoprotein |
| IQR | Interquartile range |
| LSM | Liver stiffness measurements |
| LSS | A liver stiffness score |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| NFS | NAFLD Fibrosis Score |
| NHANES | National Health and Nutrition Examination Surveys |
| SAFE | Steatosis-associated fibrosis estimator |
References
- Canivet, C.M.; Boursier, J. Screening for Liver Fibrosis in the General Population: Where Do We Stand in 2022? Diagnostics 2022, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Cramb, R.; Davison, S.M.; Dillon, J.F.; Foulerton, M.; Godfrey, E.M.; Hall, R.; Harrower, U.; Hudson, M.; Langford, A.; et al. Guidelines on the management of abnormal liver blood tests. Gut 2018, 67, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, J.; Patel, J.; Caldwell, H.; Davies, S.; Hebditch, V.; Hollywood, C.; Hubscher, S.; Karkhanis, S.; Lester, W.; Roslund, N.; et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut 2020, 69, 1382–1403. [Google Scholar] [CrossRef]
- Sumida, Y.; Nakajima, A.; Itoh, Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2014, 20, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.X.; Pradhan, F.; Zimmer, S.; Niu, S.; Crotty, P.; Tracey, J.; Schneider, C.; Heitman, S.J.; Kaplan, G.G.; Swain, M.G.; et al. The feasibility and reliability of transient elastography using Fibroscan: A practice audit of 2335 examinations. Can. J. Gastroenterol. Hepatol. 2014, 28, 143–149. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and Fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.S.; Yamada, G.; Vuppalanchi, R.; Van Natta, M.; Loomba, R.; Guy, C.; Brandman, D.; Tonascia, J.; Chalasani, N.; Neuschwander-Tetri, B.; et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin. Gastroenterol. Hepatol. 2019, 17, 1877–1885. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a Simple Noninvasive Index to Predict Significant Fibrosis in Patients with HIV/HCV Coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chang, D.; Kodali, S.; Harrison, S.A.; Ghobrial, M.; Alkhouri, N.; Noureddin, M. Degree of Discordance Between FIB-4 and Transient Elastography: An Application of Current Guidelines on General Population Cohort. Clin. Gastroenterol. Hepatol. 2024, 22, 1453–1461.E2. [Google Scholar] [CrossRef] [PubMed]
- Graupera, I.; Thiele, M.; Serra-Burriel, M.; Caballeria, L.; Roulot, D.; Wong, G.L.; Fabrellas, N.; Guha, I.N.; Arslanow, A.; Expósito, C.; et al. Low accuracy of FIB-4 and NAFLD fibrosis scores for screening for liver fibrosis in the population. Clin. Gastroenterol. Hepatol. 2022, 20, 2567–2576. [Google Scholar] [CrossRef] [PubMed]
- Viganò, M.; Pugliese, N.; Cerini, F.; Turati, F.; Cimino, V.; Ridolfo, S.; Rocchetto, S.; Foglio, F.; Terrin, M.; La Vecchia, C.; et al. Accuracy of FIB-4 to detect elevated liver stiffness measurements in patients with nonalcoholic fatty liver disease: A cross-sectional study in referral centers. Int. J. Mol. Sci. 2022, 23, 12489. [Google Scholar] [CrossRef] [PubMed]
- US CDC; National Center for Health Statistics. National Health and Nutrition Examination Survey (NHANES) Comprehensive Data List. Available online: https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component (accessed on 4 June 2024).
- Kanwal, F.; Shubrook, J.H.; Adams, L.A.; Pfotenhauer, K.; Wong, V.W.-S.; Wright, E.; Abdelmalek, M.F.; Harrison, S.A.; Loomba, R.; Mantzoros, C.S.; et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 2021, 161, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Neuschwander-Tetri, B.A.; Siddiqui, M.S.; Abdelmalek, M.F.; Caldwell, S.; Barb, D.; Kleiner, D.E.; Loomba, R. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023, 77, 1797–1835. [Google Scholar] [CrossRef] [PubMed]
- Boursier, J.; Zarski, J.; de Ledinghen, V.; Rousselet, M.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- van Kleef, L.A.; Sonneveld, M.J.; Zhu, F.; Ikram, M.A.; Kavousi, M.; de Knegt, R.J. Liver stiffness is associated with excess mortality in the general population driven by heart failure: The Rotterdam Study. Liver Int. 2023, 43, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.J.; Keyserling, T.C.; Ammerman, A.S.; Berkowitz, S.A. Diet quality trends among adults with diabetes by socioeconomic status in the US: 1999–2014. BMC Endocr. Disord. 2019, 19, 54. [Google Scholar] [CrossRef]
- Berkowitz, S.A.; Baggett, T.P.; Wexler, D.J.; Huskey, K.W.; Wee, C.C. Food insecurity and metabolic control among US adults with diabetes. Diabetes Care 2013, 36, 3093–3099. [Google Scholar] [CrossRef]
- Sun, H.; Weaver, C.M. Trends in Diet Quality and Increasing Inadequacies of Micronutrients Vitamin C, Vitamin B12, Iron and Potassium in US Type 2 Diabetic Adults. Nutrients 2023, 15, 1980. [Google Scholar] [CrossRef] [PubMed]
- Bonder, A.; Afdhal, N. Utilization of FibroScan in clinical practice. Curr. Gastroenterol. Rep. 2014, 16, 372. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Petta, S.; Pisano, G.; Dongiovanni, P.; Rinaldi, L.; Adinolfi, L.E.; Acierno, C.; Valenti, L.; Boemi, R.; Spatola, F.; et al. FibroScan identifies patients with nonalcoholic fatty liver disease and cardiovascular damage. Clin. Gastroenterol. Hepatol. 2020, 18, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Sripongpun, P.; Kim, W.R.; Mannalithara, A.; Charu, V.; Vidovszky, A.; Asch, S.; Desai, M.; Kim, S.H.; Kwong, A.J. The steatosis-associated fibrosis estimator (SAFE) score: A tool to detect low-risk NAFLD in primary care. Hepatology 2023, 77, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Kariyama, K.; Kawanaka, M.; Nouso, K.; Hiraoka, A.; Toyoda, H.; Tada, T.; Ishikawa, T.; Wakuta, A.; Miyake, N.; Murakami, S.; et al. Fibrosis-3 index: A new score to predict liver fibrosis in patients with nonalcoholic fatty liver disease without age as a factor. Gastro Hep Adv. 2022, 1, 1108–1113. [Google Scholar] [CrossRef]
- Unal, I. Defining an optimal cut-point value in ROC analysis: An alternative approach. Comput. Math. Methods Med. 2017, 2017, 3762651. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Younossi, Z.; LaVine, J.E.; Diehl, A.M.; Brunt, E.M.; Cusi, K.; Charlton, M.; Sanyal, A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012, 55, 2005–2023. [Google Scholar] [CrossRef]
- Ratziu, V.; Bellentani, S.; Cortez-Pinto, H.; Day, C.; Marchesini, G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J. Hepatol. 2010, 53, 372–384. [Google Scholar] [CrossRef]
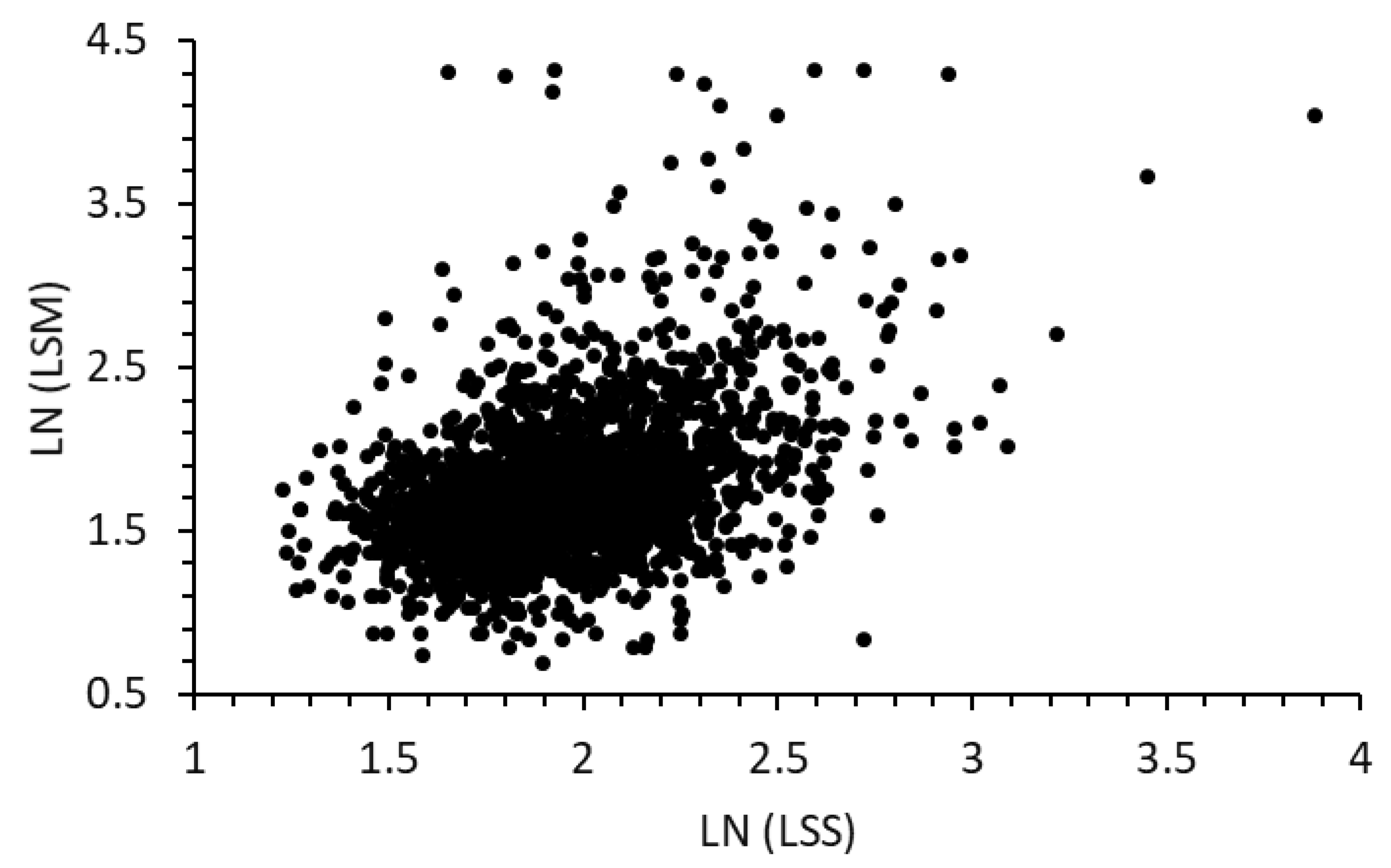
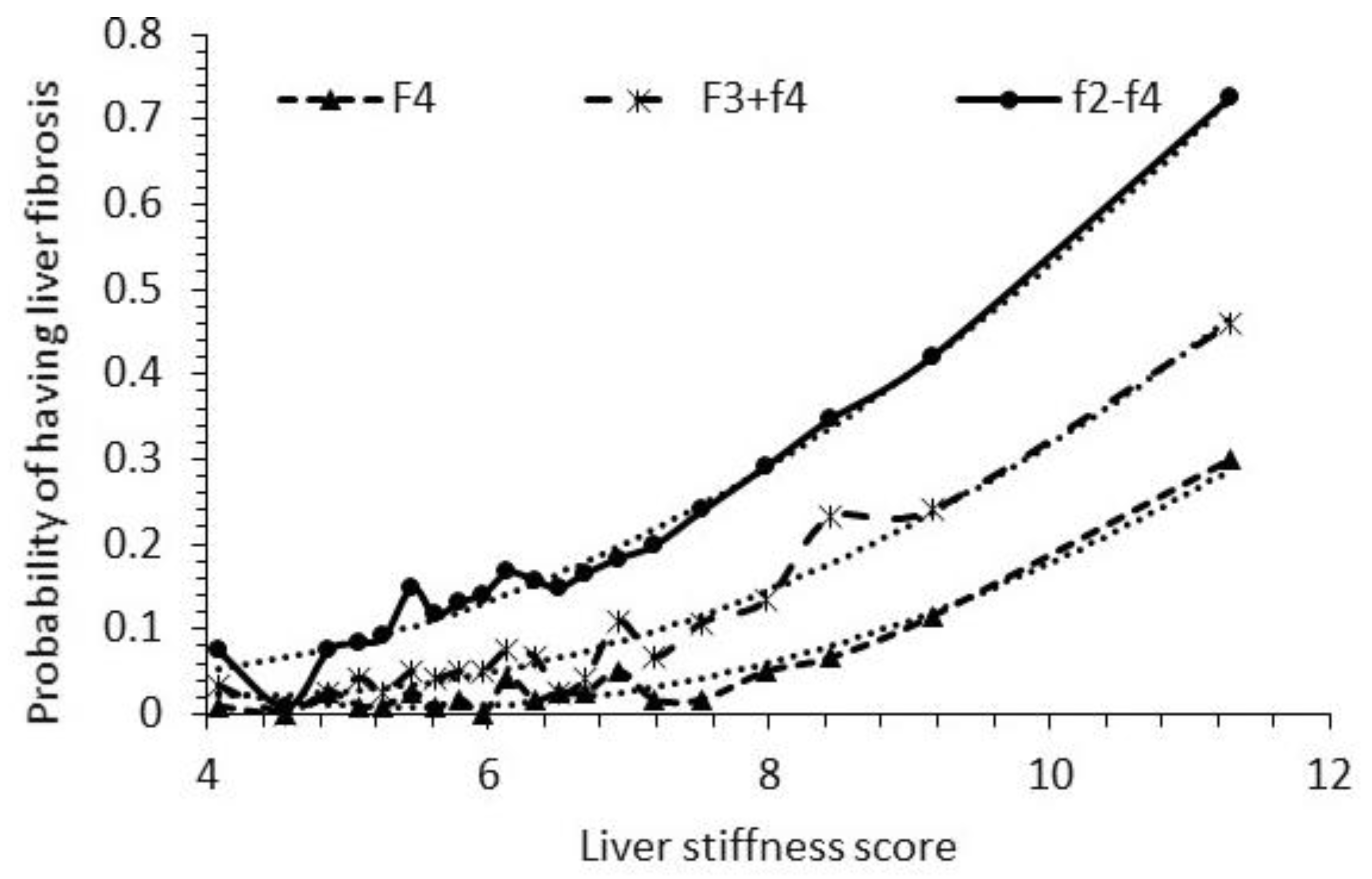
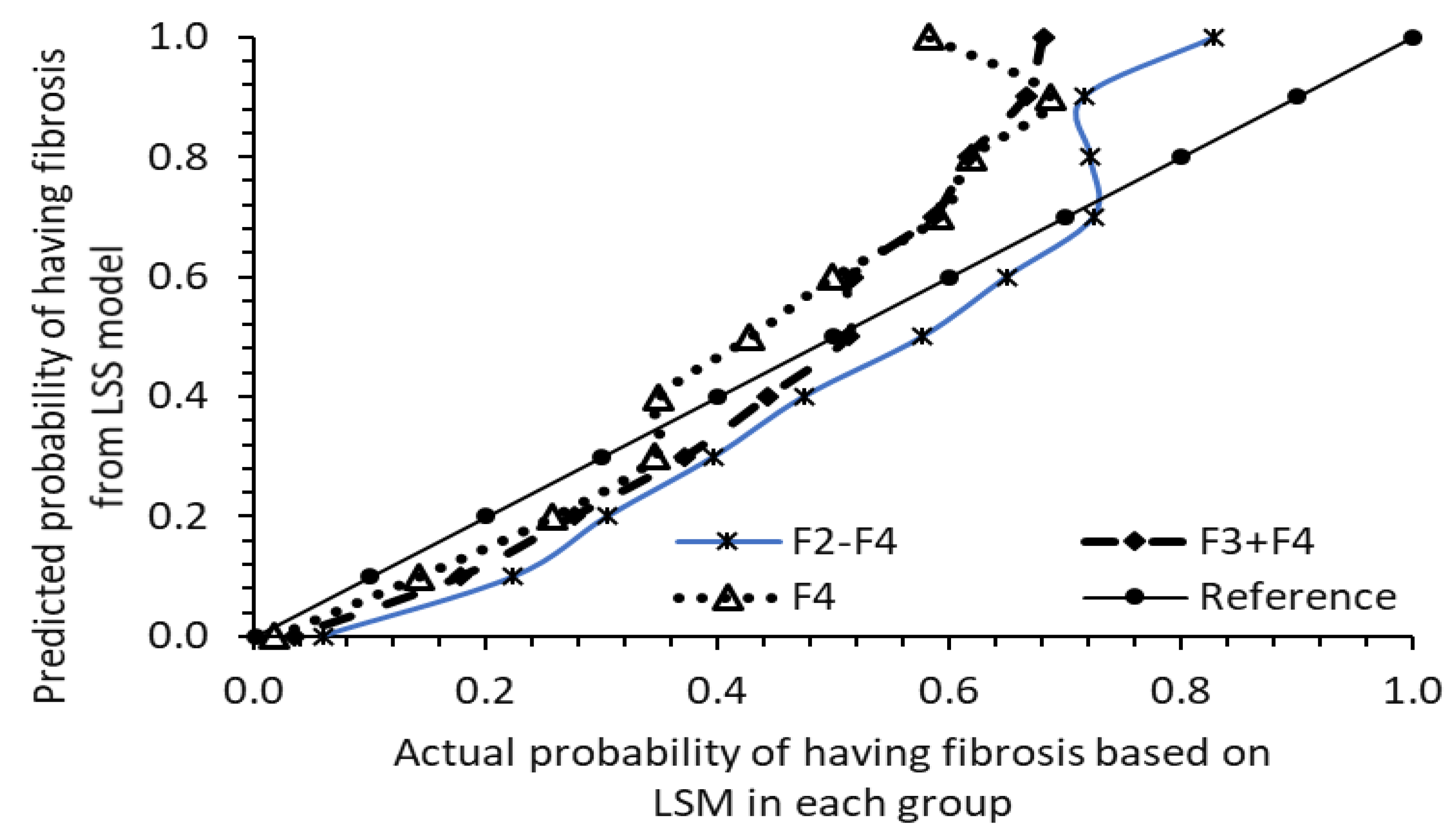



| Average Age, Body Measurements, and Blood Chemistry Data Examined | |||||
|---|---|---|---|---|---|
| 2017–2020 | 2017–2018 | 2017–2020 | 2017–2018 | ||
| Age (years) | 55.8 ± 0.3 | 56.1 ± 0.4 | GGT (IU/L) | 38.5 ± 1 | 38.7 ± 1.2 |
| Waist circ. (cm) | 90.8 ± 0.5 | 89.7 ± 0.6 | Platelet count (1000 cells/UL) | 244.3 ± 1.4 | 239.7 ± 1.7 |
| Weight (kg) | 108.3 ± 0.3 | 107.8 ± 0.4 | Albumin (g/dL) | 40.4 ± 0.1 | 40.3 ± 0.1 |
| BMI | Triglycerides (mg/dL) | 171.9 ± 2.4 | 180.5 ± 3.3 | ||
| HbA1C (%) | 6.4 ± 0.03 | 6.4 ± 0.03 | HDL | ||
| ALT (IU/L) | 27.8 ± 0.4 | 28.1 ± 0.5 | Systolic BP (mm Hg) | 131.8 ± 0.4 | 132.6 ± 0.5 |
| AST (IU/L) | 23.8 ± 0.3 | 24.3 ± 0.4 | Diastolic BP (mm Hg) | 75.6 ± 0.2 | 74.2 ± 0.3 |
| Counts and percentages of fibrosis stage | Counts of races | ||||
| 1 Fibrosis score | 2017–2020 | 2017–2018 | 2017–2020 | 2017–2018 | |
| F0-F1 (LSM < 7.1) | 1870 (77.5%) | 1205 (77.1%) | Mexican Americans | 331 | 244 |
| Ft (7.1–7.5) | 72 (3%) | 43 (2.8%) | 2 NMA Hispanic | 281 | 163 |
| F2 (7.5–10) | 247 (10.2%) | 167 (10.7%) | White | 767 | 497 |
| F3 (10–14) | 126 (5.2%) | 81 (5.2%) | Black | 615 | 342 |
| F4 (>14) | 99 (4.1%) | 66 (4.2%) | Asian | 308 | 233 |
| Total | 2414 | 1562 | Others | 112 | 83 |
| Counts of men in two sample cycles | Counts of women in two sample cycles | ||||
| men | 1320 | 841 | women | 1094 | 721 |
| F4 | F3 + F4 | F2–F4 | |
|---|---|---|---|
| LSS | 0.79 (0.73–0.86) | 0.78 (0.74–0.82) | 0.73 (0.7–0.77) |
| NFS | 0.76 (0.7–0.81) | 0.7 (0.65–0.74) | 0.65 (0.62–0.69) |
| FIB-4 | 0.71 (0.64–0.78) | 0.61 (0.56–0.67) | 0.57 (0.53–0.61) |
| SAFE | 0.77 (0.7–0.84) | 0.71 (0.66–0.76) | 0.67 (0.63–0.7) |
| FIB-3 | 0.75 (0.68–0.81) | 0.65 (0.6–0.7) | 0.59 (0.55–0.63) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H. An Alternative Non-Invasive Screening Model for Liver Fibrosis among US Adults at Risk of MASLD. Diseases 2024, 12, 150. https://doi.org/10.3390/diseases12070150
Sun H. An Alternative Non-Invasive Screening Model for Liver Fibrosis among US Adults at Risk of MASLD. Diseases. 2024; 12(7):150. https://doi.org/10.3390/diseases12070150
Chicago/Turabian StyleSun, Hongbing. 2024. "An Alternative Non-Invasive Screening Model for Liver Fibrosis among US Adults at Risk of MASLD" Diseases 12, no. 7: 150. https://doi.org/10.3390/diseases12070150
APA StyleSun, H. (2024). An Alternative Non-Invasive Screening Model for Liver Fibrosis among US Adults at Risk of MASLD. Diseases, 12(7), 150. https://doi.org/10.3390/diseases12070150





