Maternal Supplementation with Lacticaseibacillus rhamnosus GG Improves Glucose Tolerance and Modulates the Intestinal Microbiota of Offspring
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling
2.2. Preparation of Bacterial Culture
2.3. Maternal Nutritional Programming Model
2.4. Body Weight, Food Intake, and Energy Efficiency
2.5. Oral Glucose Tolerance Test (OGTT)
2.6. Analysis of Biochemical Parameters
2.7. Tissue Weight and Body Fat
2.8. DNA Extraction and 16S rRNA Gene Amplification
2.9. Library Preparation and Sequencing
2.10. Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
3.1. Effects of L. rhamnosus GG on Food Consumption and Body Weight of the Offspring
3.2. The Effects of L. rhamnosus GG Supplementation on OGTT in the Offspring of the Experimental Groups
3.3. Supplementing L. rhamnosus GG During Maternal Programming Mitigates the Effects of the CAF Diet on the Lipid Profile and Weight of Visceral Fat Tissue and Modulates the Gut Microbiota in the Offspring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, V.X.; Patel, S.; Jones, H.F.; Dale, R.C. Maternal immune activation and neuroinflammation in human neurodevelopmental disorders. Nat. Rev. Neurol. 2021, 17, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Grote, V.; Kirchberg, F.F.; Prell, C.; Rzehak, P.; Uhl, O.; Weber, M. Long-Term Health Impact of Early Nutrition: The Power of Programming. Ann. Nutr. Metab. 2017, 70, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Perrone, S.; Santacroce, A.; Picardi, A.; Buonocore, G. Fetal programming and early identification of newborns at high risk of free radical-mediated diseases. World J. Clin. Pediatr. 2016, 5, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming? A lifetime health is under the control of in utero health. Obs. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef]
- Lewis, R.M.; Desoye, G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann. Nutr. Metab. 2017, 70, 228–231. [Google Scholar] [CrossRef]
- Li, Y. Epigenetic Mechanisms Link Maternal Diets and Gut Microbiome to Obesity in the Offspring. Front. Genet. 2018, 9, 342. [Google Scholar] [CrossRef]
- Amorín, R.; Liu, L.; Moriel, P.; DiLorenzo, N.; Lancaster, P.A.; Peñagaricano, F. Maternal diet induces persistent DNA methylation changes in the muscle of beef calves. Sci. Rep. 2023, 13, 1587. [Google Scholar] [CrossRef]
- Kannan, A.; Davila, J.; Gao, L.; Rattan, S.; Flaws, J.A.; Bagchi, M.K.; Bagchi, I.C. Maternal high-fat diet during pregnancy with concurrent phthalate exposure leads to abnormal placentation. Sci. Rep. 2021, 11, 16602. [Google Scholar] [CrossRef]
- Ceasrine, A.M.; Devlin, B.A.; Bolton, J.L.; Green, L.A.; Jo, Y.C.; Huynh, C.; Patrick, B.; Washington, K.; Sanchez, C.L.; Joo, F.; et al. Maternal diet disrupts the placenta–brain axis in a sex-specific manner. Nat. Metab. 2022, 4, 1732–1745. [Google Scholar] [CrossRef]
- Daliry, A.; Pereira, E.N.G.d.S. Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders. Nutrients 2021, 13, 3533. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Miyamoto, J.; Ohue-Kitano, R.; Watanabe, K.; Yamada, T.; Onuki, M.; Aoki, R.; Isobe, Y.; Kashihara, D.; Inoue, D.; et al. Maternal gut microbiota in pregnancy influences offspring metabolic phenotype in mice. Science 2020, 367, eaaw8429. [Google Scholar] [CrossRef] [PubMed]
- Sinha, T.; Brushett, S.; Prins, J.; Zhernakova, A. The maternal gut microbiome during pregnancy and its role in maternal and infant health. Curr. Opin. Microbiol. 2023, 74, 102309. [Google Scholar] [CrossRef] [PubMed]
- Di Gesù, C.M.; Matz, L.M.; Buffington, S.A. Diet-induced dysbiosis of the maternal gut microbiome in early life programming of neurodevelopmental disorders. Neurosci. Res. 2021, 168, 3–19. [Google Scholar] [CrossRef]
- Dawson, S.L.; O’Hely, M.; Jacka, F.N.; Ponsonby, A.L.; Symeonides, C.; Loughman, A.; Collier, F.; Moreno-Betancur, M.; Sly, P.; Burgner, D.; et al. Maternal prenatal gut microbiota composition predicts child behaviour. eBioMedicine 2021, 68, 103400. [Google Scholar] [CrossRef]
- Obuchowska, A.; Gorczyca, K.; Standyło, A.; Obuchowska, K.; Kimber-Trojnar, Ż.; Wierzchowska-Opoka, M.; Leszczyńska-Gorzelak, B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 8253. [Google Scholar] [CrossRef]
- Kim, B.; Park, K.Y.; Ji, Y.; Park, S.; Holzapfel, W.; Hyun, C.K. Protective effects of Lactobacillus rhamnosus GG against dyslipidemia in high-fat diet-induced obese mice. Biochem. Biophys. Res. Commun. 2016, 473, 530–536. [Google Scholar] [CrossRef]
- Cardenas-Perez, R.E.; Fuentes-Mera, L.; De La Garza, A.L.; Torre-Villalvazo, I.; Reyes-Castro, L.A.; Rodriguez-Rocha, H.; Garcia-Garcia, A.; Corona-Castillo, J.C.; Tovar, A.R.; Zambrano, E.; et al. Maternal overnutrition by hypercaloric diets programs hypothalamic mitochondrial fusion and metabolic dysfunction in rat male offspring. Nutr. Metab. 2018, 15, 38. [Google Scholar] [CrossRef]
- Li, N.; Russell, W.M.; Douglas-Escobar, M.; Hauser, N.; Lopez, M.; Neu, J. Live and Heat-Killed Lactobacillus rhamnosus GG: Effects on Proinflammatory and Anti-Inflammatory Cytokines/Chemokines in Gastrostomy-Fed Infant Rats. Pediatr. Res. 2009, 66, 203–207. [Google Scholar] [CrossRef]
- Milton-laskibar, I.; Marcos-zambrano, L.J.; Gómez-zorita, S.; Fernández-quintela, A.; de Santa Pau, E.C.; Martínez, J.A.; Portillo, M.P. Gut microbiota induced by pterostilbene and resveratrol in high-fat-high-fructose fed rats: Putative role in steatohepatitis onset. Nutrients 2021, 13, 1738. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhan, L.; Tang, W.; Wang, Q.; Dai, Z.; Zhou, L.; Feng, T.; Chen, M.; Wu, T.; Hu, E.; et al. MicrobiotaProcess: A comprehensive R package for deep mining microbiome. Innovation 2023, 4, 100388. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, 255. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Ginestet, C. Ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2009; p. 212. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 5 May 2023).
- Cetin, A.K.; Buyukdere, Y.; Gulec, A.; Akyol, A. Taurine supplementation reduces adiposity and hepatic lipid metabolic activity in adult offspring following maternal cafeteria diet. Nutr. Res. 2023, 1, 15–29. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Garza-Cuellar, M.A.; Silva-Hernandez, I.A.; Cardenas-Perez, R.E.; Reyes-Castro, L.A.; Zambrano, E.; Gonzalez-Hernandez, B.; Garza-Ocañas, L.; Fuentes-Mera, L.; Camacho, A. Maternal flavonoids intake reverts depression-like behaviour in rat female offspring. Nutrients 2019, 11, 572. [Google Scholar] [CrossRef]
- Camacho-Morales, A.; Caballero-Benitez, A.; Vázquez-Cruz, E.; Maldonado-Ruiz, R.; Cárdenas-Tueme, M.; Rojas-Martinez, A.; Caballero-Hernández, D. Maternal programming by high-energy diets primes ghrelin sensitivity in the offspring of rats exposed to chronic immobilization stress. Nutr. Res. 2022, 107, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Mori, J.; Nishida, N.; Miyagaki, S.; Kawabe, Y.; Ota, T.; Morimoto, H.; Tsuma, Y.; Fukuhara, S.; Ogata, T.; et al. High-fat diet during pregnancy lowers fetal weight and has a long-lasting adverse effect on brown adipose tissue in the offspring. J. Dev. Orig. Health Dis. 2023, 14, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Seki, Y.; Vuguin, P.M.; Quan Du, X.; Fiallo, A.; Glenn, A.S.; Singer, S.; Breuhahn, K.; Katz, E.B.; Charron, M.J. High-Fat Intake During Pregnancy and Lactation Exacerbates High-Fat Diet-Induced Complications in Male Offspring in Mice. Endocrinology 2013, 154, 3565–3576. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.E.; Abrams, B.; Barbour, L.A.; Catalano, P.; Christian, P.; Friedman, J.E.; Hay, W.W.; Hernandez, T.L.; Krebs, N.F.; Oken, E.; et al. The importance of nutrition in pregnancy and lactation: Lifelong consequences. Am. J. Obstet. Gynecol. 2022, 226, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Pullar, J.; Wickramasinghe, K.; Demaio, A.R.; Roberts, N.; Perez-Blanco1, K.M.; Noonan, K.; Townsend, N. The impact of maternal nutrition on offspring’s risk of non-communicable diseases in adulthood: A systematic review. J. Glob. Health 2019, 9, 020405. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Morita, H.; Toh, H.; Oshima, K.; Murakami, M.; Taylor, T.D.; Igimi, S.; Hattori, M. Complete Genome Sequence of the Probiotic Lactobacillus rhamnosus ATCC 53103. J. Bacteriol. 2009, 191, 7630–7631. [Google Scholar] [CrossRef]
- Segers, M.E.; Lebeer, S. Towards a better understanding of Lactobacillus rhamnosus GG—Host interactions. Microb. Cell Fact. 2014, 13, S7. [Google Scholar] [CrossRef]
- Arellano-García, L.; Trepiana, J.; Martínez, J.A.; Portillo, M.P.; Milton-Laskibar, I. Beneficial Effects of Viable and Heat-Inactivated Lactobacillus rhamnosus GG Administration on Oxidative Stress and Inflammation in Diet-Induced NAFLD in Rats. Antioxidants 2023, 12, 717. [Google Scholar] [CrossRef]
- Darby, T.M.; Naudin, C.R.; Luo, L.; Jones, R.M. Lactobacillus rhamnosus GG–induced Expression of Leptin in the Intestine Orchestrates Epithelial Cell Proliferation. Cell Mol. Gastroenterol. Hepatol. 2020, 9, 627–639. [Google Scholar] [CrossRef]
- Gastiazoro, M.P.; Rossetti, M.F.; Schumacher, R.; Stoker, C.; Durando, M.; Zierau, O.; Ramos, J.G.; Varayoud, J. Epigenetic disruption of placental genes by chronic maternal cafeteria diet in rats. J. Nutr. Biochem. 2022, 106, 109015. [Google Scholar] [CrossRef]
- Crew, R.C.; Waddell, B.J.; Mark, P.J. Maternal obesity induced by a ‘cafeteria’ diet in the rat does not increase inflammation in maternal, placental or fetal tissues in late gestation. Placenta 2016, 39, 33–40. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.A.; Ito, Y.; Waki, H.; Uchida, S. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Melo, N.C.; Cuevas-Sierra, A.; Arellano-Garcia, L.; Portillo, M.P.; Milton-Laskibar, I.; Alfredo Martinez, J. Oral administration of viable or heat-inactivated Lacticaseibacillus rhamnosus GG influences on metabolic outcomes and gut microbiota in rodents fed a high-fat high-fructose diet. J. Funct. Foods 2023, 109, 105808. [Google Scholar] [CrossRef]
- Arellano-García, L.; Macarulla, M.T.; Cuevas-Sierra, A.; Martínez, J.A.; Portillo, M.P.; Milton-Laskibar, I. Lactobacillus rhamnosus GG administration partially prevents diet-induced insulin resistance in rats: A comparison with its heat-inactivated parabiotic. Food Funct. 2023, 14, 8865–8875. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, K.Y.; Kim, B.; Kim, E.; Hyun, C.K. Lactobacillus rhamnosus GG improves insulin sensitivity and reduces adiposity in high-fat diet-fed mice through enhancement of adiponectin production. Biochem. Biophys. Res. Commun. 2013, 431, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Portela-Cidade, J.P.; Borges-Canha, M.; Leite-Moreira, A.F.; Pimentel-Nunes, P. Systematic Review of the Relation Between Intestinal Microbiota and Toll-Like Receptors in the Metabolic Syndrome: What Do We Know So Far? GE Port. J. Gastroenterol. 2015, 22, 240–258. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. eBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef]
- Politi, C.; Mobrici, M.; Parlongo, R.M.; Spoto, B.; Tripepi, G.; Pizzini, P.; Cutrupi, S.; Franco, D.; Tino, R.; Farruggio, G.; et al. Role of Gut Microbiota in Overweight Susceptibility in an Adult Population in Italy. Nutrients 2023, 15, 2834. [Google Scholar] [CrossRef] [PubMed]
- Ale, E.C.; Rojas, M.F.; Reinheimer, J.A.; Binetti, A.G. Lactobacillus fermentum: Could EPS production ability be responsible for functional properties? Food Microbiol. 2020, 90, 103465. [Google Scholar] [CrossRef]
- Mahalak, K.K.; Firrman, J.; Bobokalonov, J.; Narrowe, A.B.; Bittinger, K.; Daniel, S.; Tanes, C.; Mattei, L.M.; Zeng WBin Soares, J.W.; Kobori, M.; et al. Persistence of the Probiotic Lacticaseibacillus rhamnosus Strain GG (LGG) in an In Vitro Model of the Gut Microbiome. Int. J. Mol. Sci. 2022, 23, 12973. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.S.; Narrowe, A.B.; Firrman, J.A.; Mahalak, K.K.; Bobokalonov, J.T.; Lemons, J.M.S.; Bittinger, K.; Daniel, S.; Tanes, C.; Mattei, L.; et al. Lacticaseibacillus rhamnosus Strain GG (LGG) Regulate Gut Microbial Metabolites, an In Vitro Study Using Three Mature Human Gut Microbial Cultures in a Simulator of Human Intestinal Microbial Ecosystem (SHIME). Foods 2023, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2020, 14, 113–124. [Google Scholar] [CrossRef]
- Vijay, A.; Valdes, A.M. Role of the gut microbiome in chronic diseases: A narrative review. Eur. J. Clin. Nutr. 2021, 76, 489–501. [Google Scholar] [CrossRef]
- Han, X.; Ma, Y.; Ding, S.; Fang, J.; Liu, G. Regulation of dietary fiber on intestinal microorganisms and its effects on animal health. Anim. Nutr. 2023, 14, 356. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. The Profound Influence of Gut Microbiome and Extracellular Vesicles on Animal Health and Disease. Int. J. Mol. Sci. 2024, 25, 4024. [Google Scholar] [CrossRef]
- Zeebone, Y.Y.; Bóta, B.; Halas, V.; Libisch, B.; Olasz, F.; Papp, P.; Keresztény, T.; Gerőcs, A.; Ali, O.; Kovács, M.; et al. Gut-Faecal Microbial and Health-Marker Response to Dietary Fumonisins in Weaned Pigs. Toxins 2023, 15, 328. [Google Scholar] [CrossRef]

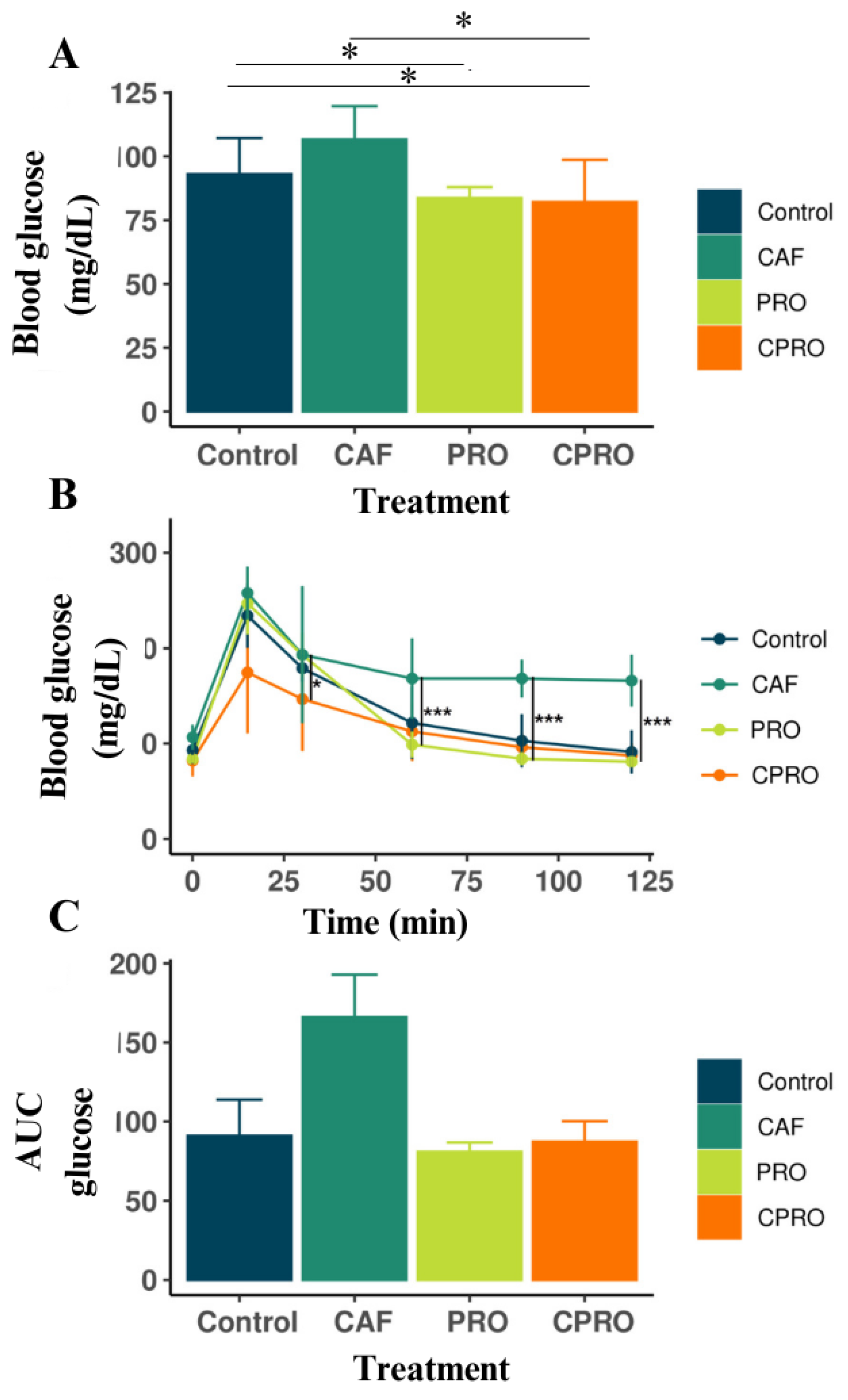
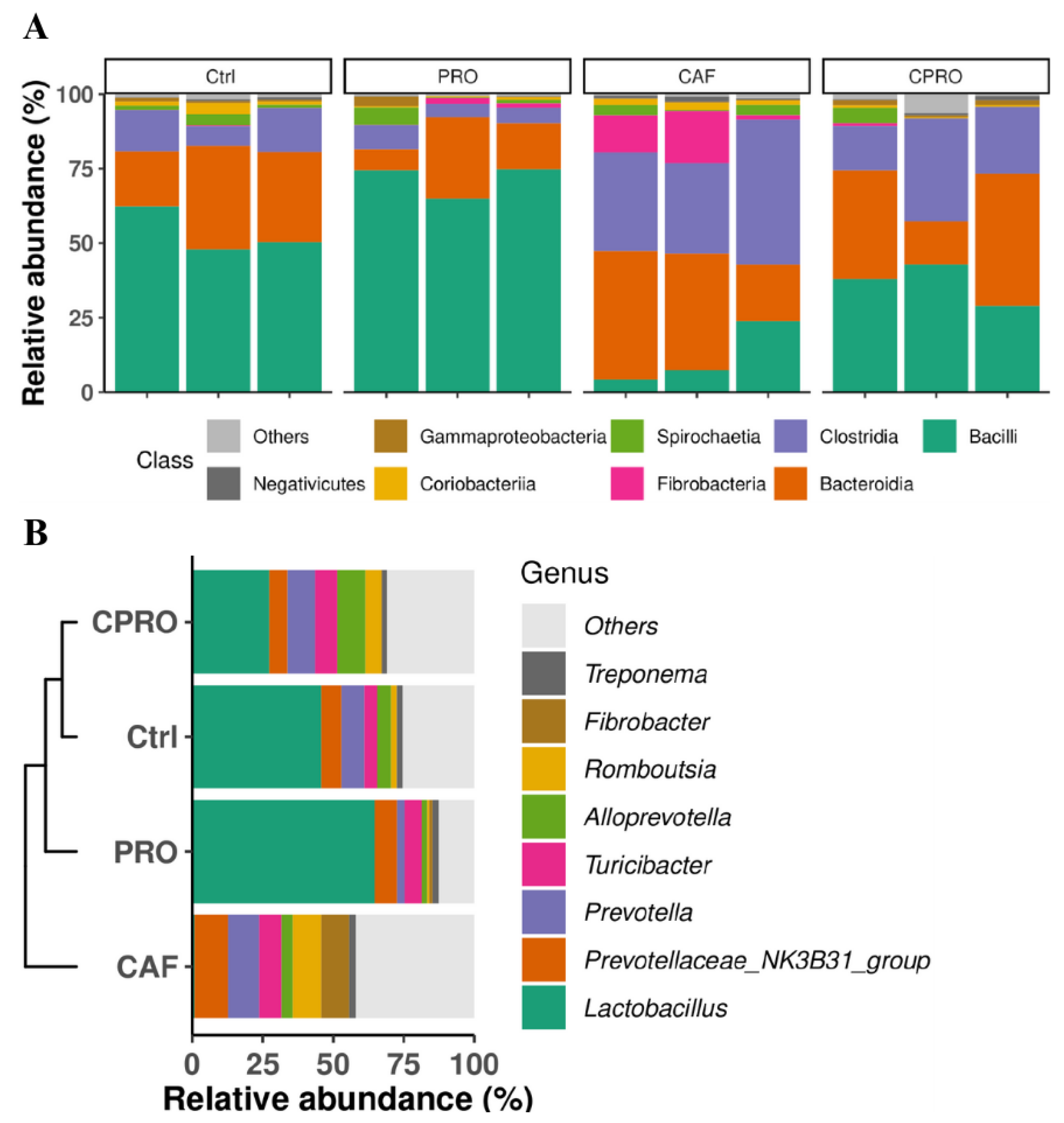


| Parameters | Control | CAF | PRO | CPRO | p-Value |
|---|---|---|---|---|---|
| Maternal (n = 4/group) | |||||
| Final body weight (g) | 279.3 ± 27 | 323.6 ± 32.7 | 283.4 ± 55.4 | 292.4 ± 24.3 | 0.61 |
| food intake (g/day) | 18.8 ± 6 | 21.3 ± 5.3 | 21.4 ± 6.6 | 25.6 ± 6.8 * | <0.01 |
| Total food intake (kcal) | 62.7 ± 19.8 | 79.2 ± 22.1 ** | 71.6 ± 20.7 | 93.6 ± 28.5 * | <0.001 |
| EEC (g/kcal) | 1.2 ± 0.8 | 0.9 ± 0.5 | 1.2 ± 0.8 | 0.6 ± 0.3 ** | <0.001 |
| Offspring (n = 8/group) | |||||
| Weight at birth | 7.2 ± 0.2 | 6.2 ± 0.1 * | 6.71 ± 0.2 | 6.81 ± 0.3 | <0.01 |
| Final body weight (g) | 203 ± 9.6 | 169 ± 21 ** | 205 ± 18.7 | 254 ± 8.0 | <0.001 |
| food intake (g/day) | 18.8 ± 6.5 | 16.8 ± 6.0 | 19.6 ± 6.2 | 11.1 ± 6.5 ** | <0.001 |
| Total food intake (kcal) | 63.3 ± 21.8 | 62.6 ± 22.5 | 65.6 ± 20.7 | 41.2 ± 20.6 ** | <0.001 |
| EEC (g/kcal) | 4.3 ± 1.4 | 3.5 ± 1.7 | 3.9 ± 1.2 | 9.7 ± 5.0 ** | <0.001 |
| Parameters | Control | CAF | PRO | CPRO | p-Value |
|---|---|---|---|---|---|
| Visceral fat weight (g) | 0.6 ± 0.2 | 3.4 ± 0.7 *** | 0.85 ± 0.6 | 0.7 ± 0.7 | <0.0001 |
| Liver weight (g) | 14.3 ± 0.9 | 14.6 ± 3.0 | 12.2 ± 1.3 | 13 ± 0.3 | 0.64 |
| Serum TC (mg/dL) | 55.9 ± 6.0 | 35.3 ± 13.2 | 35.8 ± 16.2 | 48.1 ± 10.4 | 0.81 |
| Serum TG (mg/dL) | 34.9 ± 14.0 | 138.4 ± 30.9 ** | 41.7 ± 3.9 | 71.9 ± 16.1 | <0.001 |
| HDL-c (mg/dL) | 45 ± 10.6 | 25.7 ± 15.0 | 35.7 ± 9.4 | 14.4 ± 6.1 ** | <0.001 |
| LDL-c (mg/dL) | 40.2 ± 19.9 | 132.2 ± 61.8 | 87.2 ± 20.0 | 10.3 ± 3.9 *** | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia Gomes, D.; Meza Alvarado, J.E.; Zamora Briseño, J.A.; Cano Sarmiento, C.; Camacho Morales, A.; Viveros Contreras, R. Maternal Supplementation with Lacticaseibacillus rhamnosus GG Improves Glucose Tolerance and Modulates the Intestinal Microbiota of Offspring. Diseases 2024, 12, 312. https://doi.org/10.3390/diseases12120312
Correia Gomes D, Meza Alvarado JE, Zamora Briseño JA, Cano Sarmiento C, Camacho Morales A, Viveros Contreras R. Maternal Supplementation with Lacticaseibacillus rhamnosus GG Improves Glucose Tolerance and Modulates the Intestinal Microbiota of Offspring. Diseases. 2024; 12(12):312. https://doi.org/10.3390/diseases12120312
Chicago/Turabian StyleCorreia Gomes, Dayane, José Enrique Meza Alvarado, Jesus Alejandro Zamora Briseño, Cynthia Cano Sarmiento, Alberto Camacho Morales, and Rubi Viveros Contreras. 2024. "Maternal Supplementation with Lacticaseibacillus rhamnosus GG Improves Glucose Tolerance and Modulates the Intestinal Microbiota of Offspring" Diseases 12, no. 12: 312. https://doi.org/10.3390/diseases12120312
APA StyleCorreia Gomes, D., Meza Alvarado, J. E., Zamora Briseño, J. A., Cano Sarmiento, C., Camacho Morales, A., & Viveros Contreras, R. (2024). Maternal Supplementation with Lacticaseibacillus rhamnosus GG Improves Glucose Tolerance and Modulates the Intestinal Microbiota of Offspring. Diseases, 12(12), 312. https://doi.org/10.3390/diseases12120312








