Trigeminal Pontine Sign: From Imaging to Diseases Beyond Trigeminal Neuralgia
Abstract
:1. Introduction
2. TN: Neuroanatomical Issues
- Mesencephalic nucleus: situated at the pons-midbrain junction, it primarily receives proprioceptive input from the mandibular division (V3).
- Spinal trigeminal nucleus: extending from the ponto-medullary junction to the upper cervical cord, this nucleus mediates pain and temperature sensations from V1–V3.
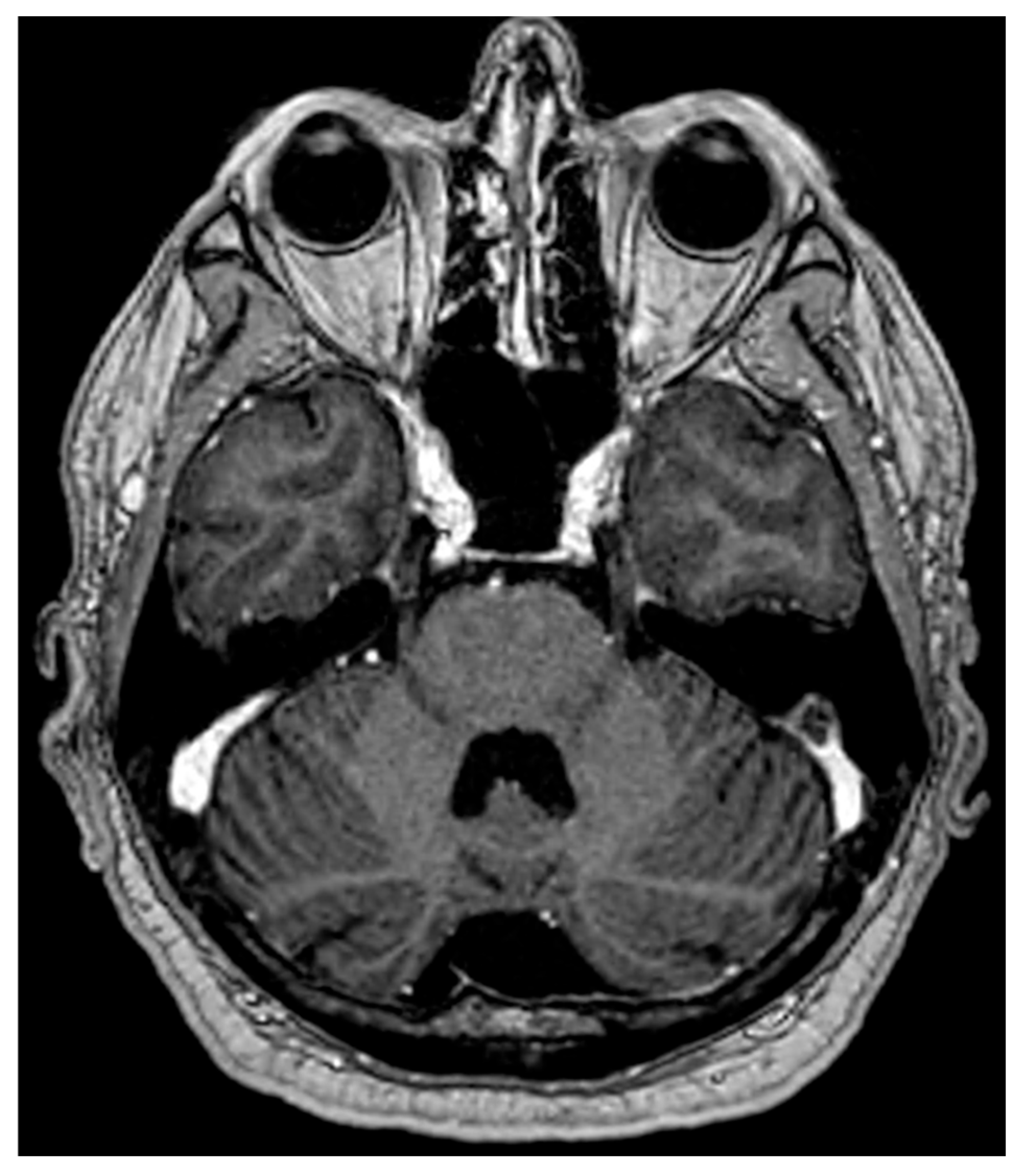
3. Trigeminal Pontine Segment: Neuroradiological Issues
- The sensory nucleus (touch and position sensation);
- The motor nucleus (muscle control for mastication);
- The mesencephalic nucleus (proprioception);
- The spinal nucleus and tract (pain and temperature sensation);
- The intrapontine fascicular part, the central pathway of trigeminal nerve fibers within the brainstem.
4. Trigeminal Pontine Sign: Definition and Etiologies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laine, F.J.; Smoker, W. Anatomy of the cranial nerves. Neuroimaging Clin. N. Am. 1998, 8, 69–100. [Google Scholar] [PubMed]
- Manzoni, G.C.; Torelli, P. Epidemiology of typical and atypical craniofacial neuralgias. Neurol. Sci. 2005, 26, s65–s67. [Google Scholar] [CrossRef] [PubMed]
- Devor, M.; Amir, R.; Rappaport, Z.H. Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin. J. Pain 2002, 18, 4–13. [Google Scholar] [CrossRef]
- Love, S.; Coakham, H.B. Trigeminal neuralgia: Pathology and pathogenesis. Brain 2001, 124 Pt 12, 2347–2360. [Google Scholar] [CrossRef]
- Nakashima, I.; Fujihara, K.; Kimpara, T.; Okita, N.; Takase, S.; Itoyama, Y. Linear pontine trigeminal root lesions in multiple sclerosis: Clinical and magnetic resonance imaging studies in 5 cases. Arch. Neurol. 2001, 58, 101–104. [Google Scholar] [CrossRef]
- Renard, D.; Tubery, A.; Castelnovo, G. Linear pontine trigeminal root and trigeminal nerve lesions in multiple sclerosis. Acta Neurol. Belg. 2015, 115, 429–430. [Google Scholar] [CrossRef]
- Mills, R.J.; Young, C.A.; Smith, E.T. Central trigeminal involvement in multiple sclerosis using high-resolution MRI at 3 T. Br. J. Radiol. 2010, 83, 493–498. [Google Scholar] [CrossRef]
- D’Amico, A.; Russo, C.; Ugga, L.; Mazio, F.; Capone, E.; D’Arco, F.; Mankad, K.; Caranci, F.; Marano, E.; Brunetti, A. Can pontine trigeminal T2-hyperintensity suggest herpetic etiology of trigeminal neuralgia? Quant. Imaging Med. Surg. 2016, 6, 490–495. [Google Scholar] [CrossRef]
- Sugiyama, A.; Mori, M.; Masuda, H.; Uchida, T.; Muto, M.; Uzawa, A.; Ito, S.; Kuwabara, S. Trigeminal root entry zone involvement in neuromyelitis optica and multiple sclerosis. J. Neurol. Sci. 2015, 355, 147–149. [Google Scholar] [CrossRef]
- Majeed, M.H.; Arooj, S.; Khokhar, M.A.; Mirza, T.; Ali, A.A.; Bajwa, Z.H. Trigeminal neuralgia: A clinical review for the general physician. Cureus 2018, 10, e3750. [Google Scholar] [CrossRef]
- Tohyama, S.; Hung, P.S.-P.; Cheng, J.C.; Zhang, J.Y.; Halawani, A.; Mikulis, D.J.; Oh, J.; Hodaie, M. Trigeminal neuralgia associated with a solitary pontine lesion: Clinical and neuroimaging definition of a new syndrome. Pain 2020, 161, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Naraghi, R.; Tanrikulu, L.; Hastreiter, P.; Fahlbusch, R.; Neundörfer, B.; Tröscher-Weber, R. Neurovascular relationship at the trigeminal root entry zone in persistent idiopathic facial pain: Findings from MRI 3D visualization. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1506–1509. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.R.; Jannetta, P.J.; Clyde, B.L.; Subach, B.R.; Comey, C.H.; Resnick, D.K. Microvascular decompression of cranial nerves: Lessons learned after 4400 operations. J. Neurosurg. 1999, 90, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Peker, S.; Kurtkaya, O.; Uzun, I.; Pamir, M.N. Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery 2006, 59, 354–359. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Moller, A.; Verlooy, J.; Cornelissen, M.; De Ridder, L. Is the root entry/exit zone important in microvascular compression syndromes? Neurosurgery 2002, 51, 427–434. [Google Scholar] [CrossRef]
- Tomii, M.; Onoue, H.; Yasue, M.; Tokudome, S.; Abe, T. Microscopic measurement of the facial nerve root exit zone from central glial myelin to peripheral schwann cell myelin. J. Neurosurg. 2003, 99, 121–124. [Google Scholar] [CrossRef]
- Sindou, M.; Howeidy, T.; Acevedo, G. Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia. Acta Neurochir. 2002, 144, 1–12; discussion 12–13. [Google Scholar] [CrossRef]
- Skinner, H.A. Some histologic features of the cranial nerves. Arch. Neurol. Psychiatry 1931, 25, 356–372. [Google Scholar] [CrossRef]
- Tarlov, I.M. Structure of the nerve root: Part 1—Nature of the junction between the central and the peripheral nervous system. Arch. Neurol. Psychiatry 1937, 37, 555–583. [Google Scholar] [CrossRef]
- Lang, J. Anatomy, length and blood vessel relations of “central” and “peripheral” paths of intracisternal cranial nerves. Zentralblatt Neurochir. 1982, 43, 217–258. (In German) [Google Scholar]
- Ziyal, I.M.; Sekhar, L.N.; Özgen, T.; Söylemezoğlu, F.; Alper, M.; Beşer, M. The trigeminal nerve and ganglion: An anatomical, histological, and radiological study addressing the transtrigeminal approach. Surg. Neurol. 2004, 61, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.B. Microvascular compression: An alternative view and hypothesis. J. Neurosurg. 1989, 70, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jannetta, P.J. Repeat operations in failed microvascular decompression for trigeminal neuralgia. Clinical study. Neurosurgery 1994, 35, 670. [Google Scholar]
- Toshiro, H.; Kondo, A.; Aoyama, I.; Nin, K.; Shimotake, K.; Nishioka, T.; Ikai, Y.; Takahashi, J. Trigeminal neuralgia caused by compressing from arteries transfixing the nerve. J. Neurosurg. 1991, 75, 783–786. [Google Scholar] [CrossRef]
- Sheth, S.; Branstetter, B.F., 4th; Escott, E.J. Appearance of normal cranial nerves on steady-state free precession MR images. RadioGraphics 2009, 29, 1045–1055. [Google Scholar] [CrossRef]
- De Marco, J.K.; Hesselink, J.R. Trigeminal neuropathy. Neuroimaging Clin. N. Am. 1993, 3, 105–128. [Google Scholar] [CrossRef]
- Donnet, A.; Moullin, G.; Tubiana, N.; Gras, R.; Robert, J.L. Lymphomatous meningitis: Neuroradiological appearances. Neuroradiology 1992, 34, 411–412. [Google Scholar] [CrossRef]
- Tien, R.D.; Dillon, W.P. Herpes trigeminal neuritis and rhombencephalitis on Gd-DTPA-enhanced MR imaging. AJNR 1990, 11, 413–414. [Google Scholar]
- Majoie, C.B.; Verbeeten, B., Jr.; Dol, J.A.; Peeters, F.L. Trigeminal neuropathy: Evaluation with MR imaging. RadioGraphics 1995, 15, 795–811. [Google Scholar] [CrossRef]
- Gonella, M.C.; Fischbein, N.J.; So, Y.T. Disorders of the trigeminal system. Semin. Neurol. 2009, 29, 36–44. [Google Scholar] [CrossRef]
- Williams, L.S.; Schmalfuss, I.M.; Sistrom, C.L.; Inoue, T.; Tanaka, R.; Seoane, E.R.; Mancuso, A.A. MR imaging of the trigeminal ganglion, nerve, and the perineural vascular plexus: Normal appearance and variants with correlation to cadaver specimens. AJNR Am. J. Neuroradiol. 2003, 24, 1317–1323. [Google Scholar] [PubMed]
- Borges, A.; Casselman, J. Imaging the trigeminal nerve. Eur. J. Radiol. 2010, 74, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Barakos, J.A.; D’Amour, P.A.; Dillon, W.P.; Newton, T.H. Trigeminal sensory neuropathy caused by cervical disc herniation. AJNR 1990, 11, 609. [Google Scholar] [PubMed]
- Saito, N.; Wamakawa, K.; Sasaki, T.; Saito, I.; Takakura, K. Intra-medullary cavernous angioma with trigeminal neuralgia: A case report and review of literature. Neurosurgery 1989, 95, 97–101. [Google Scholar]
- Walker, H.K. Chapter 61cranial nerve V: The trigeminal nerve. In Clinical Methods the History, Physical, and Laboratory Examinations, 3rd ed.; Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Gass, A.; Kitchen, N.; MacManus, D.G.; Moseley, I.F.; Hennerici, M.G.; Miller, D.H. Trigeminal neuralgia in patients with multiple sclerosis. Lesion localization with magnetic resonance imaging. Neurology 1997, 49, 1142–1144. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, C.; Lunskens, S.; Deryck, O.; Casselman, J.; Vanopdenbosch, L. MRI characteristics of trigeminal nerve involvement in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2013, 2, 200–203. [Google Scholar] [CrossRef]
- Ferroli, P.; Farina, L.; Franzini, A.; Milanese, C.; Broggi, G. Linear pontine and trigeminal root lesions and trigeminal neuralgia. Arch. Neurol. 2001, 58, 1311–1312. [Google Scholar] [CrossRef]
- Jenssen, T.S.; Rasmussen, P.; Reske-Nielsen, E. Association of trigeminal neuralgia with multiple sclerosis: Clinical and pathological features. Acta Neurol. Scand. 1982, 65, 182–189. [Google Scholar] [CrossRef]
- Pichiecchio, A.; Bergamaschi, R.; Tavazzin, E.; Romani, A.; Todeschini, A.; Bastianello, S. Bilateral trigeminal enhancement on magnetic resonance imaging in a patient with multiple sclerosis and trigeminal neuralgia. Mult. Scler. 2007, 13, 814–816. [Google Scholar] [CrossRef]
- da Silva, C.J.; da Rocha, A.J.; Mendes, M.F.; Maia, A.C., Jr.; Braga, F.T.; Tilbery, C.P. Trigeminal involvement in multiple sclerosis: Magnetic resonance imaging findings with clinical correlation in a series of patients. Mult. Scler. 2005, 11, 282–285. [Google Scholar] [CrossRef]
- Seixas, D.; Foley, P.; Palace, J.; Lima, D.; Ramos, I.; Tracey, I. Pain in multiple sclerosis: A systematic review of neuroimaging studies. NeuroImage Clin. 2014, 5, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Balasa, R.; Bajko, Z. Trigeminal neuralgia in multiple sclerosis patients: A clinical comparison of trigeminal neuralgia in patients with and without underlying multiple sclerosis. Rom. J. Neurol. 2010, 9, 68–73. [Google Scholar] [CrossRef]
- Eldridge, P.R.; Sinha, A.K.; Javadpour, M.; Littlechild, P.; Varma, T.R. Microvascular decompression for trigeminal neuralgia in patients with multiple sclerosis. Stereotact. Funct. Neurosurg. 2003, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.R.; Chang, J.; Dublin, A.B.; Vijayan, N. The association of brainstem lesions with migraine-like headache: An imaging study of multiple sclerosis. Headache 2005, 45, 670–677. [Google Scholar] [CrossRef]
- Meaney, J.F.; Watt, J.W.; Eldridge, P.R.; Whitehouse, G.H.; Wells, J.C.; Miles, J.B. Association between trigeminal neuralgia and multiple sclerosis: Role of magnetic resonance imaging. J. Neurol. Neurosurg. Psychiatry 1995, 59, 253–259. [Google Scholar] [CrossRef]
- Svendsen, K.B.; Sørensen, L.; Jensen, T.S.; Hansen, H.J.; Bach, F.W. MRI of the central nervous system in MS patients with and without pain. Eur. J. Pain 2011, 15, 395–401. [Google Scholar] [CrossRef]
- Tortorella, P.; Rocca, M.A.; Colombo, B.; Annovazzi, P.; Comi, G.; Filippi, M. Assessment of MRI abnormalities of the brainstem from patients with migraine and multiple sclerosis. J. Neurol. Sci. 2006, 244, 137–141. [Google Scholar] [CrossRef]
- Yetimalar, Y.; Seçil, Y.; Inceoglu, A.K.; Eren, S.; Başoğlu, M. Unusual primary manifestations of multiple sclerosis. N. Z. Med. J. 2008, 121, 47–59. [Google Scholar]
- Andrade, C.; Massano, J.; Guimarães, J.; Garrett, M.C. Stretching the limbs? Tonic spasms in multiple sclerosis. BMJ Case Rep. 2012, 2012, 3023208828. [Google Scholar] [CrossRef]
- Deppe, M.; Müller, D.; Kugel, H.; Ruck, T.; Wiendl, H.; Meuth, S.G. DTI detects water diffusion abnormalities in the thalamus that correlate with an extremity pain episode in a patient with multiple sclerosis. NeuroImage Clin. 2013, 2, 258–262. [Google Scholar] [CrossRef]
- Kister, I.; Caminero, A.B.; Monteith, T.S.; Soliman, A.; Bacon, T.E.; Bacon, J.H.; Kalina, J.T.; Inglese, M.; Herbert, J.; Lipton, R.B. Migraine is comorbid with multiple sclerosis and associated with a more symptomatic MS course. J. Headache Pain 2010, 11, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Broggi, G.; Ferroli, P.; Franzini, A.; Nazzi, V.; Farina, L.; La Mantia, L.; Milanese, C. Operative findings and outcomes ofmicrovascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery 2004, 55, 830–838. [Google Scholar] [CrossRef] [PubMed]
- González-Quintanilla, V.; Oterino, A.; Toriello, M.; de Pablos, C.; Wu, Y.; de Marco, E.; Pascual, J. Cluster-tic syndrome as the initial manifestation of multiple sclerosis. J. Headache Pain 2012, 13, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Leandri, M.; Cruccu, G.; Gottlieb, A. Cluster headache-like pain in multiple sclerosis. Cephalalgia Int. J. Headache 1999, 19, 732–734. [Google Scholar] [CrossRef]
- Cordella, R.; Franzini, A.; La Mantia, L.; Marras, C.; Erbetta, A.; Broggi, G. Hypothalamic stimulation for trigeminal neuralgia in multiple sclerosis patients: Efficacy on the paroxysmal ophthalmic pain. Mult. Scler. 2009, 15, 1322–1328. [Google Scholar] [CrossRef]
- Jing, J.; Zhang, Z.; Su, L.; Gao, C.; Guo, A.; Liu, X.; Wang, H.; Zhang, X.; Liu, Y.; Comi, G.; et al. Central vein sign and trigeminal lesions of multiple sclerosis visualised by 7T MRI. J. Neurol. Neurosurg. Psychiatry 2024, 95, 761–766. [Google Scholar] [CrossRef]
- Sati, P.; Oh, J.; Constable, R.T.; Evangelou, N.; Guttmann, C.R.; Henry, R.G.; Klawiter, E.C.; Mainero, C.; Massacesi, L.; McFarland, H.; et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: A consensus statement from the North American imaging in multiple sclerosis cooperative. Nat. Rev. Neurol. 2016, 12, 714–722. [Google Scholar] [CrossRef]
- Truini, A.; Prosperini, L.; Calistri, V.; Fiorelli, M.; Pozzilli, C.; Millefiorini, E.; Frontoni, M.; Cortese, A.; Caramia, F.; Cruccu, G. A dual concurrent mechanism explains trigeminal neuralgia in patients with multiple sclerosis. Neurology 2016, 86, 2094–2099. [Google Scholar] [CrossRef]
- Dalaqua, M.; do Nascimento, F.B.P.; Miura, L.K.; Reis, F.; Garcia, M.R.T.; Barbosa Júnior, A.A. Magnetic resonance imaging of the cranial nerves in congenital, traumatic, and vascular diseases: A pictorial essay. Radiol. Bras. 2021, 54, 185–192. [Google Scholar] [CrossRef]
- Herweh, C.; Kress, B.; Rasche, D.; Tronnier, V.; Troger, J.; Sartor, K.; Stippich, C. Loss of anisotropy in trigeminal neuralgia revealed by diffusion tensor imaging. Neurology 2007, 68, 776–778. [Google Scholar] [CrossRef]
- Leal, P.R.; Roch, J.A.; Hermier, M.; Souza, M.A.; Cristino-Filho, G.; Sindou, M. Structural abnormalities of the trigeminal root revealed by diffusion tensor imaging in patients with trigeminal neuralgia caused by neurovascular compression: A prospective, double-blind, controlled study. Pain 2011, 152, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Linn, J.; Mehrkens, J.H.; Thon, N.; Stahl, R.; Seelos, K.; Bruckmann, H.; Holtmannspotter, M. Trigeminal neuralgia due to neurovascular compression: High-spatial-resolution diffusion-tensor imaging reveals microstructural neural changes. Radiology 2011, 258, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.R.L.; Roch, J.; Hermier, M.; Berthezene, Y.; Sindou, M. Diffusion tensor imaging abnormalities of the trigeminal nerve root in patients with classical trigeminal neuralgia: A pre- and postoperative comparative study 4 years after microvascular decompression. Acta Neurochir. 2019, 161, 1415–1425. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef]
- Song, S.K.; Sun, S.W.; Ramsbottom, M.J.; Chang, C.; Russell, J.; Cross, A.H. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002, 17, 1429–1436. [Google Scholar] [CrossRef]
- Song, S.K.; Sun, S.W.; Ju, W.K.; Lin, S.J.; Cross, A.H.; Neufeld, A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003, 20, 1714–1722. [Google Scholar] [CrossRef]
- Song, S.K.; Yoshino, J.; Le, T.Q.; Lin, S.J.; Sun, S.W.; Cross, A.H.; Armstrong, R.C. Demyelination increases radial diffusivity in corpus callosum of mousebrain. Neuroimage 2005, 26, 132–140. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, B.; Qiu, W.; Kang, Z.; Shen, L.; Long, Y.; Huang, J.; Hu, X. Comparative brain stem lesions on MRI of acute disseminated encephalomyelitis, neuromyelitis optica, and multiple sclerosis. PLoS ONE 2011, 6, e22766. [Google Scholar] [CrossRef]
- Pittock, S.J.; Weinshenker, B.G.; Lucchinetti, C.F.; Wingerchuk, D.M.; Corboy, J.R.; Lennon, V.A. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch. Neurol. 2006, 63, 964–968. [Google Scholar] [CrossRef]
- Kremer, L.; Mealy, M.; Jacob, A.; Nakashima, I.; Cabre, P.; Bigi, S.; Paul, F.; Jarius, S.; Aktas, O.; Elsone, L.; et al. Brainstem manifestations in neuromyelitis optica: A multicenter study of 258 patients. Mult. Scler. J. 2014, 20, 843–847. [Google Scholar] [CrossRef]
- Takai, Y.; Misu, T.; Nakashima, I.; Takahashi, T.; Itoyama, Y.; Fujihara, K.; Aoki, M. Two cases of lumbosacralmyeloradiculitis with anti-aquaporin-4 antibody. Neurology 2012, 79, 1826–1828. [Google Scholar] [CrossRef] [PubMed]
- Laakso, S.M.; Hekali, O.; Kurdo, G.; Martola, J.; Sairanen, T.; Atula, S. Trigeminal neuralgia in multiple sclerosis: Prevalence and association with demyelination. Acta Neurol. Scand. 2020, 142, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Nagane, Y.; Utsugisawa, K.; Yonezawa, H.; Tohgi, H. A case with trigeminal herpes zoster manifesting a long lesion of the spinal trigeminal nucleus and tract on MR T2-weighted image. Rinsho Shinkeigaku 2001, 41, 56–59. [Google Scholar] [PubMed]
- Kidd, D.; Duncan, J.S.; Thompson, E.J. Pontine inflammatory lesion due to shingles. J. Neurol. Neurosurg. Psychiatry 1998, 65, 208. [Google Scholar] [CrossRef] [PubMed]
- Aribandi, M.; Aribandi, L. MRI of trigeminal zoster. Neurology 2005, 65, 1812. [Google Scholar] [CrossRef]
- Haanpää, M.; Dastidar, P.; Weinberg, A.; Levin, M.; Miettinen, A.; Lapinlampi, A.; Laippala, P.; Nurmikko, T. CSF and MRI findings in patients with acute herpes zoster. Neurology 1998, 51, 1405–1411. [Google Scholar] [CrossRef]
- Kost, R.G.; Straus, S.E. Postherpetic neuralgia—Pathogenesis, treatment, and prevention. N. Engl. J. Med. 1996, 335, 32–42. [Google Scholar] [CrossRef]
- Chakravorty, B.G. Association of trigeminal neuralgia with multiple sclerosis. Arch. Neurol. 1966, 14, 95–99. [Google Scholar] [CrossRef]
- Olafson, R.A.; Rushton, J.G.; Sayre, G.P. Trigeminal neuralgia in a patient with multiple sclerosis. J. Neurosurg. 1966, 24, 755–759. [Google Scholar] [CrossRef]
- Brisman, R. Trigeminal neuralgia and multiple sclerosis. Arch. Neurol. 1987, 44, 379–381. [Google Scholar] [CrossRef]
- Meaney, J.F.M.; Miles, J.B.; Nixon, T.E.; Whitehouse, G.H.; Ballentyne, E.S.; Eldridge, P.R. Vascular contact with the fifth cranial nerve at the pons in patients with trigeminal neuralgia: Detection with 3D FISP imaging. AJR Am. J. Roentgenol. 1994, 163, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Jannetta, P.J. Neurovascular compression in cranial nerve and systemic disease. Ann. Surg. 1980, 192, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Pollack, I.F.; Jannetta, P.J.; Bissonette, D.J. Bilateral trigeminal neuralgia: A 14-year experience with microvascular decompression. J. Neurosurg. 1988, 68, 559–565. [Google Scholar] [CrossRef]
- Fromm, G.H.; Terrence, C.F.; Maroon, J.C. Trigeminal neuralgia. Current concepts regarding etiology and pathogenesis. Arch. Neurol. 1984, 41, 1204–1207. [Google Scholar] [CrossRef]
- Kerr, F.W.L. Correlated light and electron microscopic observations on the normal trigeminal ganglion and sensory root in man. J. Neurosurg. 1967, 26, 168–172. [Google Scholar] [CrossRef]
- Harris, W. Rare forms of paroxysmal trigeminal neuralgia and their relation to disseminated sclerosis. BMJ 1950, 2, 1015–1019. [Google Scholar] [CrossRef]
- Itoyama, Y.; Sekizawa, T.; Openshaw, H.; Kogure, K.; Goto, I. Early loss of astrocytes in herpes simplex virus-induced central nervous system demyelination. Ann. Neurol. 1991, 29, 285–292. [Google Scholar] [CrossRef]
- Townsend, J.J.; Baringer, J.R. Central nervous system susceptibility to herpes simplex infection. J. Neuropathol. Exp. Neurol. 1978, 37, 255–262. [Google Scholar] [CrossRef]
- Kristensson, K.; Vahlne, A.; Persson, L.A.; Lycke, E. Neural spread of herpes simplex virus types 1 and 2 in mice after corneal or subcutaneous (footpad) inoculation. J. Neurol. Sci. 1978, 35, 331–340. [Google Scholar] [CrossRef]
- Sanders, V.J.; Felisan, S.; Waddell, A.; Tourtellotte, W.W. Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. J. Neurovirol. 1996, 2, 249–258. [Google Scholar] [CrossRef]
- Sanders, V.J.; Waddell, A.E.; Felisan, S.L.; Li, X.; Conrad, A.J.; Tourtellotte, W.W. Herpes simplex virus in postmortem multiple sclerosis brain tissue. Arch. Neurol. 1996, 53, 125–133. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedde, M.; Pascarella, R. Trigeminal Pontine Sign: From Imaging to Diseases Beyond Trigeminal Neuralgia. Diseases 2024, 12, 327. https://doi.org/10.3390/diseases12120327
Zedde M, Pascarella R. Trigeminal Pontine Sign: From Imaging to Diseases Beyond Trigeminal Neuralgia. Diseases. 2024; 12(12):327. https://doi.org/10.3390/diseases12120327
Chicago/Turabian StyleZedde, Marialuisa, and Rosario Pascarella. 2024. "Trigeminal Pontine Sign: From Imaging to Diseases Beyond Trigeminal Neuralgia" Diseases 12, no. 12: 327. https://doi.org/10.3390/diseases12120327
APA StyleZedde, M., & Pascarella, R. (2024). Trigeminal Pontine Sign: From Imaging to Diseases Beyond Trigeminal Neuralgia. Diseases, 12(12), 327. https://doi.org/10.3390/diseases12120327




