Eco-Friendly and COVID-19 Friendly? Decreasing the Carbon Footprint of the Operating Room in the COVID-19 Era
Abstract
:1. Introduction
- Identify the ecological footprint of surgery and its contribution to healthcare waste;
- Review the impact of COVID-19 on the carbon footprint of operating rooms;
- Identify eco-friendly practices that can be implemented in operating rooms to decrease their carbon footprint;
- Evaluate the feasibility and effectiveness of these eco-friendly practices in the context of the COVID-19 pandemic;
- Provide recommendations for healthcare facilities to adopt eco-friendly practices in their operating rooms to reduce their carbon footprint.
2. Materials and Methods
3. Results
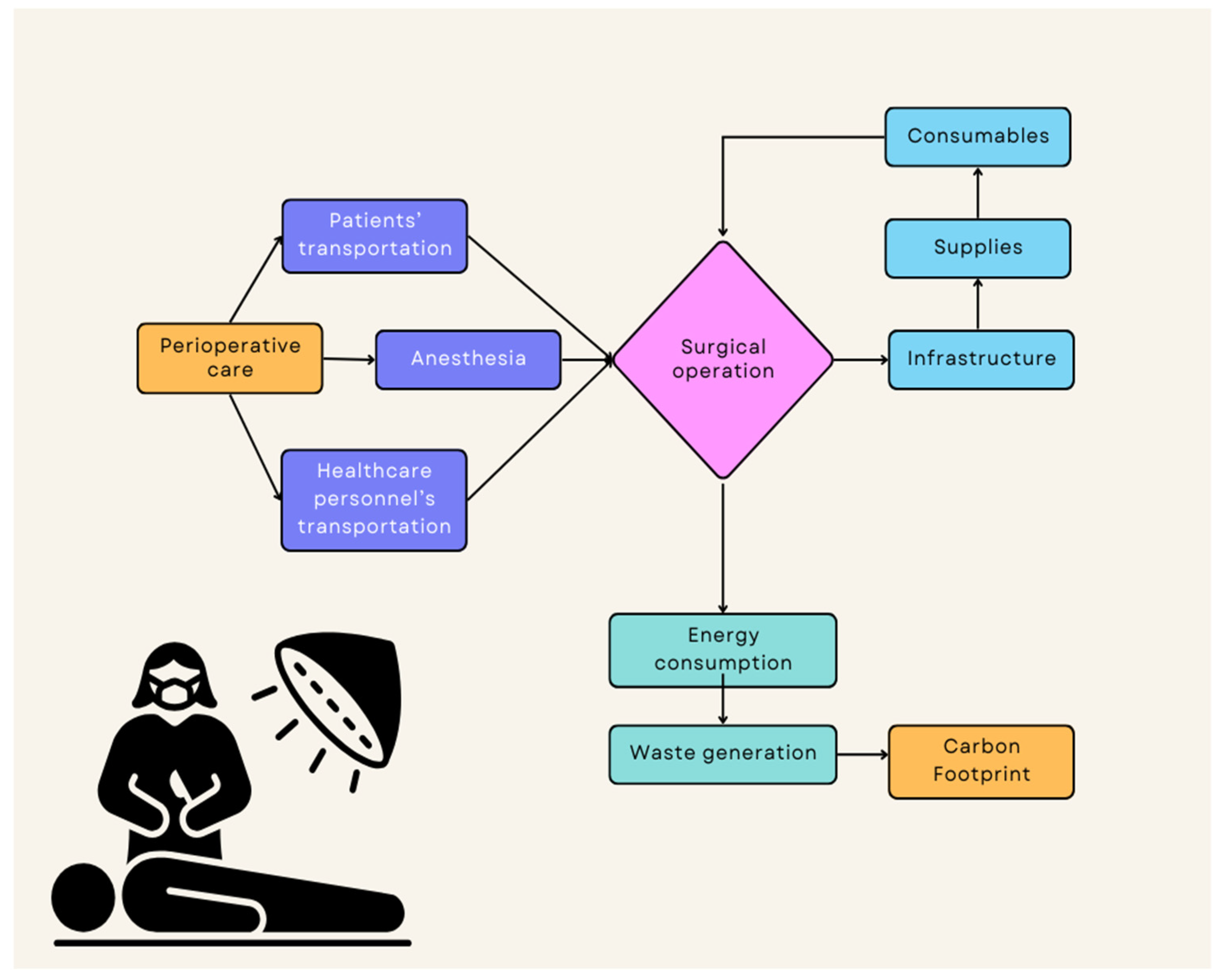
3.1. The Carbon Footprint of Surgical Operations
3.2. Review of Previous Recommendations
3.3. Application of Previous Recommendations during COVID-19 OR and What Are the New Steps Forward
3.4. Operative Treatment for Patients with COVID-19 and Other Airborne Pathogens
3.5. Making Operating Theaters Sustainable during the COVID-19 Pandemic
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rizan, C.; Steinbach, I.; Nicholson, R.; Lillywhite, R.; Reed, M.; Bhutta, M.F. The Carbon Footprint of Surgical Operations: A Systematic Review. Ann. Surg. 2020, 272, 986–995. [Google Scholar] [CrossRef]
- MacNeill, A.J.; Lillywhite, R.; Brown, C.J. The Impact of Surgery on Global Climate: A Carbon Footprinting Study of Operating Theatres in Three Health Systems. Lancet Planet Health 2017, 1, e360–e367. [Google Scholar] [CrossRef]
- Mattingly, A.S.; Rose, L.; Eddington, H.S.; Trickey, A.W.; Cullen, M.R.; Morris, A.M.; Wren, S.M. Trends in US Surgical Procedures and Health Care System Response to Policies Curtailing Elective Surgical Operations During the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2138038. [Google Scholar] [CrossRef]
- Frountzas, M.; Nikolaou, C.; Schizas, D.; Toutouzas, K.G. Personal Protective Equipment against COVID-19: Vital for Surgeons, Harmful for Patients? Am. J. Surg. 2020, 221, 772–774. [Google Scholar] [CrossRef]
- Coccolini, F.; Perrone, G.; Chiarugi, M.; Di Marzo, F.; Ansaloni, L.; Scandroglio, I.; Marini, P.; Zago, M.; De Paolis, P.; Forfori, F.; et al. Surgery in COVID-19 Patients: Operational Directives. World J. Emerg. Surg. 2020, 15, 25. [Google Scholar] [CrossRef]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B.; Blunt, H.; Brigham, T.; Chang, S.; et al. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef]
- Rizan, C.; Lillywhite, R.; Reed, M.; Bhutta, M.F. The Carbon Footprint of Products Used in Five Common Surgical Operations: Identifying Contributing Products and Processes. J. R Soc. Med. 2023, 116, 199–213. [Google Scholar] [CrossRef]
- ICAO Carbon Emissions Calculator (ICEC). Available online: https://www.icao.int/environmental-protection/Carbonoffset/Pages/default.aspx (accessed on 11 October 2023).
- Morris, D.S.; Wright, T.; Somner, J.E.A.; Connor, A. The Carbon Footprint of Cataract Surgery. Eye 2013, 27, 495–501. [Google Scholar] [CrossRef]
- Romanello, M.; Eckelman, M.J.; Tennison, I.; Roschnik, S.; Ashby, B.; Boyd, R.; Hamilton, I.; Oreszczyn, T.; Owen, A.; Romanello, M.; et al. Health Care’s Response to Climate Change: A Carbon Footprint Assessment of the NHS in England. Artic. Lancet Planet Health 2021, 5, 84–92. [Google Scholar]
- Van Demark, R.E.; Smith, V.J.S.; Fiegen, A. Lean and Green Hand Surgery. J. Hand Surg. 2018, 43, 179–181. [Google Scholar] [CrossRef]
- Reuse of Disposables. Reprocessing Issues Taking Users down “Slippery Slope”. OR Manag. 1996, 12, 7.
- Kenny, C.; Priyadarshini, A. Review of Current Healthcare Waste Management Methods and Their Effect on Global Health. Healthcare 2021, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.T.; Malmrose, J.; Riley, C.A.; Tolisano, A.M. Operating Room Waste Generated Across Otolaryngology Cases. Mil. Med. 2021, 188, e1697–e1700. [Google Scholar] [CrossRef]
- Kwakye, G.; Brat, G.A.; Makary, M.A. Green Surgical Practices for Health Care. Arch. Surg. 2011, 146, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Allegranzi, B.; Bischoff, P.; de Jonge, S.; Kubilay, N.Z.; Zayed, B.; Gomes, S.M.; Abbas, M.; Atema, J.J.; Gans, S.; van Rijen, M.; et al. New WHO Recommendations on Preoperative Measures for Surgical Site Infection Prevention: An Evidence-Based Global Perspective. Lancet Infect. Dis. 2016, 16, e276–e287. [Google Scholar] [CrossRef]
- Sehulster, L.; Chinn, R.Y.W. Guidelines for Environmental Infection Control in Health-Care Facilities Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm. Rep. 2003, 52, 1–42. [Google Scholar] [PubMed]
- Nastase, I.; Croitoru, C.; Vartires, A.; Tataranu, L. Indoor Environmental Quality in Operating Rooms: An European Standards Review with Regard to Romanian Guidelines. Energy Procedia 2016, 85, 375–382. [Google Scholar] [CrossRef]
- Katz, J.D. Control of the Environment in the Operating Room. Anesth. Analg. 2017, 125, 1214–1218. [Google Scholar] [CrossRef]
- Su, X.; Sutarlie, L.; Loh, X.J. Sensors and Analytical Technologies for Air Quality: Particulate Matters and Bioaerosols. Chem. Asian J. 2020, 15, 4241–4255. [Google Scholar] [CrossRef]
- Gormley, T.; Markel, T.A.; Jones, H.W.; Wagner, J.; Greeley, D.; Clarke, J.H.; Abkowitz, M.; Ostojic, J. Methodology for Analyzing Environmental Quality Indicators in a Dynamic Operating Room Environment. Am. J. Infect. Control. 2017, 45, 354–359. [Google Scholar] [CrossRef]
- Guide to Infection Control in the Healthcare Setting—ISID. Available online: https://isid.org/guide/ (accessed on 9 October 2023).
- Infection Prevention and Control during Health Care for Probable or Confirmed Cases of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Infection. Available online: https://www.who.int/publications-detail-redirect/10665-174652 (accessed on 9 October 2023).
- Reich, P.; Elward, A. Infection Prevention during the Coronavirus Disease 2019 Pandemic. Infect. Dis. Clin. N. Am. 2022, 36, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.F.; Köhler, F.; Germer, C.T.; Flemming, S.; Wiegering, A. Impact of COVID-19 on Elective and Emergency Colorectal Surgery. Chirurg 2021, 92, 924–928. [Google Scholar] [CrossRef]
- Nepogodiev, D.; Omar, O.M.; Glasbey, J.C.; Li, E.; Simoes, J.F.F.; Abbott, T.E.F.; Ademuyiwa, A.O.; Biccard, B.M.; Chaudhry, D.; Davidson, G.H.; et al. Elective Surgery Cancellations Due to the COVID-19 Pandemic: Global Predictive Modelling to Inform Surgical Recovery Plans. Br. J. Surg. 2020, 107, 1440–1449. [Google Scholar] [CrossRef]
- Livingston, E.H. Surgery in a Time of Uncertainty: A Need for Universal Respiratory Precautions in the Operating Room. J. Am. Med. Assoc. 2020, 323, 2254–2255. [Google Scholar] [CrossRef] [PubMed]
- Bhangu, A.; Lawani, I.; Ng-Kamstra, J.S.; Wang, Y.; Chan, A.; Futaba, K.; Ng, S.; Ebele, E.; Lederhuber, H.; Tabiri, S.; et al. Global Guidance for Surgical Care during the COVID-19 Pandemic. Br. J. Surg. 2020, 107, 1097–1103. [Google Scholar] [CrossRef]
- Søreide, K.; Hallet, J.; Matthews, J.B.; Schnitzbauer, A.A.; Line, P.D.; Lai, P.B.S.; Otero, J.; Callegaro, D.; Warner, S.G.; Baxter, N.N.; et al. Immediate and Long-Term Impact of the COVID-19 Pandemic on Delivery of Surgical Services. Br. J. Surg. 2020, 107, 1250–1261. [Google Scholar] [CrossRef]
- Jayaweera, M.; Perera, H.; Gunawardana, B.; Manatunge, J. Transmission of COVID-19 Virus by Droplets and Aerosols: A Critical Review on the Unresolved Dichotomy. Environ. Res. 2020, 188, 109819. [Google Scholar] [CrossRef]
- Jessop, Z.M.; Dobbs, T.D.; Ali, S.R.; Combellack, E.; Clancy, R.; Ibrahim, N.; Jovic, T.H.; Kaur, A.J.; Nijran, A.; O’Neill, T.B.; et al. Personal Protective Equipment for Surgeons during COVID-19 Pandemic: Systematic Review of Availability, Usage and Rationing. Br. J. Surg. 2020, 107, 1262–1280. [Google Scholar] [CrossRef]
- Brat, G.A.; Hersey, S.; Chhabra, K.; Gupta, A.; Scott, J. Protecting Surgical Teams During the COVID-19 Outbreak: A Narrative Review and Clinical Considerations. Ann. Surg. 2023, 278, e957–e959. [Google Scholar] [CrossRef]
- Perrone, G.; Giuffrida, M.; Bellini, V.; Lo Coco, A.; Pattonieri, V.; Bonati, E.; Del Rio, P.; Bignami, E.G.; Catena, F. Operating Room Setup: How to Improve Health Care Professionals Safety During Pandemic COVID-19-A Quality Improvement Study. J. Laparoendosc. Adv. Surg. Tech. A 2021, 31, 85–89. [Google Scholar] [CrossRef]
- Prakash, L.; Dhar, S.A.; Mushtaq, M. COVID-19 in the Operating Room: A Review of Evolving Safety Protocols. Patient Saf. Surg. 2020, 14, 30. [Google Scholar] [CrossRef]
- Coccia, M. Preparedness of Countries to Face COVID-19 Pandemic Crisis: Strategic Positioning and Factors Supporting Effective Strategies of Prevention of Pandemic Threats. Environ. Res. 2022, 203, 111678. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Pastore, C.B.P.; Deckers, P.; Halla, I.K.M.W.; Dias, A.L.R.; da Mata, M.V.M.; do Nascimento Martins, A.; Viu, M.M.; Lopez, R.V.M.; Yamada, A.D. Oncological Surgery During the COVID-19 Pandemic: Effectiveness of Preoperative Screening and Factors Associated with Postoperative SARS-CoV-2 Infection. Ann. Surg. Oncol. 2022, 29, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Hoernke, K.; Djellouli, N.; Andrews, L.; Lewis-Jackson, S.; Manby, L.; Martin, S.; Vanderslott, S.; Vindrola-Padros, C. Frontline Healthcare Workers’ Experiences with Personal Protective Equipment during the COVID-19 Pandemic in the UK: A Rapid Qualitative Appraisal. BMJ Open 2021, 11, e046199. [Google Scholar] [CrossRef]
- Leyva Moraga, F.A.; Leyva Moraga, E.; Leyva Moraga, F.; Juanz González, A.; Ibarra Celaya, J.M.; Ocejo Gallegos, J.A.; Barreras Espinoza, J.A. Aerosol Box, An Operating Room Security Measure in COVID-19 Pandemic. World J. Surg. 2020, 44, 2049–2050. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lei, J.; Li, J.; Zhang, Z.; Yu, Z.; Du, C. Design Characteristics on the Indoor and Outdoor Air Environments of the COVID-19 Emergency Hospital. J. Build. Eng. 2022, 45, 103246. [Google Scholar] [CrossRef]
- Urban, M.J.; Patel, T.R.; Raad, R.; LoSavio, P.; Stenson, K.; Al-Khudari, S.; Nielsen, T.; Husain, I.; Smith, R.; Revenaugh, P.C.; et al. Implementation of Preoperative Screening Protocols in Otolaryngology During the COVID-19 Pandemic. Otolaryngol. Head Neck Surg. 2020, 163, 265–270. [Google Scholar] [CrossRef]
- Black, J.R.M.; Bailey, C.; Przewrocka, J.; Dijkstra, K.K.; Swanton, C. COVID-19: The Case for Health-Care Worker Screening to Prevent Hospital Transmission. Lancet 2020, 395, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- COVIDSurg Collaborative SARS-CoV-2 Vaccination Modelling for Safe Surgery to Save Lives: Data from an International Prospective Cohort Study—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7995808/ (accessed on 9 October 2023).
- Thiel, C.L.; Woods, N.C.; Bilec, M.M. Strategies to Reduce Greenhouse Gas Emissions from Laparoscopic Surgery. Am. J. Public Health 2018, 108, S158–S164. [Google Scholar] [CrossRef] [PubMed]
- The Paris Agreement|UNFCCC. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 27 October 2023).
- Engström, G.; Gars, J.; Jaakkola, N.; Lindahl, T.; Spiro, D.; van Benthem, A.A. What Policies Address Both the Coronavirus Crisis and the Climate Crisis? Environ. Resour. Econ. 2020, 76, 789. [Google Scholar] [CrossRef]
- Tanaka, K.; Azar, C.; Boucher, O.; Ciais, P.; Gaucher, Y.; Johansson, D.J.A. Paris Agreement Requires Substantial, Broad, and Sustained Policy Efforts beyond COVID-19 Public Stimulus Packages. Clim. Change 2022, 172, 1. [Google Scholar] [CrossRef]
- Banhidy, F.P.; Banhidy, N.F. The Role and Duty of Global Surgery in Increasing Sustainability and Improving Patient Care in Low and Middle-Income Countries. Cureus 2022, 14, e30023. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, S.S.; Ngangue, P.; Soubeiga, D.; Barro, A.; Pilabré, A.H.; Bationo, N.; Pafadnam, Y.; Drabo, K.M.; Hien, H.; Savadogo, G.B.L. Barriers and Facilitators for the Sustainability of Digital Health Interventions in Low and Middle-Income Countries: A Systematic Review. Front. Digit. Health 2022, 4, 1014375. [Google Scholar] [CrossRef]
- Yang, C.; Hao, Y.; Irfan, M. Energy Consumption Structural Adjustment and Carbon Neutrality in the Post-COVID-19 Era. Struct. Change Econ. Dyn. 2021, 59, 442. [Google Scholar] [CrossRef] [PubMed]
- Andrijevic, M.; Schleussner, C.F.; Gidden, M.J.; McCollum, D.L.; Rogelj, J. COVID-19 Recovery Funds Dwarf Clean Energy Investment Needs. Science 2020, 370, 298–300. [Google Scholar] [CrossRef]
- Ng-Kamstra, J.S.; Greenberg, S.L.M.; Abdullah, F.; Amado, V.; Anderson, G.A.; Cossa, M.; Costas-Chavarri, A.; Davies, J.; Debas, H.T.; Dyer, G.S.M.; et al. Global Surgery 2030: A Roadmap for High Income Country Actors. BMJ Glob. Health 2016, 1, e000011. [Google Scholar] [CrossRef]
- Sharma, M.G.; Popli, H. Challenges for Lower-Middle-Income Countries in Achieving Universal Healthcare: An Indian Perspective. Cureus 2023, 15, e33751. [Google Scholar] [CrossRef]
- Cunha, M.F.; Pellino, G. Environmental Effects of Surgical Procedures and Strategies for Sustainable Surgery. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 399. [Google Scholar] [CrossRef] [PubMed]
- Guetter, C.R.; Williams, B.J.; Slama, E.; Arrington, A.; Henry, M.C.; Möller, M.G.; Tuttle-Newhall, J.E.; Stein, S.; Crandall, M. Greening the Operating Room. Am. J. Surg. 2018, 216, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, G.A.; Reiter, A.J.; Hu, A.; Smith, C.; Storton, K.; Gulack, B.C.; Shah, A.N.; Dsida, R.; Raval, M.V. Operating Room Recycling: Opportunities to Reduce Carbon Emissions Without Increases in Cost. J. Pediatr. Surg. 2023, 58, 2187–2191. [Google Scholar] [CrossRef]
- Petit, H.J.; Sullivan, G.A.; Hughes, I.M.; Pittman, K.L.; Myers, J.A.; Cocoma, S.M.; Gulack, B.C.; Shah, A.N. Exploring Barriers and Facilitators to Reducing the Environmental Impact of the Operating Room. J. Surg. Res. 2023, 292, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Cleary, S.M.; Wilkinson, T.; Tamandjou Tchuem, C.R.; Docrat, S.; Solanki, G.C. Cost-Effectiveness of Intensive Care for Hospitalized COVID-19 Patients: Experience from South Africa. BMC Health Serv. Res. 2021, 21, 82. [Google Scholar] [CrossRef]
- Page BKin, B.M.; Urbach, D.R.; Brull, R. Optimizing Timing of Completion of the Surgical Safety Checklist to Account for Emergence from Anesthesia. CMAJ Can. Med. Assoc. J. 2022, 194, E650. [Google Scholar] [CrossRef] [PubMed]
- Vinden, C.; Malthaner, R.; McGee, J.; McClure, J.A.; Winick-Ng, J.; Liu, K.; Nash, D.M.; Welk, B.; Dubois, L. Teaching Surgery Takes Time: The Impact of Surgical Education on Time in the Operating Room. Can. J. Surg. 2016, 59, 87. [Google Scholar] [CrossRef] [PubMed]
- McGee, J.; Winick-Ng, J.; McClure, J.A.; Penava, D.; Lovett, E.; Vinden, C.; McKeown, M. Resident Trainees Increase Surgical Time: A Comparison of Obstetric and Gynaecologic Procedures in Academic Versus Community Hospitals. J. Obstet. Gynaecol. Can. 2020, 42, 430–438.e2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsagkaris, C.; Saeed, H.; Laubscher, L.; Eleftheriades, A.; Stavros, S.; Drakaki, E.; Potiris, A.; Panagiotopoulos, D.; Sioutis, D.; Panagopoulos, P.; et al. Eco-Friendly and COVID-19 Friendly? Decreasing the Carbon Footprint of the Operating Room in the COVID-19 Era. Diseases 2023, 11, 157. https://doi.org/10.3390/diseases11040157
Tsagkaris C, Saeed H, Laubscher L, Eleftheriades A, Stavros S, Drakaki E, Potiris A, Panagiotopoulos D, Sioutis D, Panagopoulos P, et al. Eco-Friendly and COVID-19 Friendly? Decreasing the Carbon Footprint of the Operating Room in the COVID-19 Era. Diseases. 2023; 11(4):157. https://doi.org/10.3390/diseases11040157
Chicago/Turabian StyleTsagkaris, Christos, Hamayle Saeed, Lily Laubscher, Anna Eleftheriades, Sofoklis Stavros, Eirini Drakaki, Anastasios Potiris, Dimitrios Panagiotopoulos, Dimos Sioutis, Periklis Panagopoulos, and et al. 2023. "Eco-Friendly and COVID-19 Friendly? Decreasing the Carbon Footprint of the Operating Room in the COVID-19 Era" Diseases 11, no. 4: 157. https://doi.org/10.3390/diseases11040157
APA StyleTsagkaris, C., Saeed, H., Laubscher, L., Eleftheriades, A., Stavros, S., Drakaki, E., Potiris, A., Panagiotopoulos, D., Sioutis, D., Panagopoulos, P., & Zil-E-Ali, A. (2023). Eco-Friendly and COVID-19 Friendly? Decreasing the Carbon Footprint of the Operating Room in the COVID-19 Era. Diseases, 11(4), 157. https://doi.org/10.3390/diseases11040157








