Abstract
Left atrial appendage occlusion affects systemic coagulation parameters, leading to additional patient-related benefits. The aim of this study was to investigate the differences in coagulation factor changes 6 months after epicardial left atrial appendage occlusion in patients with different LAA morphometries. This is the first study to analyze these relationships in detail. A prospective study of 22 consecutive patients was performed. Plasminogen, fibrinogen, tPA concentration, PAI-1, TAFI and computed tomography angiograms were performed. Patients were divided into subgroups based on left atrial appendage body and orifice diameter enlargement. The results of blood tests at baseline and six-month follow-up were compared. In a population with normal LAA body size and normal orifice diameter size, a significant decrease in analyzed clotting factors was observed between baseline and follow-up for all parameters except plasminogen. A significant decrease between baseline and follow-up was observed with enlarged LAA body size in all parameters except TAFI, in which it was insignificant and plasminogen, in which a significant increase was observed. Occlusion of the left atrial appendage is beneficial for systemic coagulation. Patients with a small LAA may benefit more from LAA closure in terms of stabilizing their coagulation factors associated with potential thromboembolic events in the future.
1. Introduction
1.1. Atrial Fibrillation Epidemiology, Complications and Treatment
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with an estimated lifetime risk of one in three patients [1]. It is estimated that approximately 15% of patients with AF are undiagnosed [2]. AF is associated with serious complications, including impaired quality of life, stroke and death [1,2]. To assess the risk of stroke in patients with AF, the CHA2DS2-VASc score is used to determine whether antithrombotic therapy should be initiated [3]. In addition, the HAS-BLED score should be evaluated to determine whether the patient is at an increased risk of bleeding [4]. Standard treatment for stroke prevention includes oral anticoagulation, with nonvitamin K antagonist oral anticoagulants being preferable to vitamin K antagonists [5]. As an alternative to lifelong anticoagulant therapy, left atrial appendage occlusion (LAAO) can be performed to eliminate the main source of thrombus in the human body—the left atrial appendage (LAA) [6]. In the past, several procoagulant factors were defined as being associated with thrombus formation in the left atrial appendage [7,8].
1.2. The Left Atrial Appendage Occlusion Procedures
Closure of the left atrial appendage can be performed in several ways, including percutaneous endocardial access (Watchman, Amplatzer) and epicardial access (Lariat [SentreHEART Inc., Redwood, CA, USA, now AtriCure Inc., USA], AtriClip [AtriCure Inc., Mason, OH, USA) [9,10,11]. It is worth noting that each method has device-specific limitations, such as the size of the LAA orifice or the anatomic shape of the LAA body, which may be associated with an increased risk of complications in certain cases [12,13].
1.3. The Left Atrial Appendage Systemic Impact
The nonthrombogenic role of LAA is still under investigation. There is still a lack of knowledge about the mechanisms associated with decreased, however, still present risk of thrombotic complications, including device-related thrombus, observed after this procedure [13,14,15,16]. To date, the neuroendocrine, hemodynamic, arrhythmogenic, and regenerative roles of LAA have been described, although the effects of LAAO on these features are not fully understood [17,18,19,20,21]. Recently, it was discovered that LAAO can influence not only stroke risk but also systemic coagulation parameters, leading to additional patient-related benefits [22]. However, it is not yet known whether patient-specific LAA anatomy can be used as a predictor of better postoperative systemic outcomes.
1.4. The Aim of the Study
In this study, we focused on well-established laboratory parameters that assess both blood coagulation and fibrinolysis. These markers not only reflect hypercoagulable states but are also involved in modulating various stages of hemostasis; hence, they may help assess not only the risk of thrombus formation but also in understanding the pathophysiology of blood coagulation and fibrinolytic disorders in patients undergoing the procedure of left atrial appendage closure.
The aim of this study was to investigate the differences in coagulation factor changes at 6 months in patients with epicardial left atrial appendage occlusion and different LAA morphometries. This is the first study to analyze these relationships in detail.
2. Materials and Methods
2.1. Characteristics of the Patients
A prospective study of 22 consecutive patients was performed. All eligible patients had electrocardiographically confirmed long-term AF and were free of LAA thrombi on transesophageal echocardiography. These patients were at high risk of stroke and bleeding from continued long-term systemic anticoagulation. The detailed patient characteristics can be found in Appendix A, Table A1.
2.2. Laboratory Tests
Laboratory investigations, blood sampling, laboratory tests, and calibrated automated thrombogram protocol were performed in accordance with a protocol published in our previous study [22]. Blood samples were collected from an antecubital vein in vacutainer tubes (containing 0.109 M sodium citrate and CTAD (buffered citrate, theophylline, adenosine and dipyridamole)). In all cases, before the procedure, anti-Xa activity (IU/mL) was measured. Tissue plasminogen activator (tPA) antigen, PAI-1 antigen, TAFI antigen and plasminogen activity were determined by ELISA (Hyphen BioMed, Neuville-Sur-Oise, France). Additionally, TAFI functional analysis was performed using retardation of clot lysis by TAFI antigen. The fibrinogen level was measured in terms of the fibrin polymerization function using the Clauss method. Plasminogen was reported as its percentage activity, while TAFI was measured as having a potency of 100%.
Patients were hospitalized for 1 or 2 days before the procedure. Patients receiving long-term vitamin K antagonists (VKAs) or direct oral anticoagulants (DOACs) were eligible if their anticoagulation was stable within the last 3 months. Prior to the procedure, bridging therapy with low-molecular-weight heparin was administered. VKA was discontinued before five days, and DOAC was discontinued at least two days before the LAAO procedure. In patients receiving aspirin or no oral anticoagulants, low-molecular-weight heparin was started at least 2 days before the procedure. The last dose of low-molecular-weight heparin was administered > 12 h before the procedure.
2.3. Left Atrial Appendage Occlusion Procedure
All LAAO were performed with a Lariat device (SentreHEART Inc., Redwood, CA, USA, now AtriCure Inc., USA). Postoperative transesophageal echocardiography was performed to ensure no postprocedural leakage. This method was described in detail in our previous study [23]. On the first postoperative day, all patients were continued on low-molecular-weight heparin until hospital discharge. All patients were discharged with aspirin monotherapy (150 mg/day).
2.4. Image Processing and Analysis
Contrast-enhanced electrocardiogram-guided computed tomography angiograms from 22 patients aged 66.2 ± 8.7 years, including 59.1% women, who were eligible for LAAO, were analyzed. The patients with heart rates greater than 70 bpm were administered 10 or 40 mg of propranolol or 40 mg of verapamil before the procedure on the recommendation of the physician. Imaging parameters for the CT were: 350–400 mA effective tube current and 100–120 kV tube voltage. The collimation was 2 × 32 × 0.6 mm, and the temporal resolution was 165 ms. During the imaging procedure, an iodine-containing contrast agent (Visipaque, GE Healthcare, Chicago, IL, USA) was injected at a dose of 1.0 mL/kg at a rate of 5 mL/s. For the test bolus, the acquisition delay was the time of maximal density of the ascending aorta with an additional 6 s. Images were reconstructed using the B26f and B46f kernels and a 512 × 512-pixel image matrix. The 70% phase of the multiphase reconstruction (10 to 100%) was assessed as the left ventricular end-diastolic (ED) phase and further investigated. The left atrium and LAA in the end-diastolic phase were segmented and processed semiautomatically using Mimics Innovation Suite 23.0 visualization and three-dimensional reconstruction software (Materialise, Plymouth, MI, USA). The LAA type was evaluated. The area of the LAA ostium, transverse length, and longitudinal length were measured. The angle of the LAA body was measured. The width and length of the LAA body were measured, and the LAA body index was calculated by multiplying the width and length (see Figure 1).
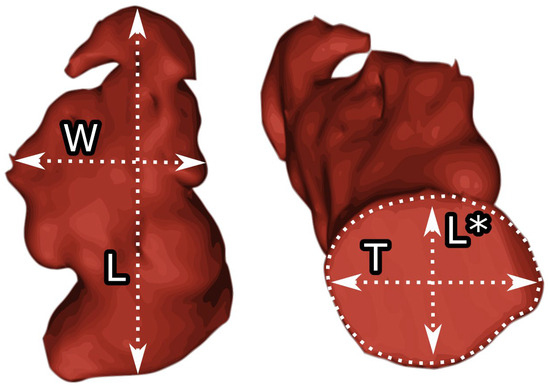
Figure 1.
Measurements of the left atrial appendage. W—body width, L—body length, T—the left atrial appendage orifice transverse diameter, L*—the left atrial appendage orifice longitudinal diameter.
2.5. Statistical Analysis
Patients were divided into two subgroups: Patients with an LAA body index above average (cutoff defined as 1000 mm2) were included in the enlarged LAA body group, and patients who did not meet this requirement were included in the small LAA body group. In addition, patients with a larger than average LAA orifice area (cutoff defined as 23.6 mm in longitudinal orifice dimension), as proposed in previous research, were classified as enlarged LAA orifice patients, whereas patients with a smaller than average LAA orifice area were included in the small LAA orifice group [24]. Collected data were tested for normality using the Shapiro–Wilk test, tabulated and reported as means with standard deviations [mean ± SD]. The U-Mann–Whitney test was used to compare LAA morphometric characteristics and coagulation factor levels in blood samples between subgroups in the previously described populations. The Wilcoxon test was used to analyze the differences between the results of the blood samples at baseline and after 6 months in each subgroup separately. Results were considered statistically significant when the p-value was less than 0.05.
3. Results
Detailed general characteristics of the LAA are provided in Appendix A, Table A2.
3.1. LAA Body Size Coagulation Impact
A significant decrease in analyzed coagulation factors between baseline and follow-up was observed in normal LAA body size for all parameters except plasminogen, for which a statistically nonsignificant decrease was observed. A significant decrease between baseline and follow-up was observed in enlarged LAA body size for all parameters except TAFI, where an insignificant decrease was observed, and plasminogen, where a statistically significant increase was observed between baseline and follow-up (see Figure 2). A detailed comparison of subgroups can be found in Table 1, and a detailed comparison of baseline and follow-up results can be found in Table 2.
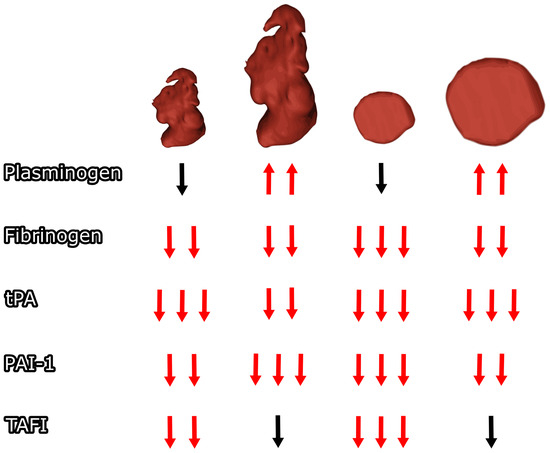
Figure 2.
The left atrial appendage orifice and body size impact on postoperative coagulation changes. Arrows for statistically significant (p < 0.05) different results compared to baseline are marked red. Different numbers of arrows represent significant differences between subgroups in follow-up. tPA—tissue plasminogen activator antigen, PAI-1—plasminogen activator inhibitor type 1 antigen, TAFI—thrombin activatable fibrinolysis inhibitor.

Table 1.
The left atrial appendage of normal and enlarged body subgroups detailed comparison. LAA—left atrial appendage, tPA—tissue plasminogen activator antigen, PAI-1—plasminogen activator inhibitor type 1 antigen, TAFI—thrombin activatable fibrinolysis inhibitor.

Table 2.
Baseline versus 6-month follow-up left atrial appendage normal and enlarged body subgroups detailed comparison. LAA—left atrial appendage, tPA—tissue plasminogen activator antigen, PAI-1—plasminogen activator inhibitor type 1 antigen, TAFI—thrombin activatable fibrinolysis inhibitor.
3.2. Effect of LAA Orifice Size on Coagulation
A significant decrease in the analyzed coagulation factors between baseline and follow-up was observed at normal LAA orifice size for all parameters except plasminogen, for which a statistically nonsignificant decrease was observed. A significant decrease between baseline and follow-up was observed with enlarged LAA openings in all parameters except TAFI, in which the observed change was not statistically significant, and plasminogen, in which a statistically significant increase was observed between baseline and follow-up (see Figure 2). A detailed comparison of baseline and follow-up results can be found in Table 3, and a detailed comparison of subgroups can be found in Table 4.

Table 3.
The left atrial appendage of normal and enlarged orifice subgroups detailed comparison. LAA—left atrial appendage, tPA—tissue plasminogen activator antigen, PAI-1—plasminogen activator inhibitor type 1 antigen, TAFI—thrombin activatable fibrinolysis inhibitor.

Table 4.
Baseline versus 6-month follow-up left atrial appendage of normal and enlarged orifice subgroups detailed comparison. LAA—Left atrial appendage, tPA—tissue plasminogen activator antigen, PAI-1—plasminogen activator inhibitor type 1 antigen, TAFI—thrombin activatable fibrinolysis inhibitor.
4. Discussion
4.1. LAA Subtypes Impact on Postoperative Coagulation Changes
It is widely known that there is a relationship between the structure of the LA, disorders of the blood coagulation process and an increased risk of prothrombotic complications [7,14]. Our study fills the gap in understanding the relationship between hemostasis and LAA structure, including laboratory parameters assessing both blood coagulation and fibrinolysis. Our study provides detailed information about the influence of preoperative LAA anatomy on the changes in coagulation factors. It is the first study to discuss this topic in detail, connecting the molecular findings with anatomical parameters of cardiac structures. In the described population, it was observed that baseline plasminogen levels were higher in LAAs with small bodies than in LAAs with larger lobes. However, this was not demonstrated to be statistically significant. In contrast, there were significant differences between patients with large and small LAA orifices. Nevertheless, in both cases, mean plasminogen levels were stabilized at standard values at the 6-month coagulation factors analysis follow-up. The significant reduction in coagulation factor reduction differences in TAFI observed between the analyzed subgroups argues for potentially greater systemic coagulation changes favoring fibrinolysis in patients with smaller lobe dimensions and smaller orifices, which may play an additional role in preventing and reducing thromboembolic complications in patients in the future. There was no significant difference in the change in TAFI levels between baseline and follow-up in both large-lobe and large-orifice subgroups. This finding may be important in determining the potential negative systemic impact on fibrinolysis of the left atrial appendage and the closure of small left atrial appendages.
The intriguing relationship we observed was the lower levels of plasminogen in enlarged LAA orifices, which requires the study of this parameter in the context of the entire fibrinolytic system, including tissue plasminogen activator (tPA). This is extremely methodologically difficult due to the instability of this laboratory parameter in plasma. We cannot say conclusively that lower plasminogen levels in these patients increase the risk of thrombotic complications, but low plasminogen levels are associated with the severity of some diseases [25,26]. Low plasminogen levels may be associated with higher plasma PAI-1 levels, which are a well-established marker for major adverse cardiovascular events [27].
4.2. Does Left Atrial Appendage Type Matter?
This may suggest that orifice size, rather than LAA shape, may increase the risk of thromboembolism. Hypothetically, patients with a narrower LAA opening might experience increased turbulent blood flow and impaired blood outflow from the LAA, increasing the risk of thromboembolism. It may turn out that the size of the LAA appendage, rather than its shape, is crucial for the risk of thrombosis. However, this theory requires further extensive studies with a larger number of patients. It has already been demonstrated that AF promotes LAA remodeling, including dilation of the LAA orifice [28]. In this case, it could be an additional factor leading to thrombus formation within the LAA. The observed normalization of coagulation parameters over a 6-month period proves that the possible adverse effects can be reversed.
4.3. Molecular Findings Patophysiological Association
Our results are related to the two main causes of Virchow’s triad—stasis of blood flow and hypercoagulability in the remodeled, dilated LAA. The changes in fluid dynamics and geometry of the LAA, previously studied in detail, prove that the enlarged LAA further restricts blood flow through its lumen, which, combined with hypercoagulability in such patients, poses a major risk for thrombus formation, leading to catastrophic complications, including cryptogenic stroke and death. These results demonstrate the critical role of LAA occlusion procedures in the long-term prevention of thrombus formation in patients with AF, especially when DOAC or VKA cannot be administered because of the patient’s increased risk of bleeding.
The mechanism of clot formation after the LARIAT procedure is not fully understood, but considering the risk of this phenomenon being difficult to predict, it seems extremely important to look for the role of these phenomena in patient care [23]. In assessing such a risk, an in-depth analysis of the blood coagulation process seems important to more efficiently and quickly predict the risk of thrombosis after this type of surgery. The concept of assessing the viscoelastometric properties of the clot seems interesting here. Although these laboratory methods are largely associated with their usefulness during cardiac surgery, the evaluation of the clot elasticity of whole blood samples after the operation, e.g., using thromboelastometry, may bring surprising results in the evaluation of the post-LARIAT procedure.
4.4. Coagulation and Fibrynolitic Factors
Plasminogen is a plasma protein that can be activated by proteolysis in its active form—plasmin, which plays a crucial role in fibrinolysis. Its activity reference is 75% to 135%. It is worth noting that, in addition to its key fibrinolytic function, plasminogen also plays a crucial role in the behavior of cells in immune and inflammatory processes, including reducing the risk of infection and providing antiviral protection [29]. Fibrinogen plays a crucial role in the coagulation process, as it is the source of fibrin. Its reference range is 2–4 g/L. Its post-translational modifications have been shown to be associated with abnormal clot formation and thrombosis and can be found in patients with increased cardiovascular risk [25]. A thrombin-activatable fibrinolysis inhibitor is a plasma protein that plays an important role in protecting fibrin clots from degradation by removing C-terminal lysines from fibrin. It is critical for maintaining the balance between fibrinolysis and coagulation. It can be activated by several plasma proteins, including thrombin, in complex with thrombomodulin and plasmin. Elevated levels are associated with a hypofibrinolytic state, which increases the risk of thrombotic and cardiovascular disease [30]. Tissue plasminogen activator is a serine protease that converts plasminogen to plasmin, which is critical for blood clot dissolution. Its biological function is used in daily practice for the treatment of ischemic diseases such as stroke, pulmonary embolism, deep vein thrombolysis and in isolated cases of myocardial infarction [31]. Plasminogen activator inhibitor-1 plays a crucial role in the regulation and inhibition of tissue- and urinary-type plasminogen activators and blocks fibrinolysis at the stage of conversion of plasminogen to plasmin. It is produced in various cells and stored in platelets. Its increased expression in vivo can lead to pathological fibrin deposition and tissue damage. PAI-1 activity is increased by mental and psychological stress. Moreover, its increased activation has been found to be related to metabolic factors and the aging process [32].
4.5. Enlarged Left Atrial Appendage—How to Determine?
To define the enlarged LAA, one would first acknowledge its dimensions in a healthy population. In a healthy population analyzed by Veinot and colleagues, the mean longitudinal dimension of the LAA orifice was 1.16 ± 0.35 cm in males, with slightly lower values (was 1.07 ± 0.32 cm) in females. The length and width were 2.59 ± 0.66 cm and 1.83 ± 0.73 cm, respectively, with similar, slightly lower values in females [33]. The left atrial appendage can be divided into two main parts: body and neck. It was recently proven that the left atrial appendage neck, crucial in terms of blood flow and LAAO procedures, does not differ between the LAA morphological types [34].
The LAA enlargement was historically connected with rheumatic heart disease and evaluated subjectively [35,36]. Nowadays, there are still no clear criteria for defining the LAA enlargement. However, the study performed by Ren and colleagues proposed the first cutoff values for the left atrial appendage orifice diameter at the level of 23.6 mm [24]. In this study, we additionally propose the cutoff point of LAA body index at the level of 1000 mm2. There is still a great need for the evaluation and definition of cutoff values for each LAA parameter for a clear definition of its enlargement.
4.6. Clinical Impact of the Left Atrial Appendage Anatomy
LAA anatomy plays an important role in patient evaluation and qualification for specific LAAO procedures. Several LAA classifications have been proposed for easy evaluation, but we have not found significant differences between specific LAA types and clotting factor changes in follow-up [28,37]. LAA anatomy changes in patients with AF [28]. It was found that LAA orifice dimensions and lobe size differ between patients with AF and healthy individuals, with significantly larger values of the LAA orifice and significantly lower values of LAA ejection fraction observed in patients with AF, but there are limited data on the relationship between LA and LAA enlargement [28]. No data are available on how AF affects LAA size and dimensions in patients suffering from AF. To facilitate the implementation of the results in clinical practice, it was suggested that the LAA body index or orifice area should be determined, and patients divided into two subgroups. These measurements can be performed daily during the preoperative assessment of patients. As presented in our study, such data may provide additional information about potential postoperative coagulation factors in patients.
4.7. Endocardial Left Atrial Appendage Closure Coagulation Impact
Previously, several studies were conducted to determine endocardial LAAO and the impact of anticoagulation treatment on coagulation changes [38,39,40]. A study performed by Sherwood and colleagues aimed to determine the clinical characteristics of patients with one of the most commonly observed endocardial LAAO complications: device-related thrombus and postoperative bleeding [38]. It was proven that the prothrombogenic profile of the patients at baseline was associated with device-related thrombosis when low platelet reactivity was observed preoperatively in the bleeding group [38]. Asmarats and colleagues discovered that preoperative LAA thrombosis was observed only in patients with antiplatelet therapy and was associated with increased activation of coagulation factors postoperatively. In patients with oral anticoagulation treatment, such complications were not observed [39]. Additionally, Duthoit and colleagues compared rivaroxaban and dual antiplatelet therapy in patients undergoing endocardial LAAO, which proved that postoperative thrombin generation was lower in patients treated with lowered oral anticoagulation treatment doses, which proves it as an alternative to double antiplatelet therapy [40].
4.8. Limitations of the Study
This was a single-center prospective study performed only on patients of Caucasian origin. In addition, only 22 patients in whom epicardial LAAO was performed with the Lariat system were analyzed because data from other systems were not currently available. The blood analysis was performed before the procedure and at the 6-month follow-up. Additional analysis should be performed to establish long-term differences in patients’ coagulation factor changes after LAAO. One limitation of our study is the noticeable, although small, variability among patients in terms of drug treatment. It would be ideal to select a cohort without anticoagulant therapy, but in clinical practice, this is unlikely. However, it seems that the greatest impact on the tested coagulation and fibrinolysis parameters would be caused by vitamin K antagonists, which were used in only three patients in our current cohort. The proposed LAA body index should be clinically standardized and evaluated on a broad population of patients for standard value development. For the proposed hypothesis, an additional prospective study with a large number of patients divided into groups based on their LAA types and different LAA body and orifice sizes should be performed. This study was performed on patients with contraindications for oral anticoagulation, and its results should be interpreted in terms of such a population.
5. Conclusions
Closure of the left atrial appendage is beneficial with respect to systemic coagulation. Patients with a small LAA may benefit more from LAA closure in terms of stabilization of their coagulation factors associated with potential thromboembolic events in the future.
Author Contributions
Conceptualization, J.B., J.R., A.S., M.B. (Marian Burysz), R.L., M.B. (Magdalena Bartuś), D.R.L., R.J.L., J.N., M.Z., B.K. and K.B.; Data curation, J.B. and J.R.; Formal analysis, J.B. and J.R.; Funding acquisition, A.S. and K.B.; Investigation, J.B. and J.R.; Methodology, J.B., J.R., A.S., M.B. (Marian Burysz), R.L., M.B. (Magdalena Bartuś), R.J.L., J.N., M.Z., B.K. and K.B.; Project administration, R.L. and K.B.; Resources, R.L.; Supervision, M.B. (Marian Burysz) and K.B.; Validation, R.L. and D.R.L.; Writing—original draft, J.B. and J.R.; Writing—review and editing, A.S., M.B. (Marian Burysz), R.L., M.B. (Magdalena Bartuś), D.R.L., R.J.L., J.N., M.Z., B.K. and K.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish National Science Center, grant number UMO-2019/33/B/NZ5/02395. The APC was funded by Artur Słomka.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Jagiellonian University Ethical Committee (No. KBET/282/B/2012).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data from the study are available upon reasonable request from the corresponding author.
Conflicts of Interest
Bartuś K and his institutions, Jagiellonian University Medical College and John Paul II Hospital in Krakow, Poland, were recipients of the above research grant from the Polish National Science Centre. Other authors declare no conflicts of interest concerning this study. R.J. Lee is a consultant and equity holder in SentreHEART, Inc. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Patients’ baseline demographic and clinical characteristics. BMI—body mass index, OAC—oral anticoagulant, SD—standard deviation, TIA—transient ischemic attack.
Table A1.
Patients’ baseline demographic and clinical characteristics. BMI—body mass index, OAC—oral anticoagulant, SD—standard deviation, TIA—transient ischemic attack.
| Variable | Patients (n = 22) |
|---|---|
| Age, years (Mean ± SD) | 66.2 ± 8.7 |
| Female | 59.1% (n = 13) |
| BMI (Mean ± SD) [kg/m2] | 26.4 ± 2.4 |
| CHADS2 score (Mean ± SD) | 2.8 ± 1.0 |
| CHA2DS2-VASc score (Mean ± SD) | 3.9 ± 1.5 |
| HAS-BLED score (Mean ± SD) | 3.6 ± 1.2 |
| Congestive heart failure | 13.6% (n = 3) |
| Hypertension | 86.4% (n = 19) |
| Hyperlipidemia | 54.5% (n = 12) |
| Diabetes mellitus type 2 | 31.8% (n = 7) |
| Previous ischemic stroke/TIA | 68.2% (n = 15) |
| Hemorrhagic stroke | 59.1% (n = 13) |
| Myocardial infarction | 9.1% (n = 2) |
| Vascular disease | 13.6% (n = 3) |
| Alcoholism | 4.5% (n = 1) |
| Preprocedural OAC | |
| 13.6% (n = 3) 50.0% (n = 11) 13.6% (n = 3) 22.7% (n = 5) |

Table A2.
Anatomical characteristics of the left atrial appendage. LA—left atrium, LAA—left atrial appendage.
Table A2.
Anatomical characteristics of the left atrial appendage. LA—left atrium, LAA—left atrial appendage.
| n (%)/Mean ± SD | ||
|---|---|---|
| LAA type | chicken wing | 9 (40.9%) |
| cauliflower | 2 (9.1%) | |
| arrowhead | 11 (50%) | |
| LAA additional lobes | 0 | 11 (50%) |
| 1 | 9 (40.9%) | |
| 2 | 2 (9.1%) | |
| Orientation of main lobe | superior anterolateral | 13 (59.1%) |
| superior posterolateral | 5 (22.7%) | |
| superior lateral | 4 (18.2%) | |
| LAA width [mm] | 21.6 ± 7.2 | |
| LAA length [mm] | 47.1 ± 10.1 | |
| LAA body index [mm2] | 1025.8 ± 462.0 | |
| Ostium transverse dimension [mm] | 28.1 ± 6.2 | |
| Ostium longitudinal dimension [mm] | 23 ± 5.9 | |
| LAA ostium area [mm2] | 532.5 ± 243.4 | |
| LAA origin angle to LA [º] | 70.0 ± 19.0 | |
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts. Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J.G.M. Refining Clinical Risk Stratification for Predicting Stroke and Thromboembolism in Atrial Fibrillation Using a Novel Risk Factor-Based Approach. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.G.M.; Lip, G.Y.H. A Novel User-Friendly Score (HAS-BLED) To Assess 1-Year Risk of Major Bleeding in Patients with Atrial Fibrillation. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. EP Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Çınar, T.; Hayıroğlu, M.İ.; Selçuk, M.; Çiçek, V.; Doğan, S.; Kılıç, Ş.; Yavuz, S.; Babaoğlu, M.; Uzun, M.; Orhan, A.L. Association of Whole Blood Viscosity with Thrombus Presence in Patients Undergoing Transoesophageal Echocardiography. Int. J. Cardiovasc. Imaging 2022, 38, 601–607. [Google Scholar] [CrossRef]
- Cinar, T.; Hayiroğlu, M.İ.; Çiçek, V.; Asal, S.; Atmaca, M.M.; Keser, N.; Orhan, A.L. Predictors of Left Atrial Thrombus in Acute Ischemic Stroke Patients without Atrial Fibrillation: A Single-Center Cross-Sectional Study. Rev. Assoc. Med. Bras. 2020, 66, 1437–1443. [Google Scholar] [CrossRef]
- Toale, C.; Fitzmaurice, G.J.; Eaton, D.; Lyne, J.; Redmond, K.C. Outcomes of Left Atrial Appendage Occlusion Using the AtriClip Device: A Systematic Review. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 655–662. [Google Scholar] [CrossRef]
- Burysz, M.; Litwinowicz, R.; Bryndza, M.; Skowronek, R.; Ogorzeja, W.; Bartus, K. Percutaneous Left Atrial Appendage Closure Using the LAmbre Device. First Clinical Results in Poland. Adv. Intervent. Cardiol. 2019, 15, 251–254. [Google Scholar] [CrossRef]
- Litwinowicz, R.; Burysz, M.; Mazur, P.; Kapelak, B.; Bartus, M.; Lakkireddy, D.; Lee, R.J.; Malec-Litwinowicz, M.; Bartus, K. Endocardial versus Epicardial Left Atrial Appendage Exclusion for Stroke Prevention in Patients with Atrial Fibrillation: Midterm Follow-up. J. Cardiovasc. Electrophysiol. 2021, 32, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Batko, J.; Rams, D.; Filip, G.; Bartoszcze, A.; Kapelak, B.; Bartuś, K.; Litwinowicz, R. Left Atrial Appendage Morphology and Course of the Circumflex Artery: Anatomical Implications for Left Atrial Appendage Occlusion Procedures. Innovat. Technol. Techniq. Cardiothorac. Vasc. Surg. 2022, 17, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Fauchier, L.; Cinaud, A.; Brigadeau, F.; Lepillier, A.; Pierre, B.; Abbey, S.; Fatemi, M.; Franceschi, F.; Guedeney, P.; Jacon, P.; et al. Device-Related Thrombosis After Percutaneous Left Atrial Appendage Occlusion for Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Simard, T.; Jung, R.G.; Lehenbauer, K.; Piayda, K.; Pracoń, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; Chun, J.K.R.; et al. Predictors of Device-Related Thrombus Following Percutaneous Left Atrial Appendage Occlusion. J. Am. Coll. Cardiol. 2021, 78, 297–313. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Ganta, N.; Filice, G.; Richard, I.; Acquah, F.; Alnabwani, D.; Patel, H.B. Embolic Cerebrovascular Accident Secondary to Device-Related Thrombus Post WATCHMAN Device Implantation. Cureus 2022, 14, e26892. [Google Scholar] [CrossRef] [PubMed]
- Pracoń, R.; Zieliński, K.; Bangalore, S.; Konka, M.; Kruk, M.; Kępka, C.; Trochimiuk, P.; Dębski, M.; Przyłuski, J.; Kaczmarska, E.; et al. Residual Stroke Risk after Left Atrial Appendage Closure in Patients with Prior Oral Anticoagulation Failure. Int. J. Cardiol. 2022, 354, 17–21. [Google Scholar] [CrossRef]
- Bartus, K.; Elbey, M.A.; Kanuri, S.H.; Lee, R.; Litwinowicz, R.; Natorska, J.; Zabczyk, M.; Bartus, M.; Kapelak, B.; Malecki, M.T.; et al. Metabolic Effects of the Left Atrial Appendage Exclusion (the Heart Hormone Study). J. Cardiovasc. Electrophysiol. 2022, 33, 2064–2071. [Google Scholar] [CrossRef]
- Dar, T.; Afzal, M.R.; Yarlagadda, B.; Kutty, S.; Shang, Q.; Gunda, S.; Samanta, A.; Thummaluru, J.; Arukala, K.S.; Kanmanthareddy, A.; et al. Mechanical Function of the Left Atrium Is Improved with Epicardial Ligation of the Left Atrial Appendage: Insights from the LAFIT-LARIAT Registry. Heart Rhythm. 2018, 15, 955–959. [Google Scholar] [CrossRef]
- Fanton, Y.; Robic, B.; Rummens, J.-L.; Daniëls, A.; Windmolders, S.; Willems, L.; Jamaer, L.; Dubois, J.; Bijnens, E.; Heuts, N.; et al. Cardiac Atrial Appendage Stem Cells Engraft and Differentiate into Cardiomyocytes in Vivo: A New Tool for Cardiac Repair after MI. Int. J. Cardiol. 2015, 201, 10–19. [Google Scholar] [CrossRef]
- Krul, S.P.J.; Berger, W.R.; Smit, N.W.; van Amersfoorth, S.C.M.; Driessen, A.H.G.; van Boven, W.J.; Fiolet, J.W.T.; van Ginneken, A.C.G.; van der Wal, A.C.; de Bakker, J.M.T.; et al. Atrial Fibrosis and Conduction Slowing in the Left Atrial Appendage of Patients Undergoing Thoracoscopic Surgical Pulmonary Vein Isolation for Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2015, 8, 288–295. [Google Scholar] [CrossRef]
- Nesapiragasan, V.; Hayıroğlu, M.İ.; Sciacca, V.; Sommer, P.; Sohns, C.; Fink, T. Catheter Ablation Approaches for the Treatment of Arrhythmia Recurrence in Patients with a Durable Pulmonary Vein Isolation. Balkan Med. J. 2023, 40, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Bartus, K.; Litwinowicz, R.; Natorska, J.; Zabczyk, M.; Undas, A.; Kapelak, B.; Lakkireddy, D.; Lee, R.J. Coagulation Factors and Fibrinolytic Activity in the Left Atrial Appendage and Other Heart Chambers in Patients with Atrial Fibrillation: Is There a Local Intracardiac Prothrombotic State? (HEART-CLOT Study). Int. J. Cardiol. 2020, 301, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Janga, P.; Pamulapati, H.; Kanmanthareddy, A.; Vallakati, A.; Gunda, S.; Bommana, S.; Jaeger, M.; Yeruva, M.R.; Lakkireddy, D. State of the Art in Left Atrial Appendage Ligation—The Lariat. J. Atr. Fibrillation 2014, 7, 1073. [Google Scholar] [PubMed]
- Ren, Z.; Zheng, Y.; Zhang, J.; Yang, H.; Wu, J.; Li, H.; Guo, R.; Meng, W.; Zhang, J.; Sun, H.; et al. Patients with Larger Left Atrial Appendage Orifice Presented Worse Prognosis Contributed by Acute Heart Failure after Left Atrial Appendage Closure. J. Am. Heart Assoc. 2022, 11, e026309. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Shapiro, A.D. Plasminogen Deficiency. Haemophilia 2008, 14, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Della-Morte, D.; Pacifici, F.; Ricordi, C.; Massoud, R.; Rovella, V.; Proietti, S.; Iozzo, M.; Lauro, D.; Bernardini, S.; Bonassi, S.; et al. Low Level of Plasminogen Increases Risk for Mortality in COVID-19 Patients. Cell Death Dis. 2021, 12, 773. [Google Scholar] [CrossRef]
- Jung, R.G.; Motazedian, P.; Ramirez, F.D.; Simard, T.; Di Santo, P.; Visintini, S.; Faraz, M.A.; Labinaz, A.; Jung, Y.; Hibbert, B. Association between Plasminogen Activator Inhibitor-1 and Cardiovascular Events: A Systematic Review and Meta-Analysis. Thromb. J. 2018, 16, 12. [Google Scholar] [CrossRef]
- Slodowska, K.M.; Batko, J.; Holda, J.P.; Dudkiewicz, D.; Koziej, M.; Litwinowicz, R.; Bartus, K.; Holda, M.K. Morphometrical Features of Left Atrial Appendage in the AF Patients Subjected to Left Atrial Appendage Closure. Folia Morphol. 2023, 82, 814–821. [Google Scholar] [CrossRef]
- Keragala, C.B.; Medcalf, R.L. Plasminogen: An Enigmatic Zymogen. Blood 2021, 137, 2881–2889. [Google Scholar] [CrossRef]
- Sillen, M.; Declerck, P.J. Thrombin Activatable Fibrinolysis Inhibitor (TAFI): An Updated Narrative Review. Int. J. Mol. Sci. 2021, 22, 3670. [Google Scholar] [CrossRef]
- Jilani, T.N.; Siddiqui, A.H. Tissue Plasminogen Activator. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cesari, M.; Pahor, M.; Incalzi, R.A. Plasminogen Activator Inhibitor-1 (PAI-1): A Key Factor Linking Fibrinolysis and Age-Related Subclinical and Clinical Conditions. Cardiovasc. Ther. 2010, 28, e72–e91. [Google Scholar] [CrossRef] [PubMed]
- Veinot, J.P.; Harrity, P.J.; Gentile, F.; Khandheria, B.K.; Bailey, K.R.; Eickholt, J.T.; Seward, J.B.; Tajik, A.J.; Edwards, W.D. Anatomy of the Normal Left Atrial Appendage. Circulation 1997, 96, 3112–3115. [Google Scholar] [CrossRef] [PubMed]
- Batko, J.; Jakiel, R.; Krawczyk–Ożóg, A.; Litwinowicz, R.; Hołda, J.; Bartuś, S.; Bartuś, K.; Hołda, M.K.; Konieczyńska, M. Definition and Anatomical Description of the Left Atrial Appendage Neck. Clin. Anat. 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Kelley, M.J.; Higgins, C.B. Etiologic Significance of Enlargement of the Left Atrial Appendage in Adults. Radiology 1982, 142, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kelley, M.J.; Elliott, L.P.; Shulman, S.T.; Ayoub, E.M.; Victorica, B.E.; Gessner, I.H. The Significance of the Left Atrial Appendage in Rheumatic Heart Disease. Circulation 1976, 54, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Di Biase, L.; Horton, R.P.; Nguyen, T.; Morhanty, P.; Natale, A. Left Atrial Appendage Studied by Computed Tomography to Help Planning for Appendage Closure Device Placement. J. Cardiovasc. Electrophysiol. 2010, 21, 973–982. [Google Scholar] [CrossRef]
- Sherwood, M.; Bliden, K.P.; Ilkhanoff, L.; Venkataraman, G.; Strickberger, A.; Yazdani, S.; McSwain, R.; Rashid, H.; Navarese, E.P.; Plummer, T.; et al. Detailed Thrombogenicity Phenotyping and 1 Year Outcomes in Patients Undergoing WATCHMAN Implantation: (TARGET-WATCHMAN) a Case–Control Study. J. Thromb. Thrombolysis 2020, 50, 484–498. [Google Scholar] [CrossRef]
- Asmarats, L.; O’Hara, G.; Champagne, J.; Paradis, J.-M.; Bernier, M.; O’Connor, K.; Beaudoin, J.; Junquera, L.; Del Val, D.; Muntané-Carol, G.; et al. Short-Term Oral Anticoagulation Versus Antiplatelet Therapy Following Transcatheter Left Atrial Appendage Closure. Circ. Cardiovasc. Interv. 2020, 13, e009039. [Google Scholar] [CrossRef]
- Duthoit, G.; Silvain, J.; Marijon, E.; Ducrocq, G.; Lepillier, A.; Frere, C.; Dimby, S.-F.; Popovic, B.; Lellouche, N.; Martin-Toutain, I.; et al. Reduced Rivaroxaban Dose Versus Dual Antiplatelet Therapy After Left Atrial Appendage Closure. Circ. Cardiovasc. Interv. 2020, 13, e008481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).