The Therapeutic Potential of Oral Everolimus for Facial Angiofibromas in Pediatric Tuberous Sclerosis Complex: A Case-Based Analysis of Efficacy
Abstract
1. Introduction
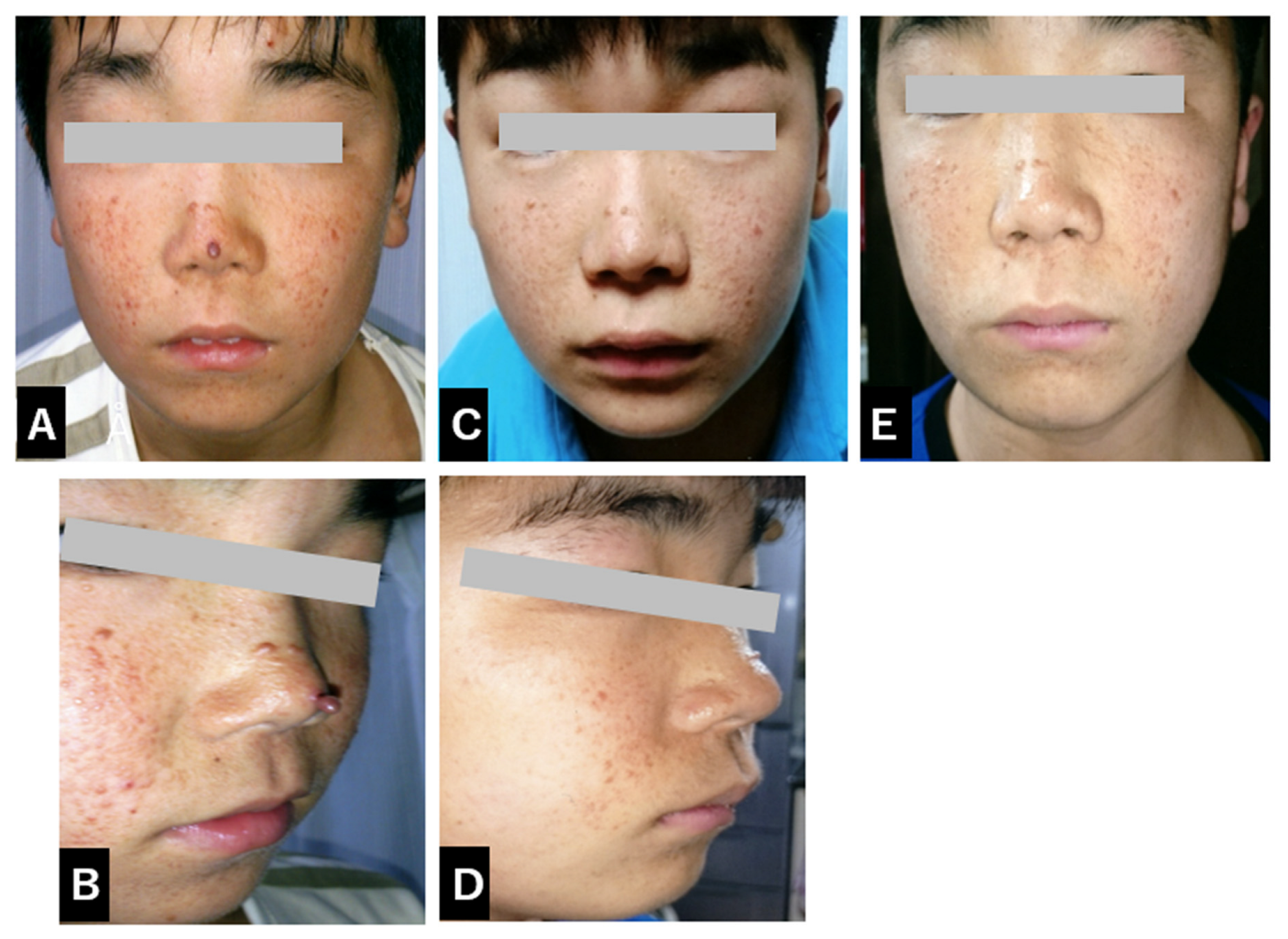
2. Case Report
3. Discussion
3.1. Everolimus for Facial Angiofibromas
3.2. Topical vs. Systemic mTOR Inhibition
3.3. Future Directions in TSC Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Northrup, H.; Krueger, D.A.; Roberds, S.; Smith, K.; Sampson, J.; Korf, B.; Kwiatkowski, D.J.; Mowat, D.; Nellist, M.; Povey, S.; et al. Tuberous sclerosis complex diagnostic criteria update: Recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr. Neurol. 2013, 49, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B.; Nathanson, K.L.; Henske, E.P. The tuberous sclerosis complex. N. Engl. J. Med. 2006, 355, 1345–1356. [Google Scholar] [CrossRef]
- Roth, J.; Roach, E.S.; Bartels, U.; Jóźwiak, S.; Koenig, M.K.; Weiner, H.L.; Franz, D.N.; Wang, H.Z. Subependymal giant cell astrocytoma in tuberous sclerosis complex: Guidelines for management. Pediatr. Neurol. 2013, 49, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Franz, D.N.; Jóźwiak, S.; Krueger, D.A.; Bissler, J.J.; Kingswood, J.C.; Belousova, V.; Frost, E.A.H.; Korf, B.K.; Koenig, M.K.; Rasmuson, J.; et al. Efficacy of everolimus for SEGAs in TSC: EXIST-1 trial. Lancet 2013, 381, 125–132. [Google Scholar] [CrossRef]
- Ehninger, D.; Silva, A.J. Rapamycin for TSC-related disorders: Mechanisms and evidence. Brain Res. Rev. 2008, 59, 293–305. [Google Scholar]
- Davies, D.M.; Johnson, S.R.; Tattersfield, A.E.; Kingswood, J.C.; Cox, J.A.; O’Callaghan, F.; Saggar, A.; Sampson, J.R.; McCartney, D.L.; De Vries, P.J. Everolimus for renal AML in TSC. Curr. Med. Res. Opin. 2017, 33, 1271–1280. [Google Scholar]
- Bissler, J.J.; Kingswood, J.C.; Radzikowska, E.; Zonnenberg, B.A.; Frost, M.; Belousova, V.; Sauter, M.; Brakemeier, S.; Nonomura, N.; Brase, J.C.; et al. Everolimus for AML in TSC or sporadic LAM: EXIST-2 trial. Nephrol. Dial. Transplant. 2016, 31, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Kingswood, J.C.; Jozwiak, S.; Belousova, V.; Frost, M.; Kaper, M.; Schillinger, E.; Flad, T.; Sauter, M.; Nonomura, N.; Brakemeier, S.; et al. Long-term everolimus in TSC-associated AML. Nephrol. Dial. Transplant. 2014, 29, 2521–2529. [Google Scholar]
- Wataya-Kaneda, M.; Tanaka, M.; Nakamura, A.; Matsumoto, S.; Katayama, I. Efficacy of sirolimus gel for FAs in TSC. JAMA Dermatol. 2017, 153, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Krueger, D.A.; Wilfong, A.A.; Holland-Bouley, K.; Anderson, A.E.; Agricola, K.; Tudor, C.; Mays, M.; Lopez, C.M.; Kim, A.H.; Franz, D.N. TSC and everolimus treatment guidelines. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163C, 91–99. [Google Scholar]
- Huang, J.; Dibble, C.C.; Matsuzaki, M.; Manning, B.D. TSC-mTOR pathway and regulation of cell growth. Nat. Rev. Mol. Cell Biol. 2008, 9, 339–350. [Google Scholar]
- Wang, J.; Peng, T.; Yin, Q.; Li, X.; Zhang, C.; Wang, Y.; Zhang, X.; Feng, Z.; Li, S. Everolimus reduces autism-like behaviors in TSC2 mice. Sci. Rep. 2015, 5, 17109. [Google Scholar]
- Yui, K.; Imataka, G.; Sasaki, H.; Shiroki, R.; Yoshihara, S. Improvement in social cognition by everolimus in TSC. Case Rep. Pediatr. 2019, 2019, 2070619. [Google Scholar]
- Koenig, M.K.; Bell, C.S.; Hebert, A.A.; Roberson, J.; Samuels, J.A.; Slopis, J.M.; Northrup, H.; Krueger, D.A.; Franz, D.N. Efficacy and safety of topical rapamycin in TSC: TREATMENT trial. JAMA Dermatol. 2018, 154, 773–780. [Google Scholar] [CrossRef]
- Pass, C.; Medley, M.; Adams, D.; Dever, D.; Patel, H.; Northrup, H. Guidelines for rapamycin gel in TSC. Dermatol. Pract. Res. 2021, 15, 225–232. [Google Scholar]
- Devaraj, N.K.; Aneesa, A.R.; Suresh, S.S.; Kumar, S.S. Topical corticosteroids in clinical practice. Med. J. Malaysia. 2019, 74, 187–189. [Google Scholar]
- Hart, C.W.; Adams, D.; Medley, M.; Pass, C.; Northrup, H. Exploring sirolimus applications in TSC dermatology. Br. J. Dermatol. 2021, 184, 741–749. [Google Scholar]
- Koenig, M.K.; Bell, C.S.; Roberson, J.; Samuels, J.A.; Slopis, J.M.; Northrup, H.; Krueger, D.A.; Franz, D.N. Everolimus and dermatologic improvements in TSC. Dermatol. Online J. 2018, 24, 13030. [Google Scholar]
- Segal, S.; Williams, R.E.; Crino, P.B.; Kwiatkowski, D.J.; Whittemore, V.H.; Sahin, M. Safety and efficacy of mTOR inhibitors in children. J. Pediatr. Pharmacol. Ther. 2020, 25, 128–135. [Google Scholar]
- Liu, M.; Ye, J.; You, X. An updated meta-analysis of effectiveness and safety of mTOR inhibitors in the management of tuberous sclerosis complex patients. Child’s Nervous System. 2024, 40, 823–829. [Google Scholar] [CrossRef]
- Lin, Y.T.; Yu, C.L.; Chi, C.C. Efficacy and Safety of Topical Mechanistic Target of Rapamycin Inhibitors for Facial Angiofibromas in Patients with Tuberous Sclerosis Complex: A Systematic Review and Network Meta-Analysis. Biomedicines 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Aguayo, A.; Rojas, M.; Carrasco, P.; Lagos, M.; Córdova, F.; Pérez, F.; García, H. Dual inhibition of mTORC1/mTORC2 in TSC. Expert. Opin. Investig. Drugs 2020, 29, 1125–1135. [Google Scholar]
- Medley, M.A.; Adams, D.; Pass, C.; Northrup, H. Current trends in mTOR inhibition for TSC. Clin. Genet. 2022, 101, 203–209. [Google Scholar]
- Lendvai, N.; Jóźwiak, S.; Kwiatkowski, D.J.; Sahin, M. Review on mTOR pathway in TSC. Curr. Opin. Neurol. 2019, 32, 737–743. [Google Scholar]
- Hussain, Z.; Feldkamp, M.M.; Johnston, D.L.; Blaser, S.; Weiss, S.K. Novel treatments targeting mTOR in TSC. Future Oncol. 2020, 16, 1035–1043. [Google Scholar]
- Bar-Peled, L.; Sabatini, D.M. Pharmacological advances in TSC management. Mol. Cancer Ther. 2019, 18, 1455–1462. [Google Scholar]
- Davies, D.M.; Johnson, S.R.; Tattersfield, A.E.; Kingswood, J.C.; Cox, J.A.; O’Callaghan, F.; Saggar, A.; Sampson, J.R.; McCartney, D.L.; De Vries, P.J. TSC pharmacology and management. Curr. Opin. Pediatr. 2019, 31, 636–642. [Google Scholar]

| Category | Everolimus (Oral) | Sirolimus (Topical) |
|---|---|---|
| Formulation | Oral tablet (oral medication) | Topical medication (topical ointment) |
| Mechanism of Action | Everolimus selectively inhibits mTORC1 (mechanistic target of rapamycin complex 1), suppressing cell cycle progression. mTORC1 is involved in regulating cell growth, proliferation, survival, and metabolism, and its inhibition suppresses the growth of tumor cells and abnormal cells. It is particularly effective for SEGA (subependymal giant cell astrocytoma), skin angiofibromas, and kidney tumors related to TSC. | Sirolimus locally inhibits mTORC1, suppressing the excessive growth of skin cells and angiogenesis, contributing to the improvement of facial angiofibromas and skin rashes. Being localized, its systemic effects are minimal. The inhibition of mTORC1 primarily suppresses cell proliferation and angiogenesis, particularly the thickening of the skin epidermis. |
| Indications | Everolimus is used in the treatment of kidney, brain (SEGA), and skin lesions associated with tuberous sclerosis complex (TSC). It is particularly effective for renal tumors and facial angiofibromas, and used for lung lesions in TSC. | Sirolimus is used in the treatment of facial angiofibromas and skin lesions associated with TSC. Aimed at improving facial skin lesions locally. |
| Pharmacological Effects | Everolimus controls cell growth, proliferation, and metabolism by inhibiting mTORC1, particularly suppressing tumor and abnormal cell proliferation. This leads to tumor size reduction and growth suppression. Efficacy has been demonstrated in shrinking renal tumors, brain tumors (SEGAs), skin angiofibromas, and lung nodules. | Sirolimus locally inhibits mTORC1, leading to an improvement in skin angiofibromas and rashes. Its localized action minimizes the risk of systemic side effects. The main effect is the suppression of cell division and angiogenesis at the skin surface. |
| Administration | Everolimus is taken orally, with a recommended daily dose, unaffected by meals. Peak plasma concentration occurs 1–2 h after administration, with a half-life of approximately 30 h. It is metabolized primarily in the liver, with metabolites excreted in the urine. | Sirolimus is applied topically, usually twice daily. The medication works locally on the skin, with minimal systemic effects. Blood concentrations are kept low. |
| Pharmacokinetics | Everolimus is absorbed from the gastrointestinal tract and metabolized in the liver by the CYP3A4 enzyme system. Its plasma concentration may fluctuate due to food intake, so regular monitoring is recommended. It is excreted after liver metabolism, with a half-life of about 30 h, requiring consistent dosing intervals. | After topical application, sirolimus acts locally with extremely low systemic absorption, minimizing the risk of systemic side effects. Data on local pharmacokinetics are limited, but the drug acts on the skin’s surface, maintaining localized effects. |
| Side Effects | Immunosuppressive effects increase the risk of infections (particularly pneumonia and urinary tract infections). Gastrointestinal symptoms (mouth ulcers, diarrhea), liver dysfunction, increased blood sugar, and hypertension are reported. Skin rashes, edema, and respiratory symptoms (coughing, shortness of breath) may also occur. | Local skin side effects (dryness, itching, redness, inflammation) are primarily observed. Systemic side effects are rare, but occasionally, skin hypersensitivity or allergic reactions are reported. |
| Clinical Trial Results | Everolimus has shown clear efficacy in treating kidney tumors, brain tumors (SEGAs), and skin lesions in TSC, especially in tumor shrinkage and disease progression inhibition. Long-term clinical trials have confirmed its therapeutic effectiveness. | Sirolimus has demonstrated localized effectiveness for facial angiofibromas, showing improvement in facial rashes and blood vessel tumors. Local treatment leads to visible improvements, with mild side effects, making it a favorable option for patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imataka, G.; Mori, S.; Yui, K.; Igawa, K.; Shiraishi, H.; Yoshihara, S. The Therapeutic Potential of Oral Everolimus for Facial Angiofibromas in Pediatric Tuberous Sclerosis Complex: A Case-Based Analysis of Efficacy. Diseases 2024, 12, 334. https://doi.org/10.3390/diseases12120334
Imataka G, Mori S, Yui K, Igawa K, Shiraishi H, Yoshihara S. The Therapeutic Potential of Oral Everolimus for Facial Angiofibromas in Pediatric Tuberous Sclerosis Complex: A Case-Based Analysis of Efficacy. Diseases. 2024; 12(12):334. https://doi.org/10.3390/diseases12120334
Chicago/Turabian StyleImataka, George, Satoshi Mori, Kunio Yui, Ken Igawa, Hideaki Shiraishi, and Shigemi Yoshihara. 2024. "The Therapeutic Potential of Oral Everolimus for Facial Angiofibromas in Pediatric Tuberous Sclerosis Complex: A Case-Based Analysis of Efficacy" Diseases 12, no. 12: 334. https://doi.org/10.3390/diseases12120334
APA StyleImataka, G., Mori, S., Yui, K., Igawa, K., Shiraishi, H., & Yoshihara, S. (2024). The Therapeutic Potential of Oral Everolimus for Facial Angiofibromas in Pediatric Tuberous Sclerosis Complex: A Case-Based Analysis of Efficacy. Diseases, 12(12), 334. https://doi.org/10.3390/diseases12120334







