The Conundrum of Cancer-Associated Thrombosis: Lesson Learned from Two Intriguing Cases and Literature Review
Abstract
1. Introduction
2. Cases Presentation
2.1. Case 1
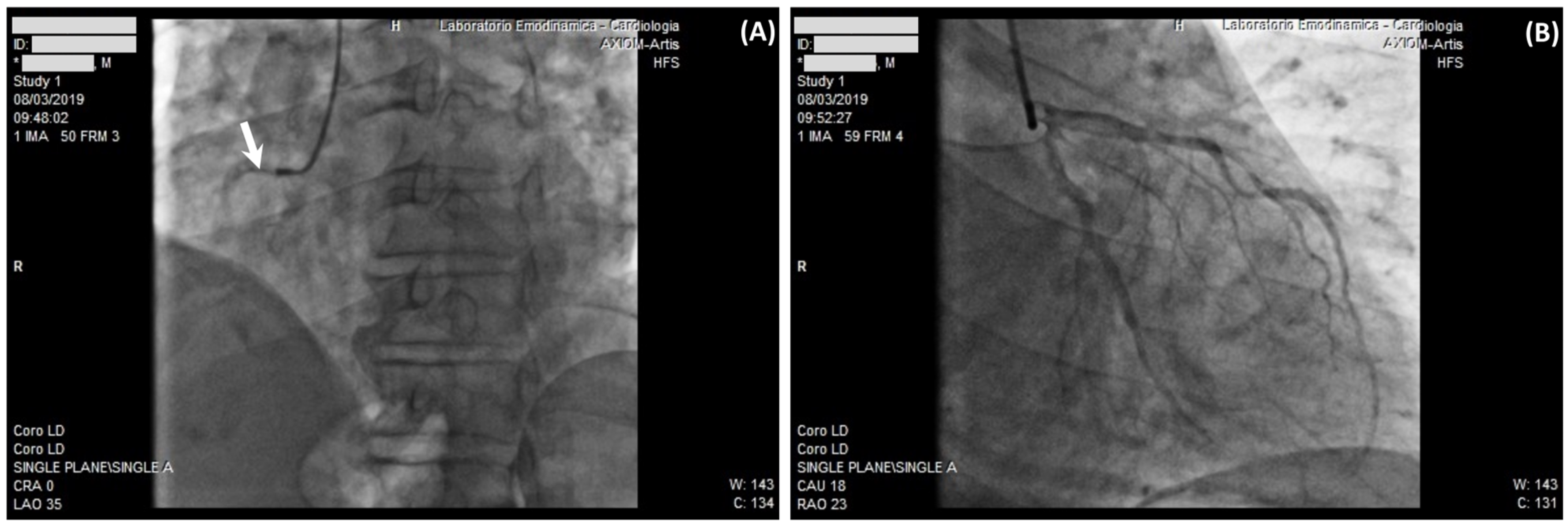
2.2. Case 2
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trousseau, A. Clinique Médicale de l’Hôtel-Dieu de Paris; Baillière: Paris, France, 1861; Volume 1. [Google Scholar]
- Bertoletti, L.; Madridano, O.; Jiménez, D.; Muriel, A.; Bikdeli, B.; Ay, C.; Trujillo-Santos, J.; Bosevski, M.; Sigüenza, P.; Monreal, M. Cancer-Associated Thrombosis: Trends in Clinical Features, Treatment, and Outcomes From 2001 to 2020. JACC CardioOncol. 2023, 5, 758–772. [Google Scholar] [CrossRef]
- Donnellan, E.; Khorana, A.A. Cancer and venous thromboembolic disease: A review. Oncologist 2017, 22, 199–207. [Google Scholar] [CrossRef]
- Lyman, G.H.; Culakova, E.; Poniewierski, M.S.; Kuderer, N.M. Morbidity, mortality and costs associated with venous thromboembolism in hospitalized patients with cancer. Thromb. Res. 2018, 164 (Suppl. S1), S112–S118. [Google Scholar] [CrossRef]
- Chatani, R.; Yamashita, Y.; Morimoto, T.; Kaneda, K.; Mushiake, K.; Kadota, K.; Nishimoto, Y.; Ikeda, N.; Kobayashi, Y.; Ikeda, S.; et al. Transition of management strategies and long-term outcomes in cancer-associated venous thromboembolism from the warfarin era to the direct oral anticoagulant era. Eur. J. Intern. Med. 2024. [Google Scholar] [CrossRef]
- Falanga, A.; Marchetti, M. Cancer-associated thrombosis: Enhanced awareness and pathophysiologic complexity. J. Thromb. Haemost. 2023, 21, 1397–1408. [Google Scholar] [CrossRef]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, A.J.; Bowman, R.L.; Park, Y.C.; O’Connor, K.; Izzo, F.; Myers, R.M.; Karzai, A.; Zaroogian, Z.; Kim, W.J.; Fernandez-Maestre, I.; et al. Jak2v617f reversible activation shows its essential requirement in myeloproliferative neoplasms. Cancer Discov. 2024. [Google Scholar] [CrossRef]
- van der Hagen, P.B.; Folsom, A.R.; Jenny, N.S.; Heckbert, S.R.; O’Meara, E.S.; Reich, L.M.; Rosendaal, F.R.; Cushman, M. Subclinical atherosclerosis and the risk of future venous thrombosis in the Cardiovascular Health Study. J. Thromb. Haemost. 2006, 4, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P. Is there a link between venous and arterial thrombosis? A reappraisal. Intern. Emerg. Med. 2020, 15, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Noumegni, S.R.; Hoffmann, C.; Tromeur, C.; Lacut, K.; Didier, R.; Couturaud, F.; Bressollette, L. Frequency and incidence of arterial events in patients with venous thromboembolism compared to the general population: A systematic review and meta-analysis of cohort studies. Thromb. Res. 2021, 203, 172–185. [Google Scholar] [CrossRef]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Tagawa, S.T.; Panageas, K.S.; DeAngelis, L.M. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019, 133, 781–789. [Google Scholar] [CrossRef]
- Carrier, M. Cancer screening in unprovoked venous thromboembolism. N. Engl. J. Med. 2015, 373, 2475. [Google Scholar] [CrossRef]
- Prandoni, P.; Bernardi, E.; Valle, F.D.; Visonà, A.; Tropeano, P.F.; Bova, C.; Bucherini, E.; Islam, M.S.; Piccioli, A. Extensive Computed Tomography versus Limited Screening for Detection of Occult Cancer in Unprovoked Venous Thromboembolism: A Multicenter, Controlled, Randomized Clinical Trial. Semin. Thromb. Hemost. 2016, 42, 884–890. [Google Scholar] [CrossRef]
- Robin, P.; Le Roux, P.-Y.; Planquette, B.; Accassat, S.; Roy, P.-M.; Couturaud, F.; Ghazzar, N.; Prevot-Bitot, N.; Couturier, O.; Delluc, A.; et al. Limited screening with versus without (18)F-fluorodeoxyglucose PET/CT for occult malignancy in unprovoked venous thromboembolism: An open-label randomised controlled trial. Lancet Oncol. 2016, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Horváth–Puhó, E.; van Es, N.; Pedersen, L.; Büller, H.R.; Bøtker, H.E.; Sørensen, H.T. Arterial thromboembolism in cancer patients. JACC CardioOncol. 2021, 3, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, Y.D.; Kim, C.H. Incidence and risk of various types of arterial thromboembolism in patients with cancer. Mayo Clin. Proc. 2021, 96, 592–600. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef]
- Streiff, M.B.; Holmstrom, B.; Angelini, D.; Ashrani, A.; Elshoury, A.; Fanikos, J.; Fertrin, K.Y.; Fogerty, A.E.; Gao, S.; Goldhaber, S.Z.; et al. Cancer-Associated Venous Thromboembolic Disease, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2021, 19, 1181–1201. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.; Parameswaran, R.; Hassoun, H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J. Clin. Oncol. 2011, 29, 3466–3473. [Google Scholar] [CrossRef]
- Fernandes, D.D.; Louzada, M.L.; Souza, C.A.; Matzinger, F. Acute aortic thrombosis in patients receiving cisplatin-based chemotherapy. Curr. Oncol. 2011, 18, e97–e100. [Google Scholar] [CrossRef]
- Libby, P. Inflammation and the pathogenesis of atherosclerosis. Vascul. Pharmacol. 2023, 154, 107255. [Google Scholar] [CrossRef]
- Prousi, G.S.; Joshi, A.M.; Atti, V.; Addison, D.; Brown, S.-A.; Guha, A.; Patel, B. Vascular inflammation, cancer, and cardiovascular diseases. Curr. Oncol. Rep. 2023, 25, 955–963. [Google Scholar] [CrossRef]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.V.; Panageas, K.S.; DeAngelis, L.M. Risk of arterial thromboembolism in patients with cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef]
- Aronson, D.; Brenner, B. Arterial thrombosis and cancer. Thromb. Res. 2018, 164 (Suppl. S1), S23–S28. [Google Scholar] [CrossRef] [PubMed]
- Grilz, E.; Posch, F.; Nopp, S.; Königsbrügge, O.; Lang, I.M.; Klimek, P.; Thurner, S.; Pabinger, I.; Ay, C. Relative risk of arterial and venous thromboembolism in persons with cancer vs. persons without cancer–A nationwide analysis. Eur. Heart J. 2021, 42, 2299–2307. [Google Scholar] [CrossRef]
- Kim, J.H.; Shim, M.J.; Lee, S.-Y.; Oh, J.; Kim, S.H. Aspirin for primary prevention of cardiovascular disease. J. Lipid Atheroscler. 2019, 8, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, C.; Basaglia, M.; Riva, M.; Meschi, M.; Meschi, T.; Castaldo, G.; Di Micco, P. Statins effects on blood clotting: A review. Cells 2023, 12, 2719. [Google Scholar] [CrossRef]
- Brenner, B.; Bikdeli, B.; Tzoran, I.; Madridano, O.; López-Reyes, R.; Suriñach, J.M.; Blanco-Molina, Á.; Tufano, A.; Núñez, J.J.L.; Trujillo-Santos, J.; et al. Arterial Ischemic Events Are a Major Complication in Cancer Patients with Venous Thromboembolism. Am. J. Med. 2018, 131, 1095–1103. [Google Scholar] [CrossRef]
- Koelwyn, G.J.; Aboumsallem, J.P.; Moore, K.J.; de Boer, R.A. Reverse cardio-oncology: Exploring the effects of cardiovascular disease on cancer pathogenesis. J. Mol. Cell. Cardiol. 2022, 163, 1–8. [Google Scholar] [CrossRef]
- Narayan, V.; Thompson, E.W.; Demissei, B.; Ho, J.E.; Januzzi, J.L.; Ky, B. Mechanistic Biomarkers Informative of Both Cancer and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2726–2737. [Google Scholar] [CrossRef]
- Nopp, S.; Moik, F.; Kraler, S.; Englisch, C.; Preusser, M.; von Eckardstein, A.; Pabinger, I.; Lüscher, T.F.; Ay, C. Growth differentiation factor-15 and prediction of cancer-associated thrombosis and mortality: A prospective cohort study. J. Thromb. Haemost. 2023, 21, 2461–2472. [Google Scholar] [CrossRef]
- Mehta, P.K.; Sharma, A.; Bremner, J.D.; Vaccarino, V. Mental Stress-Induced Myocardial Ischemia. Curr. Cardiol. Rep. 2022, 24, 2109–2120. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Rapala-Kozik, M. Neutrophil extracellular traps (NETs)—Formation and implications. Acta Biochim. Pol. 2013, 60, 277–284. [Google Scholar] [CrossRef]
- Warnatsch, A.; Ioannou, M.; Wang, Q.; Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015, 349, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.L.; Demers, M.; Martinod, K.; Gallant, M.; Wang, Y.; Goldfine, A.B.; Kahn, C.R.; Wagner, D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015, 21, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Hisada, Y.; Lundström, S.; Mackman, N.; Wallén, H. Neutrophil Extracellular Traps: Villains and Targets in Arterial, Venous, and Cancer-Associated Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1724–1738. [Google Scholar] [CrossRef]
- Franck, G.; Mawson, T.L.; Folco, E.J.; Molinaro, R.; Ruvkun, V.; Engelbertsen, D.; Liu, X.; Tesmenitsky, Y.; Shvartz, E.; Sukhova, G.K.; et al. Roles of PAD4 and netosis in experimental atherosclerosis and arterial injury: Implications for superficial erosion. Circ. Res. 2018, 123, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef]
- Alfaro, C.; Teijeira, A.; Oñate, C.; Pérez, G.; Sanmamed, M.F.; Andueza, M.P.; Alignani, D.; Labiano, S.; Azpilikueta, A.; Rodriguez-Paulete, A.; et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin. Cancer Res. 2016, 22, 3924–3936. [Google Scholar] [CrossRef]
- Tohme, S.; Yazdani, H.O.; Al-Khafaji, A.B.; Chidi, A.P.; Loughran, P.; Mowen, K.; Wang, Y.; Simmons, R.L.; Huang, H.; Tsung, A. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016, 76, 1367–1380. [Google Scholar] [CrossRef]
- Zarà, M.; Guidetti, G.F.; Camera, M.; Canobbio, I.; Amadio, P.; Torti, M.; Tremoli, E.; Barbieri, S.S. Biology and role of extracellular vesicles (evs) in the pathogenesis of thrombosis. Int. J. Mol. Sci. 2019, 20, 2840. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, Q. The effect of extracellular vesicles on thrombosis. J. Cardiovasc. Transl. Res. 2022, 16, 682–697. [Google Scholar] [CrossRef]
- Debbie Jiang, M.D.; Alfred Ian Lee, M.D. Thrombotic Risk from Chemotherapy and Other Cancer Therapies. Cancer Treat. Res. 2019, 179, 87–101. [Google Scholar] [CrossRef]
- Strauss, L.; Mahmoud, M.A.A.; Weaver, J.D.; Tijaro-Ovalle, N.M.; Christofides, A.; Wang, Q.; Pal, R.; Yuan, M.; Asara, J.; Patsoukis, N.; et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020, 5, eaay1863. [Google Scholar] [CrossRef]
- Fernandez, D.M.; Rahman, A.H.; Fernandez, N.F.; Chudnovskiy, A.; Amir, E.-A.D.; Amadori, L.; Khan, N.S.; Wong, C.K.; Shamailova, R.; Hill, C.A.; et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019, 25, 1576–1588. [Google Scholar] [CrossRef]
- Bu, D.; Tarrio, M.; Maganto-Garcia, E.; Stavrakis, G.; Tajima, G.; Lederer, J.; Jarolim, P.; Freeman, G.J.; Sharpe, A.H.; Lichtman, A.H. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1100–1107. [Google Scholar] [CrossRef]
- Lee, J.; Zhuang, Y.; Wei, X.; Shang, F.; Wang, J.; Zhang, Y.; Liu, X.; Yang, Y.; Liu, L.; Zheng, Q. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J. Mol. Cell. Cardiol. 2009, 46, 169–176. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sasaki, N.; Yamashita, T.; Emoto, T.; Kasahara, K.; Mizoguchi, T.; Hayashi, T.; Yodoi, K.; Kitano, N.; Saito, T.; et al. Overexpression of Cytotoxic T-Lymphocyte-Associated Antigen-4 Prevents Atherosclerosis in Mice. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1141–1151. [Google Scholar] [CrossRef]
- Khorana, A.A.; Palaia, J.; Rosenblatt, L.; Pisupati, R.; Huang, N.; Nguyen, C.; Barron, J.; Gallagher, K.; Bond, T.C. Venous thromboembolism incidence and risk factors associated with immune checkpoint inhibitors among patients with advanced non-small cell lung cancer. J. Immunother. Cancer 2023, 11, e006072. [Google Scholar] [CrossRef]
- Moik, F.; Ay, C. Venous and arterial thromboembolism in patients with cancer treated with targeted anti-cancer therapies. Thromb. Res. 2022, 213 (Suppl. S1), S58–S65. [Google Scholar] [CrossRef]
- Moik, F.; Riedl, J.M.; Englisch, C.; Ay, C. Update on Thrombosis Risk in Patients with Cancer: Focus on Novel Anticancer Immunotherapies. Hamostaseologie 2024. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laurino, S.; Russi, S.; Omer, L.C.; D’Angelo, A.; Bozza, G.; Gallucci, G.; Falco, G.; Roviello, G.; Bochicchio, A.M. The Conundrum of Cancer-Associated Thrombosis: Lesson Learned from Two Intriguing Cases and Literature Review. Diseases 2024, 12, 47. https://doi.org/10.3390/diseases12030047
Laurino S, Russi S, Omer LC, D’Angelo A, Bozza G, Gallucci G, Falco G, Roviello G, Bochicchio AM. The Conundrum of Cancer-Associated Thrombosis: Lesson Learned from Two Intriguing Cases and Literature Review. Diseases. 2024; 12(3):47. https://doi.org/10.3390/diseases12030047
Chicago/Turabian StyleLaurino, Simona, Sabino Russi, Ludmila Carmen Omer, Alberto D’Angelo, Giovanni Bozza, Giuseppina Gallucci, Geppino Falco, Giandomenico Roviello, and Anna Maria Bochicchio. 2024. "The Conundrum of Cancer-Associated Thrombosis: Lesson Learned from Two Intriguing Cases and Literature Review" Diseases 12, no. 3: 47. https://doi.org/10.3390/diseases12030047
APA StyleLaurino, S., Russi, S., Omer, L. C., D’Angelo, A., Bozza, G., Gallucci, G., Falco, G., Roviello, G., & Bochicchio, A. M. (2024). The Conundrum of Cancer-Associated Thrombosis: Lesson Learned from Two Intriguing Cases and Literature Review. Diseases, 12(3), 47. https://doi.org/10.3390/diseases12030047







